Abstract
Canine cutaneous mast cell tumour (CMCT) is a common cutaneous tumour in dog, with a higher incidence than in human. CMCT is classified in three subgroups, well and intermediately differentiated (G1 and G2), corresponding to a benign disease, and poorly differentiated (G3), corresponding to a malignant disease, which metastasize to lymph nodes, liver, spleen and bone marrow. In this study, we have evaluated serum (S), platelet-poor plasma (P-PP), plasma-activated platelet rich (P-APR) and cytosol vascular endothelial growth factor (VEGF) concentrations, microvascular density (MVD) and mast cell density (MCD) in a series of 86 CMCTs and we have correlated these parameters with each other, by means of ELISA detection of VEGF and immunohistochemistry. Results show that VEGF level from cytosol P-APR and MVD were significantly higher in G3 CMCTs as compared to G1 or G2 subgroups. Moreover, a significantly strong correlation among VEGF levels from P-PAR and cytosol, MVD and MCD was found in G3 subgroup. Because VEGF levels from P-APR well correlated with MVD and malignancy grade in CMCT, we suggest that VEGF might be secreted from MCs and it may be a suitable surrogate inter-species angiogenetic markers of tumour progression in CMCT. Finally, CMCT seems to be a useful model to study the role of MCs in tumour angiogenesis and inhibition of MCs degranulation or activation might be a new anti-angiogenic strategy worthy to further investigations.
Keywords: angiogenesis, canine mast cell tumour, mast cells, microvascular density, plasma-activated platelets rich, vascular endothelial growth factor
Introduction
Angiogenesis is a complex process involved in both growth and progression of several human and animal tumours [1, 2]. This process is regulated by several pro-angiogenic factors, such as vascular endothelial growth factor (VEGF), thymidine phosphory-lase (TP), fibroblast growth factor-2 (FGF-2) and anti-angiogenic factors, such as thrombospondin-1, angiostatin and endostatin [3, 4]. Anti-angiogenesis is a therepeutic strategy in the treatment of tumours. Among various molecules, a recombinant humanized monoclonal antibody against VEGF has been approved for the treatment of human tumours [5].
Inflammatory cells, such as macrophages, lymphocytes and mast cells (MCs), play a major role in tumour angiogenesis by means of angiogenic cytokines stored in their cytoplasm [6–9]. MCs are involved in neovascularization in experimentally induced tumour, accumulate near to tumour cells before the angiogenesis onset and participate in the metastatic spreading of primary tumours [10–13]. Moreover, MCs' involvement in tumour angiogenesis has been demonstrated in several human solid and haematological malignancies [14–23].
Cutaneous canine mast cell tumour (CMCT) is a common cutaneous tumour in dog, with an incidence much higher than that found in human [24–27]. CMCT is classified in three subgroups, well and intermediately differentiated (G1 and G2), corresponding to a benign disease, and poorly differentiated (G3), corresponding to a malignant disease which metastasize to lymph nodes, liver, spleen and bone marrow with a short overall survival [28].
In this study, we have evaluated serum (S), platelet-poor plasma (P-PP), plasma-activated platelet rich (P-APR) and cytosol VEGF concentrations, microvascular density (MVD) and mast cell density (MCD) in a series of 86 CMCTs and we have correlated them with each other.
Material and methods
Blood samples
Blood samples were obtained from a series of 86 cases of CMCT. Venous blood was dispensed into a serum separator tube (Becton Dickinson Vacutainer Systems, Plymouth, UK) for S, into a sodium citrate, theophylline, adenosine, dipyridamole tubes for plasma (CTAD) (Becton Dickinson Hemogard Vacutainer Systems) for each case, respectively [29, 30]. In order to generate S, blood was allowed to coagulate for at least 30 min. at 37°C and then centrifuged at 1500 × g × 15 min. [29]. CTAD samples were centrifuged at 180 × g × 10 min. [30]. The supernatant was carefully removed from the centre portion of the liquid phase using a polyethylene Pasteur pipette to obtain a platelet-rich plasma. After removing the supernatant, the samples were again centrifuged at 1500 × gx15 min. to obtain a P-PP After subjecting the platelet-rich plasma to platelet count (Automated Haematology Analyser SE-9000 RET/SYSMEX Corporation, Kobe, Japan), it was treated with thrombin (80 ml/ml) and incubated for 30 min. at room temperature. After centrifugation at 1500 × g × 15 min., the aggregated platelet sediment was eliminated and the supernatant, P-APR, was obtained. All samples were stored at −80°C until VEGF detection [29–31].
