Abstract
Psoriasis is a common chronic inflammatory disease of the skin characterized by epidermal hyperplasia and angiogenesis. Recently, vascular endothelial growth factor receptors (VEGFRs, including VEGFR-1, VEGFR-2 and VEGFR-3) were found to be expressed in normal human epidermis and associated with proliferation and migration of keratinocytes. The purpose of this study is to investigate the expression of VEGFRs on psoriatic keratinocytes and the roles of calcium and VEGF in regulating VEGFR expression. Skin samples from 17 patients with chronic plaque psoriasis and 11 normal controls were included. The expression of VEGFRs in psoriatic keratinocytes at mRNA and protein levels was determined by reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analysis. Localization of the VEGFRs in skin lesions was determined by immuno-fluorescent method. Since keratinocyte proliferation and differentiation rely on calcium concentrations, and VEGF is overexpressed in psoriatic epidermis, we further investigated the roles of calcium and VEGF in regulating the expression of VEGFRs. Overexpression of VEGFR-1, VEGFR-2 and VEGFR-3 in psoriatic epidermis was demonstrated both at mRNA and protein levels in vitro. VEGFRs were strongly labeled in non-lesional, perilesional and lesional psoriatic keratinocytes in all viable epidermal stratums in vivo. Furthermore, both exogenous VEGF165 and calcium enhanced the expression of VEGFRs. Calcium also enhanced the expression of VEGF in non-lesional psoriatic keratinocytes, while targeted blockade of VEGF activity by bevacizum-ab could not inhibit calcium-induced up-regulation of protein levels of VEGFRs. We conclude from these results that VEGFRs are overexpressed in lesional psoriatic epidermal keratinocytes. Both calcium and VEGF regulate VEGFRs expression in psoriatic epidermis. More importantly, calcium is a potential regulator for VEGFR independent of VEGF.
Keywords: psoriasis, keratinocyte, VEGFR, VEGF, calcium
Introduction
Psoriasis is a chronic inflammatory skin disease characterized by epidermal hyperplasia, impaired epidermal differentiation, and accumulation of distinct leukocyte subpopulations [1–3]. Vascular endothelial growth factor (VEGF) was identified as a major epidermis-derived vessel-specific growth factor that was strongly up-regulated in psoriatic skin lesions [4,5]. The role of VEGF in the pathogenesis of psoriasis was further supported by studies on VEGF transgenic mice [6,7] and one modifier VEGF gene [8,9]. Therefore, VEGF may play a key causative role in mechanisms underlying the development of psoriasis [7].
The biological effects of VEGF are mediated by VEGF receptors (VEGFRs, including VEGFR-1 [flt-1], VEGFR-2 [KDR/flk-1], VEGFR-3 [flt-4]) and neuropilins (NRPs, including NRP-1and NRP-2) [10–13]. Recently, VEGFR-1 has been detected in murine keratinocytes during wound repair and in normal human epidermal keratinocytes [14]. We have detected that all identified VEGFRs are expressed on keratinocytes in normal human epidermis [15] and that VEGFR-2 is expressed on the HaCaT keratinocyte cell line [16] and involved in the proliferation and migration of normal keratinocytes [15]. It is possible that VEGFRs may play an important role in regulating psoriatic keratinocyte activity. Based on this hypothesis, we studied the expression of VEGFRs on psoriatic epidermal keratinocytes. Given the importance of VEGF in the pathogenesis of psoriasis [1, 4, 6, 7]; the calcium gradient within the epidermis is known to be directly implicated in the homeostasis of the skin, regulating keratinocyte growth and differentiation [17–21] and; that VEGF can enhance expression of VEGFR-2 in HaCaT cells [16], we further investigated the interactions between calcium, VEGF and VEGFRs in non-lesional psoriatic keratinocytes.
Materials and methods
Human participants
Patients with moderate to severe active chronic plaque psoriasis (n= 17, including 10 females and seven males, aged 19–65) were recruited to the study from the department of Dermatology at the Second Affiliated Hospital, Zhejiang University School of Medicine. All patients gave informed written consent. During 1 month prior to biopsy, they had received no therapy for the psoriasis. Biopsies were taken from perilesional (within 2 cm from the lesion), non-lesional (at least 5 cm from the lesion) healthy-looking skin and lesional skin. Healthy age-matched participants (n= 11) obtained from cosmetic surgery served as controls. After removing subcutaneous elements, the specimens were divided into two parts. One part was snap frozen and embedded in optimal cutting temperature (OCT) compound (Miles, Naperville, IL, USA) and then stored in liquid nitrogen until processed. The other was placed into 0.5% dispase (Gibco, Invitrogen, USA) immediately. This study was conducted in agreement with the Declaration of Helsinki and performed under the guidelines of the institutional review committee of Zhejiang University.
