Abstract
Objective
Cervical adenocarcinoma in situ (AIS) is increasing in incidence among reproductive-age women. Cervical conization is an alternative to hysterectomy that allows future fertility, however reports regarding the risk of residual AIS and underlying adenocarcinoma are conflicting. The purpose of this study was to determine the outcomes of a large cohort of women treated for AIS.
Methods
The medical records of 180 women with cervical AIS evaluated at the University of Texas MD Anderson Cancer Center and its outlying clinics between 1983 and 2011 were reviewed for demographic information, treatment history, pathologic findings and outcomes.
Results
The mean age at diagnosis was 33.8 years (range 17.6-76.1 years). 172 of the 180 women had at least one cone biopsy performed, with 110 (64.0%) undergoing a cold knife cone (CKC), and 62 (36.0%) undergoing a loop electrosurgical excision procedure (LEEP) as their initial method of treatment. Positive margins were noted in 35.0% of patients undergoing CKC compared with 55.6% undergoing LEEP (p=0.017). 71 patients ultimately underwent hysterectomy with residual disease noted in 10 patients (14.1%), 8 patients (11.3%) with residual AIS and 2 patients (2.8%) with invasive carcinoma. Of the 101 patients who did not undergo hysterectomy, 2 patients (2.0%) developed recurrent AIS at a median of 27.5 months (range 18- 37 months) from the last cone, and none developed invasive carcinoma.
Conclusion
Patients undergoing conservative management for AIS with cervical conization alone should be monitored closely and counseled regarding the potential risks of residual and recurrent disease, even when negative cone margins are obtained.
Introduction
Cervical adenocarcinoma in situ (AIS) was initially described in 1952 by Hepler et al. [1], and is recognized as a precursor to invasive cervical adenocarcinoma. Similar to squamous cervical intraepithelial neoplasia (CIN), infection with human papillomavirus (HPV) is necessary, yet insufficient, for the development of AIS [2,3]. As the mean age at diagnosis of AIS is in the mid to late 30s, many patients have not completed childbearing and a more conservative approach is preferred [4,5,6,7,8]. Although hysterectomy is the definitive therapy for AIS, cervical conization is considered an acceptable alternative in women who desire fertility preservation. There have been conflicting reports regarding rates of recurrence and residual disease in women undergoing cervical conization for AIS [2,4,5,7,9-14]. Therefore, the ideal management of women with AIS remains controversial.
In this study, we report the outcomes of a large cohort of patients with AIS, treated by either conservative means with cervical cone alone or with hysterectomy. The purpose of the study was to determine the rate of residual disease in patients undergoing hysterectomy, as well as to estimate the recurrence rate of AIS and progression to invasive adenocarcinoma in women managed conservatively.
Materials and Methods
We performed a retrospective cohort study at The University of Texas MD Anderson Cancer Center and its outlying clinics. Institutional Review Board approval was obtained with a waiver of informed consent. All patients diagnosed with cervical AIS between 1983 and 2011 were identified using computerized databases from the Departments of Gynecologic Oncology and Pathology. Pathology reports were reviewed and patients with AIS on Papanicolaou (Pap) test, cervical biopsy, cone specimen, or hysterectomy specimen were included. Patients with invasive disease were excluded. All pathology slides were read by a gynecologic pathologist with expertise in cervical cancer and pre-invasive disease.
One hundred and eighty patients were identified. Medical records were reviewed for demographic data, treatment history, pathologic findings, and outcomes. A positive margin was defined as AIS within 1 mm of the surgical margin. Residual disease was defined as AIS found in the pathology specimen of the subsequent procedure if performed within 3 months of the prior procedure. If the subsequent procedure was performed after 3 months, this was considered recurrent disease. The follow-up period was defined as the time between initial AIS diagnosis and the date of last contact.
Demographic and clinical characteristics were summarized using descriptive statistics. Fisher’s exact test was used to compare groups of interest for various outcomes. All p-values are 2-sided, and were considered significant if <0.05. All analyses were performed using SAS 9.1 for Windows (Copyright © 2002-2003 by SAS Institute Inc., Cary, NC) and StatXact-7© for Windows (Copyright © 2005, 1989-2005, Cytel Software Corporation, Cambridge, Massachusetts).