VEGF ELISA detection
Circulating VEGF levels were detected using the Quantikine Human VEGF-enzyme-linked immuno-absorbent assay (ELISA) (R&D Systems Inc. Minneapolis, MN, USA), which recognize VEGF165[32–34]. Briefly 100 μl of S, P-PP and P-APR samples diluted in 100 μl of buffer solutions and serially diluted standard solutions (VEGF) were added to 96-well microtitre plates precoated with murine anti-VEGF monoclonal antibody and incubated at room temperature for 2 hrs. After incubation, 200 μl of the secondary antibody, an enzyme-linked VEGF-specific polyclonal goat antibody, was added and incubation continued for 2 hrs at room temperature. Substrate solution was added and the reaction continued for 30 min. Optical density was determined on a microplate reader (Labsystems Multiskan MCC/340; Labsystems Thermo Fisher Scientific, Waltham, MA, USA) at 450 nm. According to the manufacturer, the minimum detectable dose of VEGF is less than 9.0 pg/ml. Values below 9 pg/ml were equalized to zero.
Tumour cytosol and VEGF ELISA detection
These analyses were performed on cryopreserved CMCTs samples collected after surgery. Briefly from the selected 86 CMCTs cases, representative tumour tissue were cut out and frozen in liquid nitrogen e next stored to −80°C for later analyses. These fresh tumour tissues were homogenized in 4 volumes of homogenization buffer [containing 10 mM Tris-hydrochloride (pH 7.4), 1.5 mM EDTA, 5 mM disodium molybdate, 100 ml/liter glycerol, and 1 mM monothioglycerol] and one-third volume of 1.0-mm glass beads (Biospec Products, Batleville, OK). Homogenization was performed at 4200 rpm for 10 s using a Mini-bead-beater (Biospec Products). Homogenates were then centrifuged at 20,000 × gat 4°C for 10 min. and the supernatants that corresponded to tumour cytosolic extracts were used for quantitative assay of VEGF. VEGF content in the tumour cytosol was measured by ELISA using the Quantikine human VEGF ELISA kit (R&D Systems, Minneapolis, MN, USA) as already described for circulating VEGF in the previously section. The concentration of VEGF in the cytosol was expressed as pg/mg.
Histochemical, immunohistochemical and double staining
A series of formalin-fixed and paraffin-embedded tissue samples obtained from 86 cases of CMCTs were utilized. Histological diagnosis was performed on almost six slides for each tumour sample stained with haematoxylin-eosin and Undritz method (Merck, Darmstadt, Germany), specific for red-blue methachromatical MCs identification [22]. Accordingly to Patnaik et al. [35], the cases were classified as follows: 31 were G1, corresponding to well differentiated CMTC, 27 were G2, corresponding to intermediate differentiated CMTC, and 28 were G3, corresponding to poorly differentiated CMTC.
For the evaluation of MVD and VEGF expression, a three-layer biotin-avidin-peroxidase system, as previously described was adopted [22, 36]. Briefly, six serial sections, for each tissue samples, were cut. After heating, slides were incubated with the rabbit polyclonal antibody anti-factor VIII-related antigen (FVIII-RA) (Dako, Glostrup, Denmark), used as an endothelial marker and with the rabbit polyclonal anti-VEGF (Santa Cruz Biotechnology, CA, USA) antibody. The bound antibodies were visualized by using biotinylated secondary antibody, avidin-biotin peroxidase complex and 3-amino-9-ethylcarbazole or fast red (Dako). Nuclear counter-stained was performed, for each tissue sample, with Gill's haematoxylin (Polysciences, Warrington, PA, USA). A double stain was also performed by using anti-FVIII-RA antibody and Undritz method to mark on the same slide both endothelial cells and MCs [22]. As a negative immunohistochemical control, no primary antibody was added.
The slides were morphometrically evaluated by using an image analysis system (Quantimet 500 Leica Microsystems, Wetzlar, Germany). Ten most vascularized areas (‘hot spot’) were selected at low magnification and single red-brown stained endothelial cells, endothelial cell clusters and microvessels, clearly separated from adjacent microvessels, tumour cells and other connective tissue elements and MC we counted at x400 fields and x1000 fields in oils [1, 20, 22]. Finally, serial sections were also evaluated for MCs reactive to VEGF.
Statistical analysis
Mean value ± standard deviations (S.D.) was evaluated for MVD, MCD, cytosol and circulating and VEGF concentrations in G1, G2 and G3 CMCTs subgroups. The significance of differences in MVD, MCD, cytosol and circulating VEGF concentrations in serum and plasma compartments, between G1 versus G2, G2 versus G3 and G3 versus G1 tumour groups, was performed by Student's t-test. Correlations among MVD, cytosol VEGF concentrations, circulating VEGF concentrations and MCD each to other were calculated using Pearson's (r) analysis. All statistical analyses were performed with the SPSS statistical software package (SPSS, Inc., Chicago, IL, USA).