Isolation and culture of epidermal keratinocytes
Epidermal keratinocytes were cultured as described previously [15]. Briefly, the tissues were incubated overnight at 4°C in 0.5% dispase. The epidermis was peeled off from the dermis and incubated in 0.25% trypsin for 10 min at 37°C. The keratinocytes suspension was filtered and cells were washed twice at 500 g for 5 min prior to resuspension in defined keratinocyte serum free medium (KSFM) supplemented with keratinocyte growth factor (KGF) (Gibco, Invitrogen, USA). Normal, psoriatic non-lesional, perilesional and lesional keratinocytes were plated into 25 cm2 tissue culture dish respectively and maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells were used at passages 2–3 in all experiments.
Indirect immunofluorescence assay
Immunofluorescence assay was carried out as described previously [15]. Briefly, psoriatic and normal skin tissue specimens embedded in OCT compound were processed into 4 μm sections. Sections were placed on the surface of coverslips incubated with 1 μg/ml poly-L-lysine. After being fixed in 4% paraformaldehyde buffer for 20 min at room temperature, the coverslips were placed into preheated sodium citrate buffer (10 mM Sodium Citrate Buffer, pH 8.5), maintained at 95°C for 20 min, then incubated with 20 μg/ml of primary monoclonal antibodies diluted with 10% rabbit serum in PBS against VEGFR-1, VEGFR-3 (R&D, USA) and VEGFR-2 (Santa Cruz Biotechnology, USA) overnight at 4°C, followed by fluoresceinisothio-cyanate (FITC)-conjugated rabbit anti-mouse secondary antibody (DakoCytomation, Denmark) diluted 1:40 with 10% rabbit serum in phosphate-buffered saline (PBS) for 2 hrs at room temperature, away from light. The nuclei were counterstained with propidium iodide (PI) (Sigma-Aldrich, USA) mounting medium. For each case, a negative control incubated with non-immune mouse IgG (Sigma-Aldrich, USA) was included.
Reverse transcription and polymerase chain reaction (RT-PCR)
Total RNA was isolated from cultured keratinocytes using 1ml Trizol (Invitrogen, USA) reagent according to the manufacturer's instructions and stored at –80°C until use. Total RNA was reverse-transcribed into first strand cDNA in a total reaction volume of 20 μl using Moloney murine leukemia virus (MMLV) reverse transcriptase (MBI Fermantas, USA). Specific primers for VEGFR-1, VEGFR-2, VEGFR-3 and VEGF were designed by using PrimerSelect program in DNAstar package (DNAStar Inc., Madison, WI, USA) (Table 1). RT-PCR amplification was performed with a PTC 225 thermal cycler (MJ Research) with 35 cycles (Table 1). In each experiment, negative control (using total RNA as the template) was included.
1.
PCR primers used in this study
| Gene | GenBank accession no. | Primer sequence (5′→ 3′) | Location | Annealing temperature (°C) | Product length (bp) |
|---|---|---|---|---|---|
| VEGFR-1 | NM_002019 | ATGGCTCCCGAATCTATCTTTGAC GCCCCGACTCCTTACTTTTACTGG | 3445-3468 4099-4076 | 57 | 655 |
| VEGFR-2 | NM_002253 | CTGGCGGCACGAAATATCCTCTTA GGGCACCATTCCACCAAAAGAT | 3388-3411 4164-4143 | 57 | 777 |
| VEGFR-3 | NM_182925 | AGGCCGGCCCACGCAGACATC TGCACGCCCCGAGGAGGTTGA C | 2424-2444 2769-2748 | 63 | 346 |
| VEGF | NM_003376 | GGAGGAGGGCAGAATCATCACGAA CACCGCCTCGGCTTGTCACAT | 1125-1148 1678-1658 | 59 | 350, 446, 482, 536 |
| GAPDH | NM_002046 | TGAAGGTCGGAGTCAACGG TGGAAGATGGTGATGGGAT | 113-131 335-317 | 57 | 223 |
| Primers for Real-time quantitative PCR | |||||
| VEGFR-1 | NM_002019 | AGCGGCTCCCTTATGATGC GCCGTGGCCCCCTCTTT | 2681-2699 2855-2839 | 175 | |
| VEGFR-2 | NM_002253 | ATGGGAACCGGAACCTCACTATC GTCTTTTCCTGGGCACCTTCTATT | 2453-2475 2585-2562 | 55 | 133 |
| VEGFR-3 | NM_182925 | CAACTGGGTGTCCTTTCC CTTGTCTATGCCTGCTCTC | 3713-3730 3900-3882 | 188 | |
| GAPDH | NM_002046 | TGCACCACCAACTGCTTAG GAGGCAGGGATGATGTTC | 556-574 731-714 | 186 | |
Real time quantitative RT-PCR (qPCR)
To quantitate the expression levels of the target genes, real-time quantitative RT-PCR was performed on the Roche LightCycler 2.0 (Roche Diagnostic, Germany) using Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, USA) with primers in Table 1. The amplification protocol consisted of incubations at 95°C for 30 sec, 55°C for 30sec and 72°C for 10 sec for 50 cycles. All qPCR reactions were performed in duplicate. A no template control is included for each measurement. The expression data for each gene were normalized for the efficiency of amplification, as determined by a standard curve included in each run. The crossing point (CP) of target gene in each sample was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for RNA amount variation and the relative quantification was calculated using qBase version 1.3.5 [22].