Results
One hundred and eighty patients were evaluated with cervical AIS between 1983 and 2011. Demographic information is shown in Table 1. The mean age at diagnosis was 33.8 years (range 17.6 to 76.1 years). Of the 150 patients with smoking history available, 31% were current or former smokers. The majority of patients (87%) were asymptomatic at the time of diagnosis. A co-existing squamous lesion was present in 99 of the 180 patients (55%).
Table 1.
Demographic and Clinical Characteristics
| Characteristic | |
|---|---|
|
| |
| Age (years) (N=180): | |
| Mean (Standard Deviation) | 33.8 (8.8) |
| Median (Range) | 32.7 (17.6-76.1) |
|
| |
| Body Mass Index (kg/m2) (N=133) | |
| Mean (Standard Deviation) | 25.9 (6.3) |
| Median (Range) | 24.0 (12.0 – 45.9) |
|
| |
| Race (N=179) | |
| African- American | 8 (4.5%) |
| Asian | 9 (5.0%) |
| Hispanic | 22 (12.3%) |
| White | 140 (78.2%) |
|
| |
| Smoking Status (N=150) | |
| Current | 25 (16.7%) |
| Previous | 21 (14.0%) |
| Never | 104 (69.3%) |
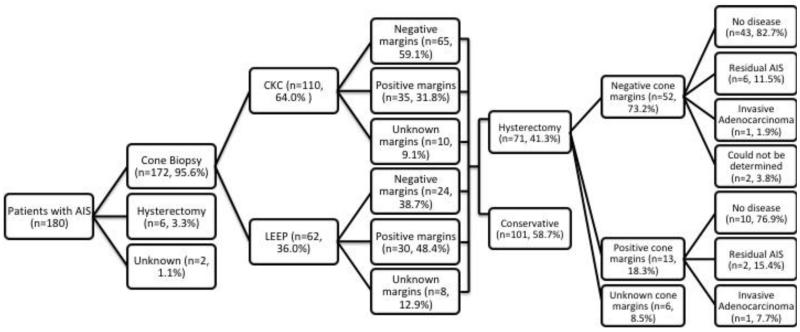
Primary management included cold knife cone (CKC) (n=110, 61.1%), loop electrosurgical excision procedure (LEEP) (n=62, 34.4%), hysterectomy (n=6, 3.3%), and was unknown in 2 patients (1.1%) (Figure 1). Of the 110 patients who underwent a CKC as their initial management, 100 had margin status available with 35 patients (35.0%) having positive cone margins, and 65 patients (65.0%) having negative cone margins (Table 2). Of the 62 patients who underwent a LEEP as their initial management, 54 had margin status available with 30 patients (55.6%) having positive cone margins and 24 patients (44.4%) had negative cone margins. LEEP was associated with a significantly higher rate of positive margins compared with CKC (p= 0.017).
Figure 1.
Patient treatment algorithm.
Table 2.
Margin status in patients undergoing cold knife cone (CKC) vs. loop electrocautery excision procedure (LEEP)
| Margin Status | CKC (N=100) | LEEP (N=54) |
|---|---|---|
| Positive | 35 (35.0%) | 30 (55.6%) |
| Negative | 65 (65.0%) | 24 (44.4%) |
p=0.017; margin status unknown in 17 patients.
An ECC was performed at first conization in 82 patients (48.0%), and was positive in 11 patients (13.4%). Of the 11 patients with a positive ECC, 10 underwent subsequent conization with 6 patients (60.0%) having residual AIS in the specimen. Of the 126 patients in whom focality was available, AIS was noted to be multifocal in 67 patients (53.2%). Patients with multifocal disease were noted to have higher rates of positive cone margins (n=38, 56.7%) compared with patients without multifocal disease (n=16, 27.1%) (p=<0.001).