Results
No significant difference was found among G1, G2 and G3 CMCTs subgroups as concerns S-VEGF and P-PP VEGF (Table 1). Otherwise, VEGF mean levels from P-APR and cytosol were significantly higher in G3 (368 ± 132 pg/ml S.D.; 776 ± 257 pg/mg S.D.) as compared to G1 (99 ± 45 pg/ml S.D.; 198 ± 106 pg/mg S.D.) or G2 (126 ± 57 pg/ml S.D.; 245 ± 152 pg/mg S.D.) (P ranging from 0.001 to 0.005) CMCTs subgroups (Table 1).
1.
All angiogenetic indexes analysed means ± standard deviations as a function of tumour malignancy grade and statistical significance of their changes between G1 versus G2, G1 versus G3 and G2 versus G3 CMCT goups by Student's t-test
| CMCTs (number of cases) | MVD 400x (0.19 mm 2) | MVD 1000x (0.06 mm 2) | MCD 400x (0.19 mm 2) | MCD 1000x (0.06 mm 2) | VEGF levels from S pg/ml | VEGF levels from P-PP pg/ml | VEGF levels from P-APR pg/ml | VEGF levels from cytosol pg/mg | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 (31) | 8 ± 4 | 4 ± 2 | 109 ± 59 | 31 ± 19 | 427 ± 261 | 29 ± 21 | 99 ± 45 | 198 ± 106 | |||||||||||||||||||||||||||||||||||
| G2 (27) | 9 ± 5 | 4 ± 3 | 105 ± 52 | 29 ± 16 | 441 ± 274 | 28 ± 19 | 126 ± 57 | 245 ± 152 | |||||||||||||||||||||||||||||||||||
| G3 (28) | 27 ± 10 | 9 ± 3 | 116 ± 63 | 33 ± 20 | 437 ± 279 | 3 1 ± 23 | 368 ± 132 | 776 ± 257 | |||||||||||||||||||||||||||||||||||
| P value (t-test) | G1 versus G2 | G1 versus G2 | G1 versus G2 | G1 versus G2 | G1 versus G2 | G1 versus G2 | G1 versus G2 | G1 versus G2 | |||||||||||||||||||||||||||||||||||
| n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||||||||||||||||||||||||||||||||
| G1 versus G3 | G1 versus G3 | G1 versus G | G1 versus G3 | G1 versus G3 | G1 versus G3 | G1 versus G3 | G1 versus G3 | ||||||||||||||||||||||||||||||||||||
| P = 0.002 | P = 0.004 | n.s. | n.s. | n.s. | n.s. | P = 0.001 | P = 0.002 | ||||||||||||||||||||||||||||||||||||
| G2 versus G3 | G2 versus G3 | G2 versus G3 | G2 versus G3 | G2 versus G3 | G2 versus G3 | G2 versus G3 | G2 versus G3 | ||||||||||||||||||||||||||||||||||||
| P = 0.003 | P = 0.004 | n.s. | n.s. | n.s. | n.s. | P = 0.002 | P = 0.003 | ||||||||||||||||||||||||||||||||||||
As concerns MVD, it was significantly higher in G3 as compared to G1 or G2 CMCTs subgroups (Figs. 1, 2 and Table 1). As concerns MC characteristics, they were often degranulated or degranulating with less or not methacromatic cytoplasmatic granules in G3 as compared to G1 or G2 CMCTs subgroups in slides stained with both immunohistochemistry and Undritz method. Furthermore, MCs were often clustered near or around to microvessels in G3 as compared to G1 or G2 CMCTs subgroups (Fig. 2). No significantly differences was found among the three subgroups in term of MCD (Table 1).
1.
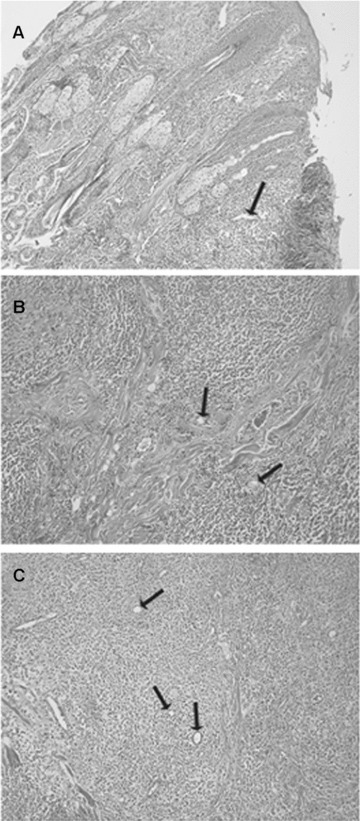
Haematoxylin-eosin staining of CMCTs in a low vascularized well-differentiated (G1) tumour (A), low vascularized intermediately differentiated (G2) tumour (B) and high vascularized poorly differentiated (G3) tumour (C). Single arrows indicate blood vessels. Original magnification: A–C, x40.