Western blot analysis
The method was performed as described previously [15]. Briefly, total cellular protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membrane (Hybond-P, Amersham, USA). Blots were blocked 30 min at room temperature in PBS (pH 7.5) containing 1% Tween-20. The membrane was probed with the respective primary antibody overnight at 4°C in blocking buffer and then incubated for 1 hr with horseradish peroxidase-conjugated sheep polyclonal antimouse IgG antibody (1:5000, Jackson, USA). The reactions were detected by using ECL plus reagent kit (Amersham, USA) and exposure for 3 min to Kodak film (Kodak, USA). A mouse monoclonal anti-GAPDH (Acris Antibodies GmbH, Germany) was used as a loading control.
Regulation of VEGFRs by VEGF165 at 10 ng/ml and bevacizumab
We detected that VEGF165 maximally enhanced the expression of VEGFR-2 mRNA and protein at a concentration of 10 ng/ml in a keratinocyte cell line [16]. Therefore, 10ng/ml VEGF165 (Chemicon, USA) was used to determine the effect of VEGF on protein levels of VEGFRs in non-lesional psoriatic keratinocytes (n= 5). The targeted, neutralizing monoclonal antibody against VEGF, bevacizumab (Avastin, Genentech, USA), which is highly active in treatment of several malignancies and has been approved for use in metastatic colon cancer therapy [23], diluted with culture medium to obtain 1.0 mg/ml (at or above the dose normally used in clinical practice [24]), was co-incubated with VEGF165 to examine its role in VEGF-induced effect on expression of VEGFRs.
Effect of calcium on the expression of VEGFRs and VEGF in non-lesional psoriatic keratinocytes
To determine the effect of calcium treatment on the expression of VEGFRs and VEGF, 80%∼90% confluent non-lesional psoriatic epidermal keratinocytes (n= 5) were incubated with calcium chloride (CaCl2) at concentrations of 0, 0.5, 1.0, 2.0, 3.0 mM in basal defined KSFM for 24 hrs at 37°C in 5% CO2. Total RNA was extracted and then reversed transcripted into cDNA. Protein was separated by SDS-PAGE (12% for VEGF, 6% for VEGFRs), respectively. To further identify whether calcium regulates expression of VEGFRs through production of VEGF, cultured non-lesion-al psoriatic keratinocytes (n= 3) were exposed to beva-cizumab at a concentration of 1.0 mg/ml in the presence of calcium at 1.0 mM.
Statistics
Statistical significance of differences in mean values was evaluated by one-way analysis of variance (anova) and Student's t-test. A value of P <0.05 was declared as significant. The levels of gene transcripts and protein were quantified as the ratio of the band intensity of the target gene to that of cor-respongding GAPDH. The data represent the mean ±SEM.
Results
Immunofluorescence analysis of VEGFRs in non-lesional,perilesional and lesional psoriatic skin
In normal skin, VEGFR-1 and VEGFR-2 were localized notably in the basal and suprabasal layer. In upper stratum spinosum and granulosum, few keratinocytes exhibited signals for VEGFR-1 and VEGFR-2. However, in psoriatic skin, VEGFR-1 and VEGFR-2 strongly labeled non-lesional, perilesional keratinocytes in all layers of the epidermis except the stratum corneum, and lesional keratinocytes in all viable layers, including the parakeratotic stratum corneum (Fig. 1). A uniform expression pattern of VEGFR-3 was detected in both normal and psoriatic epidermis except for stratum corneum. Interestingly, parakeratotic keratinocytes in lesional psoriatic stratum corneum also showed intense fluorescence for VEGFRs (Fig. 1). In good agreement with a previous study [5], papillary dermal microvascular endothelial cells (PDMECs) were also strongly stained for VEGFRs, which served as internal positive controls.