Seventy-one of the 172 patients (41.3%) initially treated with LEEP or CKC ultimately underwent a simple hysterectomy. Final hysterectomy specimen showed residual disease in 10 patients (14.1%), eight patients (11.3%) with residual AIS and two patients (2.8%) with invasive carcinoma. Both patients with invasive carcinoma underwent simple hysterectomy with the cancer diagnosis made following surgery. Both patients required adjuvant radiation therapy. Among the 65 patients with known margin status on final LEEP/CKC prior to hysterectomy, residual AIS or invasive carcinoma was noted in 23.1% (3/13, n=3 CKC and n=0 LEEP) of women with positive margins, and also in 13.5% (7/52, n=5 CKC and n=2 LEEP) of women with negative margins (Table 3).
Table 3.
Margin status on final CKC/LEEP procedure prior to hysterectomy.
| Hysterectomy Findings |
Negative Margins (N=52) |
Positive Margins (N=13) |
p-value |
|---|---|---|---|
| Invasive carcinoma | 1 (1.9%) | 1 (7.7%) | NS |
| Residual AIS | 6 (11.5%) | 2 (15.4%) | NS |
| Total | 7 (13.5%) | 3 (23.1%) | NS |
Margin status unknown in 6 patients.
NS= not significant
Of the 172 patients that had a cervical cone, 101 (58.7%) did not have a subsequent hysterectomy and were managed conservatively. To achieve negative cone margins; 72 (71.4%) required one cone biopsy, 26 (25.6%) required two cone biopsies, two (2.0%) patients required three cone biopsies, and one (1.0%) patient required four cone biopsies. Fifty percent of the repeat cone biopsies performed for positive or unevaluable margins contained residual AIS. Nine patients who were initially treated by LEEP underwent a subsequent LEEP or CKC despite having achieved negative margins and two (22.2%) were found to have residual AIS in the cone specimen. Of these 101 patients who did not undergo hysterectomy, two patients (2.0%) developed recurrent AIS at a median of 27.5 months (range 18-37 months) from the last cone. Both patients had negative cone margins on prior conization. None of the patients managed conservatively developed invasive carcinoma.
The median follow-up for all patients was 43.7 months (range 0.3-286.5 months). There were no deaths from disease in our study. However, one patient underwent a CKC for AIS with negative margins. A simple hysterectomy was then performed with final pathologic findings showing no residual AIS or invasive carcinoma. She was followed with annual cytology with no abnormalities noted. Fourteen years later, she presented with diffuse lymphadenopathy and underwent an excisional biopsy of a left inguinal lymph node, which showed metastatic poorly differentiated adenocarcinoma consistent with a cervical primary. She was treated with radiotherapy and chemotherapy [15].
Discussion
The principal findings of our study include that AIS is associated with high rates of positive margins on conization, with LEEP having a significantly higher rate of positive margins (55.6%) compared with CKC (35.0%) (p=0.017). In addition, 11.3% of patients undergoing hysterectomy after initial treatment with CKC/LEEP had residual AIS and 2.8% had invasive cancer. Furthermore, 2.0% of patients managed conservatively with cone biopsy developed recurrent AIS, but none progressed to invasive carcinoma. Surprisingly, 13.5% of patients with negative cone margins had residual disease in the final hysterectomy specimen including one patient with invasive cancer.
Hysterectomy has been recommended as the definitive management of AIS. However, given the high incidence of AIS in young women, fertility sparing treatment has become more common [8]. Previous studies have reported residual disease rates of up to 50% in patients with positive margins on final conization prior to hysterectomy [4]. Achieving negative margins is therefore recommended if conservative management is chosen. Similar to our findings, previous studies have shown that patients who undergo a LEEP are more likely to have positive margins than those who undergo CKC [4,5,16]. Kietpeerakool and colleagues [17] reported a higher incidence of positive margins in women who underwent a LEEP compared to CKC (56.8% vs. 26.1%). In addition, approximately 7% of patients with positive margins and 2% of patients with negative margins will have invasive adenocarcinoma noted at the time of hysterectomy [2,5,7,9-14]. The findings from our study and others suggest that CKC has lower rates of positive margins compared with LEEP in women with AIS who desire fertility-sparing conservative management.