2.
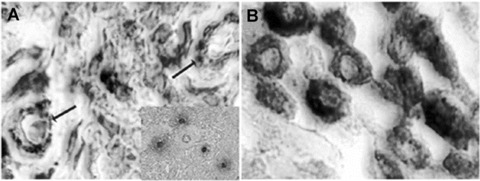
Highly vascularized poorly differentiated (G3) CMCT (A) and low vascularized well-differentiated (G1) CMCT (B) double stained with immuno-histochemical method for vessels identification by using an antibody-anti FVIII-RA and with histochemical Undritz method for specific mast cells identification. In (A), arrows indicate two microvessels (red-brown colour) among several irregular pleomorphic blue degranulated (in inset in red) mast cells. In (B), several regular monomorphic blue granulated mast cells are recognizable. Original magnification: A, B, x1000.
As concerns VEGF immunoreactivity, both MCs and microvessels were positive to VEGF in G3 CMCTs subgroup (Fig. 3).
3.
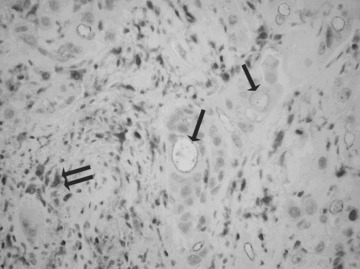
A double staining of microvessels (arrows) and mast cells (double arrow) by using an antibody anti-VEGF in highly vascularized poorly differentiated (G3) CMCT. Original magnification: ×160.
A significantly correlation has been established between these parameters: circulating VEGF from P-APR and VEGF from cytosol (r = 0.83, P= 0.001); circulating VEGF from P-APR and MVD (r = 0.82, P= 0.001); circulating VEGF from P-APR and MCD (r = 0.76, P= 0.001); VEGF from cytosol and MVD (r = 0.71, P= 0.002); VEGF from cytosol and MCD (r = 0.69, P= 0.003); and MVD and MCD (r = 0.71, P= 0.002), only in G3 CMCT subgroup (Fig. 4).
4.
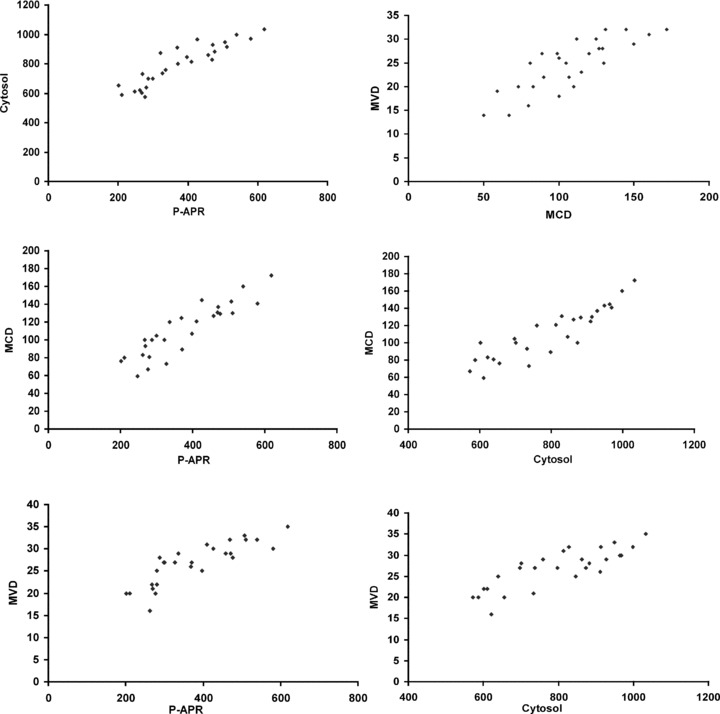
Correlation analysis in highly vascularized poorly differentiated (G3) CMCT subgroup between VEGF concentrations from P-APR and VEGF from cytosol (r = 0.83, P= 0.001); VEGF concentrations from P-APR and MVD (r = 0.82, P= 0.001); VEGF concentrations from P-APR and MCD (r = 0.76, P= 0.001); VEGF concentrations from cytosol and MVD (r = 0.71, P= 0.002); VEGF concentrations from cytosol and MCD (r = 0.69, P= 0.003) MVD and MCD (r = 0.71, P= 0.002).