Cervical AIS has been associated with multifocal lesions, commonly referred to as “skip lesions” that extend beyond the margin of resection [8,18]. It has been reported that a cone biopsy with a small focus of AIS in a very superficial gland has a lower risk of recurrence when managed conservatively compared with a cone biopsy containing extensive multifocal disease involving glands that are not on the surface and extending close to the cone margins [19]. In the present study, patients with multifocal disease were significantly more likely than patients with unifocal disease to have positive margins on CKC/LEEP. Furthermore, there were no significant differences between patients with positive and negative cone margins with regards to residual disease rates on hysterectomy specimen or recurrence rates in patients managed conservatively with cone biopsy alone.
There are limited data regarding long-term follow-up of patients undergoing conservative treatment for cervical AIS. Previous studies have suggested that high risk HPV testing performed in conjunction with cervical cytology offers an advantage during surveillance after conservative treatment for AIS [20]. Costa et al. [21] reported that AIS clearance could be predicted if follow-up cytology was normal, HPV testing was negative, and if the squamocolumnar junction was visible on colposcopy. It is recommended that patients treated conservatively with conization alone be followed closely with a combination of cervical cytology, HPV testing, and colposcopy with endocervical sampling [22]. However, the sensitivity of cervical cytology and ECC to detect AIS is only 50%, making it difficult to effectively monitor women with AIS managed conservatively [5,10]. Patients with positive margins who desire future fertility should be counseled on the risks of recurrence. Repeat conizations should be performed until negative margins are obtained. In the present study, 28.6% of patients required more than one conization to achieve negative margins. Patients should be counseled on the adverse obstetrical outcomes encountered when multiple conizations, including preterm delivery and very low birth weight infants [23-25]. We noted that 2.0% of patients managed conservatively with conization alone developed recurrent AIS, however none developed invasive adenocarcinoma. This provides further evidence that patients desiring future fertility can be managed conservatively, but should be monitored closely and definitive therapy with hysterectomy should be performed once child bearing is complete.
The finding of invasive adenocarcinoma on hysterectomy specimen following cone biopsy for AIS has been described [16,26,27]. Salani et al. [8] reported that 3.5% of patients who were initially treated conservatively with conization and subsequently underwent a hysterectomy were found to have invasive adenocarcinoma. This correlates with the findings in our study. Costa et al. [21] reported that greater than 12% of patients remain at increased risk for recurrence and even progression to invasive disease within 36 months from their first conization. It has been suggested that women who have been treated for cervical AIS require long-term surveillance for at least 10 years regardless of whether they have undergone hysterectomy or conservative management, as the risk of invasive cervical cancer remains five times greater than the general population [28,29]. To date, the longest surveillance reported in a series of patients treated by conization alone was eight years [30].
In summary, our findings provide further support that conservative management of AIS by cone biopsy is a reasonable option in women desiring future fertility. Our study is limited by retrospective data collection, a long study period and data from a single institution with possible referral bias, and no centralized pathology review was performed specifically for this study. Despite these limitations, this study included a large number of patients with AIS. Our findings suggest that LEEP is associated with a higher rate of positive margins and CKC should be considered for the initial management for AIS. In addition, long-term surveillance is warranted regardless of margin status, and all patients should be counseled on the risks of residual disease and the potential risk of recurrence associated with conservative management. They should also be advised of the adverse obstetrical outcomes associated with multiple conizations. Furthermore, given the high rate of residual disease noted in our study, definitive therapy with hysterectomy should be considered once childbearing is complete.
Highlights.
LEEP is associated with a higher rate of positive margins compared with CKC in patients with AIS
There is a high rate of residual disease at hysterectomy following cone biopsy for AIS
Patients undergoing conservative management for AIS should be counseled regarding the potential risks of residual and recurrent disease
Acknowledgments
This work was supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have any conflicts of interest to disclose.