Discussion
This is the first report describing the relationship between cytosol and circulating VEGF levels, MVD and MCD in regulating tumour angiogenesis and progression of CMCT spontaneous model. MCs' involvement in tumour angiogenesis has been demonstrated in several human solid and haematological malignancies [14–23]. Although MCs can secrete several pro-angiogenic molecules, including fibroblast growth factor-2 (FGF-2), tumour necrosis factor alpha (TNF-α), interleukin-8 (IL-8), transforming growth factor beta (TGF-β), tryptase and chymase, we have focused our attention on VEGF due to its main role in tumour angiogenesis, and to the possibility to inhibit angiogen-esis by using a humanized anti-VEGF antibody, as it has been demonstrated in pre-clinical and clinical setting [5].
Several reports in human tumours have suggested that serum VEGF could be unsuitable for sampling VEGF [29, 30, 37]. It has been demonstrated that VEGF is stored in the alpha granules and is released from platelets during clotting [38–40]. Therefore, serum VEGF may be an inaccurate indicator of circulating VEGF due to its release during sampling. Accordingly, in this study no correlations between serum VEGF and MVD and between serum VEGF and malignancy grade of CMCTs has been found.
As concerns P-PP blood fraction, we found low VEGF levels and no statistically significant differences between P-PP and other angiogenic and grading parameters. The low VEGF levels in P-PP blood fraction suggests that VEGF from serum samples is derived from platelets during the coagulation process. Accordingly, VEGF levels in the P-PP could reflect the excess of circulating VEGF to the steady state with platelets levels without any biological clinical significance.
VEGF levels in P-APR serve to discriminate between cancer patients and healthy controls, suggesting that VEGF from P-APR may play a role in cancerogenesis [29, 30]. Human MCs can synthesize and secrete VEGF contained in their secretory granules, whereas platelets represent the main reservoir of circulating blood VEGF and they can endocytose and concentrate circulating plasma VEGF [41–49].
In this study, we have shown that VEGF level from both cytosol and P-APR is higher in G3 as compared to G2 or G1 CMCTs subgroups. It has been demonstrated that neoplastic MCs in dog mastocytomas and in the dog mastocytoma cell line C2 express the VEGF protein and VEGF mRNA expression in C2 cells was counteracted by LY294002 and rapamycin, suggesting an involvement of the PI3-kinase/mTOR pathway. In addition, rapamycin decreased the expression of VEGF in C2 cells at the mRNA and protein level without suppressing their proliferation [50].
Our data suggest that MCs of CMCTs are the main source of circulating VEGF protein, which may be released by degranulation and then stored in platelets. In fact, we have demonstrated that MCs in G3 CMCTs subgroup contained few or no cytoplasmatic granules and this parameter correlated with high MVD, high VEGF level from cytosol and high VEGF level from P-APR. On the contrary, MCs in G1 or G2 CMCTs were less degranulated, showing more methacromatic granules in their cytoplasm and these data correlate with low angiogenesis. Overall, we suggest that VEGF might be secreted by MCs and it may be a suitable surrogate inter-species angiogenic markers of malignancy.
In conclusion, spontaneous CMCTs seems to be a useful model to study the role of MCs in the angiogenic pathway and the inhibition of MC degranulation or activation might be a new anti-angiogenic strategy worthy to further investigations [51–54].
Acknowledgments
This work was supported in part by grants from Alleanza Contro il Cancro – Istituto Superiore di Sanità.
References
- 1.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 2.Patruno R, Zizzo N, Zito AF, Catalano V, Valerio P, Pellecchia V, D'Errico E, Mazzone F, Ribatti D, Ranieri G. Microvascular density and endothelial area correlate with Ki-67 proliferative rate in the canine non-Hodgkin's lymphoma spontaneous model. Leuk Lymphoma. 2006;47:1138–43. doi: 10.1080/10428190600565859. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis and tissue factor. Nat Med. 1996;2:209–15. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 4.Ranieri G, Grammatica L, Patruno R, Zito AF, Valerio P, Iacobellis S, Gadaleta C, Gasparini G, Ribatti D. A possible role of thymidine phosphorylase expression and 5-fluorouracil increased sensitivity in oropharyngeal cancer patients. J Cell Mol Med. 2007;11:362–8. doi: 10.1111/j.1582-4934.2007.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem. 2006;13:1845–57. doi: 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- 6.Nienartowicz A, Sobaniec-Lotowska ME, Jarocka-Cyrta E, Lemancewicz D. Mast cells in neoangiogenesis. Med Sci Monit. 2006;12:53–6. [PubMed] [Google Scholar]
- 7.Ribatti D, Nico B, Vacca A. Importance of the bone marrow microenvironment in inducing the angiogenic response in multiple myeloma. Oncogene. 2006;25:4257–66. doi: 10.1038/sj.onc.1209456. [DOI] [PubMed] [Google Scholar]
- 8.Polverini PJ. How the extracellular matrix and macrophages contribute to angiogenesis-dependent diseases. Eur J Cancer. 1996;32:2430–7. doi: 10.1016/s0959-8049(96)00386-3. [DOI] [PubMed] [Google Scholar]
- 9.Ribatti D, Nico B, Crivellato E, Vacca A. Macrophages and tumor angiogenesis. Leukemia. 2007;21:2085–9. doi: 10.1038/sj.leu.2404900. [DOI] [PubMed] [Google Scholar]
- 10.Kessler DA, Langer RS, Pless NA, Folkman J. Mast cells and tumor angiogenesis. Int J Cancer. 1976;18:703–9. doi: 10.1002/ijc.2910180520. [DOI] [PubMed] [Google Scholar]
- 11.Meininger CJ, Zetter BR. Mast cells and angiogenesis. Semin Cancer Biol. 1992;3:73–9. [PubMed] [Google Scholar]
- 12.Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355–71. doi: 10.1034/j.1600-0463.2002.100501.x. [DOI] [PubMed] [Google Scholar]
- 13.Ch'ng S, Wallis RA, Yuan L, Davis PF, Tan ST. Mast cells and cutaneous malignancies. Mod Pathol. 2006;19:149–59. doi: 10.1038/modpathol.3800474. [DOI] [PubMed] [Google Scholar]
- 14.Ribatti D, Molica S, Vacca A, Nico B, Crivellato E, Roccaro AM, Dammacco F. Tryptase-positive mast cells correlate positively with bone marrow angiogenesis in B-cell chronic lymphocytic leukemia. Leukemia. 2003;17:1428–30. doi: 10.1038/sj.leu.2402970. [DOI] [PubMed] [Google Scholar]
- 15.Ribatti D, Vacca A, Nico B, Quondamatteo F, Ria R, Minischetti M, Marzullo A, Herken R, Roncali L, Dammacco F. Bone marrow angiogenesis and mast cell density increase simultaneously with progression of human multiple myeloma. Br J Cancer. 1999;79:451–5. doi: 10.1038/sj.bjc.6690070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takanami I, Takeuchi K, Naruke M. Mast cell density is associated with angiogenesis and poor prognosis in pulmonary adenocarcinoma. Cancer. 2000;88:2686–92. [PubMed] [Google Scholar]
- 17.Elpek GO, Gelen T, Aksoy NH, Erdogan A, Dertsiz L, Demircan A, Keles N. The prognostic relevance of angiogenesis and mast cells in squamous cell carcinoma of the oesophagus. J Clin Pathol. 2001;54:940–4. doi: 10.1136/jcp.54.12.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo K, Muramatsu M, Okamoto Y, Jin D, Takai S, Tanigawa N, Miyazaki M. Expression of chymase-positive cells in gastric cancer and its correlation with the angiogenesis. J Surg Oncol. 2006;93:36–42. doi: 10.1002/jso.20394. [DOI] [PubMed] [Google Scholar]
- 19.Tuna B, Yorukoglu K, Unlu M, Mungan MU, Kirkali Z. Association of mast cells with microvessel density in renal cell carcinomas. Eur Urol. 2006;50:530–4. doi: 10.1016/j.eururo.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Ranieri G, Labriola A, Achille G, Florio G, Zito AF, Grammatica L, Paradiso A. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. Int J Oncol. 2002;21:1317–23. [PubMed] [Google Scholar]