References
- [1].Hepler T, Dockerty MB, Randall LM. Primary adenocarcinoma of the cervix. Am J Obstet Gynecol. 1952;63:800–8. doi: 10.1016/s0002-9378(16)38860-3. [DOI] [PubMed] [Google Scholar]
- [2].Shin CH, Schorge JO, Lee KR, Sheets EE. Conservative management of adenocarcinoma in situ of the cervix. Gynecol Oncol. 2000 Oct;79(1):6–10. doi: 10.1006/gyno.2000.5962. [DOI] [PubMed] [Google Scholar]
- [3].Madeleine MM, Daling JR, Schwartz SM, et al. Human papillomavirus and long-term oral contraceptive use increase the risk of adenocarcinoma in situ of the cervix. Cancer Epidemiol Biomarkers Prev. 2001;10:171–177. [PubMed] [Google Scholar]
- [4].Denehy TR, Gregori CA, Breen JL. Endocervical curettage, cone margins, and residual adenocarcinoma in situ of the cervix. Obstet Gynecol. 1997;90:1–6. doi: 10.1016/S0029-7844(97)00122-1. [DOI] [PubMed] [Google Scholar]
- [5].Kennedy AW, Biscotti CV. Further study of the management of cervical adenocarcinoma in situ. Gynecol Oncol. 2002;86:361–364. doi: 10.1006/gyno.2002.6771. [DOI] [PubMed] [Google Scholar]
- [6].Andersen ES, Nielsen K. Adenocarcinoma in situ of the cervix: A prospective study of conization as definitive treatment. Gyneol Oncol. 2002;86:365–369. doi: 10.1006/gyno.2002.6758. [DOI] [PubMed] [Google Scholar]
- [7].McHale MT, Le TD, Burger RA, Gu M, Rutgers JL, Monk BJ. Fertility sparing treatment for in situ and early invasive adenocarcinoma of the cervix. Obstet Gynecol. 2001 Nov;98(5 Pt 1):726–31. doi: 10.1016/s0029-7844(01)01544-7. [DOI] [PubMed] [Google Scholar]
- [8].Salani R, Puri I, Bristow RE. Adenocarcinoma in situ of the uterine cervix: a metaanalysis of 1278 patients evaluating the predictive value of conization margin status. Am J Obstet Gynecol. 2009;200:182.e1–182.e5. doi: 10.1016/j.ajog.2008.09.012. [DOI] [PubMed] [Google Scholar]
- [9].Bryson P, Stulberg R, Sheperd L, McLelland K, Jeffrey J. Is electrosurgical loop excision with negative margins sufficient treatment for cervical ACIS. Gynecol Oncol. 2004;93:465–468. doi: 10.1016/j.ygyno.2004.01.028. [DOI] [PubMed] [Google Scholar]
- [10].Wolf JK, Levenback C, Malpica A, Morris M, Burke T, Mitchell MF. Adenocarcinoma in situ of the cervix: significance of cone biopsy margins. Obstet Gynecol. 1996 Jul;88(1):82–6. doi: 10.1016/0029-7844(96)00083-X. [DOI] [PubMed] [Google Scholar]
- [11].Poynor EA, Barakat RR, Hoskins WJ. Management and follow-up of patients with adenocarcinoma in situ of the uterine cervix. Gynecol Oncol. 1995;57:158–164. doi: 10.1006/gyno.1995.1118. [DOI] [PubMed] [Google Scholar]
- [12].Muntz HG. Can cervical adenocarcinoma in situ be safely managed by conization alone. Gynecol Oncol. 1996;61:301–303. doi: 10.1006/gyno.1996.0146. [DOI] [PubMed] [Google Scholar]
- [13].Bull-Phelps SL, Garner EI, Walsh CS, Gehrig PA, Miller DS, Schorge JO. Fertility-sparing surgery in 101 women with adenocarcinoma in situ of the cervix. Gynecol Oncol. 2007 Nov;107(2):316–9. doi: 10.1016/j.ygyno.2007.06.021. [DOI] [PubMed] [Google Scholar]
- [14].Im DD, Duska LR, Rosenshein NB. Adequacy of conization margins in adenocarcinoma in situ of the cervix as a predictor of residual disease. Gynecol Oncol. 1995 Nov;59(2):179–82. doi: 10.1006/gyno.1995.0003. [DOI] [PubMed] [Google Scholar]