- 21.Iamaroon A, Pongsiriwet S, Jittidecharaks S, Pattanaporn K, Prapayasatok S, Wanachantararak S. Increase of mast cells and tumor angiogenesis in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32:195–9. doi: 10.1034/j.1600-0714.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 22.Ranieri G, Passantino L, Patruno R, Passantino G, Jirillo F, Catino A, Mattioli V, Gadaleta C, Ribatti D. The dog mast cell tumour as a model to study the relationship between angiogenesis, mast cell density and tumour malignancy. Oncol Rep. 2003;10:1189–93. [PubMed] [Google Scholar]
- 23.Preziosi R, Sarli G, Paltrinieri M. Prognostic value of intratumoral vessel density in cutaneous mast cell tumors of the dog. J Comp Pathol. 2004;130:143–51. doi: 10.1016/j.jcpa.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Dobson JM, Scase TJ. Advances in the diagnosis and management of cutaneous mast cell tumours in dogs. J Small Anim Pract. 2007;48:424–31. doi: 10.1111/j.1748-5827.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 25.Horny HP, Valent P. Histopathological and immunohistochemical aspects of mastocytosis. Int Arch Allergy Immunol. 2002;127:115–7. doi: 10.1159/000048180. [DOI] [PubMed] [Google Scholar]
- 26.Wimazal F, Jordan JH, Sperr WR, Chott A, Dabbass S, Lechner K, Horny HP, Valent P. Increased angiogenesis in the bone marrow of patients with systemic mastocytosis. Am J Pathol. 2002;160:1639–45. doi: 10.1016/S0002-9440(10)61111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tefferi A, Pardanani A. Systemic mastocytosis: current concepts and treatment advances. Curr Hematol Rep. 2004;3:197–202. [PubMed] [Google Scholar]
- 28.Newman SJ, Mrkonjich L, Walker KK, Rohrbach BW. Canine subcutaneous mast cell tumour: diagnosis and prognosis. J Comp Pathol. 2007;136:231–9. doi: 10.1016/j.jcpa.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Ranieri G, Coviello M, Patruno R, Valerio P, Martino D, Milella P, Catalano V, Scotto F, De Ceglie A, Quaranta M, Ribatti D, Pellecchia A. Vascular endothelial growth factor concentrations in the plasma-activated platelets rich (P-APR) of healthy controls and colorectal cancer patients. Oncol Rep. 2004;12:817–20. [PubMed] [Google Scholar]
- 30.Ranieri G, Coviello M, Chiriatti A, Stea B, Montemurro S, Quaranta M, Dittadi R, Paradiso A. Vascular endothelial growth factor assessment in different blood fractions of gastrointestinal cancer patients and healthy controls. Oncol Rep. 2004;11:435–9. [PubMed] [Google Scholar]
- 31.Kato Y, Asano K, Mogi T, Kutara K, Teshima K, Edamura K, Tsumagari S, Hasegawa A, Tanaka S. Clinical significance of circulating vascular endothelial growth factor in dogs with mammary gland tumors. J Vet Med Sci. 2007;69:77–80. doi: 10.1292/jvms.69.77. [DOI] [PubMed] [Google Scholar]
- 32.Wergin MC, Roos M, Inteeworn N, Laluhova D, Allemann K, Kaser-Hotz B. The influence of fractionated radiation therapy on plasma vascular endothelial growth factor (VEGF) concentration in dogs with spontaneous tumors and its impact on outcome. Radiother Oncol. 2006;79:239–44. doi: 10.1016/j.radonc.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Troy GC, Huckle WR, Rossmeisl JH, Panciera D, Lanz O, Robertson JL, Ward DL. Endostatin and vascular endothelial growth factor concentrations in healthy dogs, dogs with selected neoplasia, and dogs with nonneoplastic diseases. J Vet Intern Med. 2006;20:144–50. doi: 10.1892/0891-6640(2006)20[144:eavegf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 34.Gentilini F, Calzolari C, Turba ME, Agnoli C, Fava D, Forni M, Bergamini PF. Prognostic value of serum vascular endothelial growth factor (VEGF) and plasma activity of matrix metalloproteinase (MMP) 2 and 9 in lymphoma-affected dogs. Leuk Res. 2005;29:1263–9. doi: 10.1016/j.leukres.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Patnaik AK, Ehler WY, MacEwen EG. Canine cutaneous mast cell tumors: morphologic grading and survival time in 83 dogs. Vet Pathol. 1984;21:469–74. doi: 10.1177/030098588402100503. [DOI] [PubMed] [Google Scholar]
- 36.Ranieri G, Patruno R, Lionetti A, Di Summa A, Mattioli E, Bufo P, Pellecchia A, Ribatti D, Zizzo N. Endothelial area and microvascular density in a canine non-Hodgkin's lymphoma: an interspecies model of tumor angiogenesis. Leuk Lymphoma. 2005;46:1639–43. doi: 10.1080/10428190500205150. [DOI] [PubMed] [Google Scholar]
- 37.Hormbrey E, Gillespie P, Turner K, Han C, Roberts A, McGrouther D, Harris AL. A critical review of vascular endothelial growth factor (VEGF) analysis in peripheral blood: is the current literature meaningful? Clin Exp Metastasis. 2002;19:651–63. doi: 10.1023/a:1021379811308. [DOI] [PubMed] [Google Scholar]