- [15].Costales AB, Eifel PJ, Deavers MT, et al. Metastatic adenocarcinoma found in inguinal, pelvicand para-aortic lymphnodes. Gynecol Oncol Reports. 2012;2:97–99. doi: 10.1016/j.gynor.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; 14 years following hysterectomy for adenocarcinoma in situ of the cervix
- [16].Widrich T, Kennedy AW, Myers TM, et al. Adenocarcinoma in Situ of the Uterine Cervix: Management and Outcome. Gynecol Oncol. 1997;61:304–308. doi: 10.1006/gyno.1996.0147. [DOI] [PubMed] [Google Scholar]
- [17].Kietpeerakool C, Khunamornpong S, Srisomboon J, et al. Predictive value of negative cone margin status for risk of residual disease among women with cervical adenocarcinoma in situ. Int J Gynecol and Obstet. 2012;119:266–269. doi: 10.1016/j.ijgo.2012.06.013. [DOI] [PubMed] [Google Scholar]
- [18].Krivak TC, Retherford B, Voskuil S, Rose GS, Alagoz T. Recurrent invasive adenocarcinoma after hysterectomy for cervical adenocarcinoma in situ. Gynecol Oncol. 2000 May;77(2):334–5. doi: 10.1006/gyno.2000.5761. [DOI] [PubMed] [Google Scholar]
- [19].Hopkins MP. Adenocarcinoma in Situ of the Cervix- The Margins Must Be Clear. Gynecol Oncol. 2000;79:4–5. doi: 10.1006/gyno.2000.5991. [DOI] [PubMed] [Google Scholar]
- [20].Costa S, Negri G, Sideri M, et al. Human Papillomavirus (HPV) test and PAP smear as predictors of outcome in conservatively treated adenocarcinoma in situ (AIS) of the uterine cervix. Gynecol Oncol. 2007;106:107–176. doi: 10.1016/j.ygyno.2007.03.016. [DOI] [PubMed] [Google Scholar]
- [21].Costa S, Venturoli S, Negri G, et al. Factors predicting the outcome of conservatively treated adenocarcinoma in situ of the uterine cervix: An analysis of 166 cases. Gynecol Oncol. 2012;124:490–495. doi: 10.1016/j.ygyno.2011.11.039. [DOI] [PubMed] [Google Scholar]
- [22].Wright TC, Massad SL, Dunton CJ, et al. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–345. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- [23].Kyrgiou M, et al. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367(9509):489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- [24].Albrechtsen S, et al. Pregnancy outcome in women before and after cervical conisation: population based cohort study. BMJ. 2008;337:a1343. doi: 10.1136/bmj.a1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arbyn M, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miller B, Dunn J, Dalrymple J, Krivak TC. Pelvic sidewall adenocarcinoma after definitive therapy for cervical adenocarcinoma in situ. Gynecol Oncol. 2005 Nov;99(2):489–92. doi: 10.1016/j.ygyno.2005.06.044. [DOI] [PubMed] [Google Scholar]
- [27].Hopkins MP, Roberts JA, Schmidt RW. Cervical adenocarcinoma in situ. Obstet Gynecol. 1988;71:842–844. [PubMed] [Google Scholar]
- [28].Soutter WP, Haidopoulos, Gornall RJ, et al. Is conservative treatment for adenocarcinoma in situ of the cervix safe. Br J Obstet Gynaecol. 2001;108:1184–1189. doi: 10.1111/j.1471-0528.2003.00277.x. [DOI] [PubMed] [Google Scholar]
- [29].Hwang DM, Lickrish GM, Chapman W, Colgan TJ. Long-term surveillance is required for all women treated for cervical adenocarcinoma in situ. Journal of Lower Genital Tract Disease. 2004;8(2):125–131. doi: 10.1097/00128360-200404000-00008. [DOI] [PubMed] [Google Scholar]
- [30].Ostor AG, Duncan A, Quinn M, Rome R. Adenocarcinoma in situ of the uterine cervix: an experience with 100 cases. Gynecol Oncol. 2000 Nov;79(2):207–10. doi: 10.1006/gyno.2000.5957. [DOI] [PubMed] [Google Scholar]