- 38.Werther K, Christensen IJ, Nielsen HJ. Determination of vascular endothelial growth factor (VEGF) in circulating blood: significance of VEGF in various leucocytes and platelets. Scand J Lab Invest. 2002;62:343–50. doi: 10.1080/00365510260296492. [DOI] [PubMed] [Google Scholar]
- 39.Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999;5:487–91. [PubMed] [Google Scholar]
- 40.Wergin MC, Kaser-Hotz B. Plasma vascular endothelial growth factor (VEGF) measured in seventy dogs with spontaneously occurring tumours. In Vivo. 2004;18:15–9. [PubMed] [Google Scholar]
- 41.Verheul HM, Lolkema MP, Qian DZ, Hilkes YH, Liapi E, Akkerman JW, Pili R, Voest EE. Platelets take up the monoclonal antibody bevacizumab. Clin Cancer Res. 2007;13:5341–7. doi: 10.1158/1078-0432.CCR-07-0847. [DOI] [PubMed] [Google Scholar]
- 42.Verheul HMW, Hoekman K, De Bakker SL, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187–90. [PubMed] [Google Scholar]
- 43.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, Lippert U, Henz BM, Moller A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–84. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;72:1227–36. doi: 10.4049/jimmunol.172.2.1227. [DOI] [PubMed] [Google Scholar]
- 45.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:665–75. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 46.Heissig B, Rafii S, Akiyama H, Ohki Y, Sato Y, Rafael T, Zhu Z, Hicklin DJ, Okumura K, Ogawa H, Werb Z, Hattori K. Low-dose irradiation promotes tissue revascularization through VEGF release from mast cells and MMP-9-mediated progenitor cell mobilization. J Exp Med. 2005;202:739–50. doi: 10.1084/jem.20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bosquiazzo VL, Ramos JG, Varayoud J, Munoz-de-Toro M, Luque EH. Mast cell degranulation in rat uterine cervix during pregnancy correlates with expression of vascular endothelial growth factor mRNA and angiogenesis. Reproduction. 2007;133:1045–55. doi: 10.1530/REP-06-0168. [DOI] [PubMed] [Google Scholar]
- 48.Russo A, Russo G, Peticca M, Pietropaolo C, Di Rosa M, Iuvone T. Inhibition of granuloma-associated angiogenesis by controlling mast cell mediator release: role of mast cell protease-5. Br J Pharmacol. 2005;145:24–33. doi: 10.1038/sj.bjp.0706112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crivellato E, Nico B, Vacca A, Ribatti D. Ultrastructural analysis of mast cell recovery after secretion by piecemeal degranulation in B-cell non-Hodgkin's lymphoma. Leuk Lymphoma. 2003;44:517–21. doi: 10.1080/1042819021000047001. [DOI] [PubMed] [Google Scholar]
- 50.Rebuzzi L, Willmann M, Sonneck K, Gleixner KV, Florian S, Kondo R, Mayerhofer M, Vales A, Gruze A, Pickl WF, Thalhammer JG, Valent P. Detection of vascular endothelial growth factor (VEGF) and VEGF receptors Flt-1 and KDR in canine mastocytoma cells. Vet Immunol Immunopathol. 2007;115:320–33. doi: 10.1016/j.vetimm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Isaji M, Miyata H, Ajisawa Y, Takehana Y, Yoshimura N. Tranilast inhibits the proliferation, chemotaxis and tube formation of human microvascular endothelial cells in vitro and angiogenesis in vivo. Br J Pharmacol. 1997;122:1061–6. doi: 10.1038/sj.bjp.0701493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xia Q, Yang S, Zhang SQ, Chen B, Wang DB, Zhu QX, Wang Y, Yan KL, He PP, Zhang XJ. The effect of mizolastine on expression of vascular endothelial cell growth factor, tumour necrosis factor-alpha and keratinocyte-derived chemokine in murine mast cells, compared with dexamethasone and loratadine. Clin Exp Dermatol. 2005;30:165–70. doi: 10.1111/j.1365-2230.2005.01721.x. [DOI] [PubMed] [Google Scholar]
- 53.Rusk A, McKeegan E, Haviv F, Majest S, Henkin J, Khanna C. Preclinical evaluation of antiangiogenic thrombospondin-1 pep-tide mimetics, ABT-526 and ABT-510, in companion dogs with naturally occurring cancers. Clin Cancer Res. 2006;12:7444–55. doi: 10.1158/1078-0432.CCR-06-0109. [DOI] [PubMed] [Google Scholar]
- 54.D'Cruz OJ, Uckun FM. Targeting mast cells in endometriosis with janus kinase 3 inhibitor, JANEX-1. Am J Reprod Immunol. 2007;58:75–97. doi: 10.1111/j.1600-0897.2007.00502.x. [DOI] [PubMed] [Google Scholar]