1.
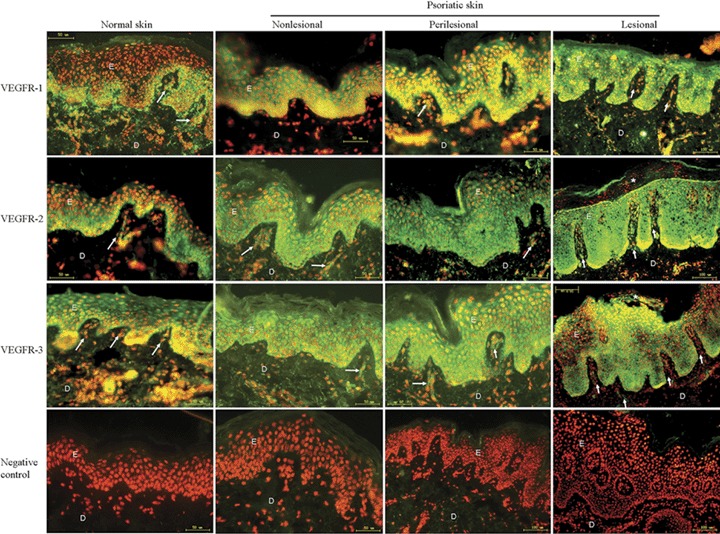
Immunofluorescence detection of VEGFRs in normal, non-lesional, perilesional and lesional psoriatic skin. The presence of VEGFRs is indicated by green fluorescence staining. All sections were counterstained with PI (red nuclear signal). The epidermal rete ridges are elongated in psoriatic lesions. Perilesional, non-lesional psoriatic and normal skins have a thin epidermis. Only small capillaries are visible in the papillary dermis of normal skin, while psoriatic plaques contain enlarged blood vessels. VEGFR-1, VEGFR-2, VEGFR-3 accumulation was found in psoriatic epidermis. VEGFRs were also observed in the parakeratotic stratum corneum of lesional psoriatic skin (asterisk). Blood vessels were stained as internal positive control (arrow head). Negative controls that were incubated with non-immune mouse IgG show no signals in any compartment of the normal or psoriatic skin. E, epidermis;D, dermis. Bars: indicated on the photo.
VEGFRs intracellular localization varied from normal to lesional psoriatic skin (Fig. 1). In normal and non-lesional psoriatic epidermal keratinocytes, VEGFRs predominantly exhibited a membranous localization, whereas in perilesional and lesional psoriatic keratinocytes, the mixed membranous and cytoplasmic localization of VEGFRs extended throughout all immunostained layers.
mRNA and protein levels of VEGFRs were overexpressed in psoriatic keratinocytes as compared to normal keratinocytes
mRNA extracted from cultured keratinocytes in different stages of psoriatic skin from the same patient was compared. RT-PCR and qPCR demonstrated that mRNA of VEGFRs was expressed in all 17 cases of psoriasis (Fig. 2A–C). mRNA level of VEGFR-1, VEGFR-2 and VEGFR-3 were found to be gradually increased from normal to non-lesional and perilesional psoriatic keratinocytes and, the most highly increased levels of VEGFRs mRNA were observed in lesional psoriatic keratinocytes (Fig. 2B and C).
2.
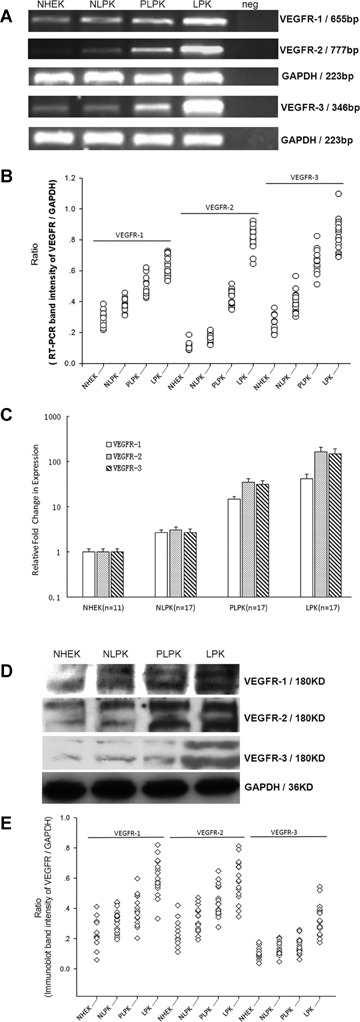
mRNA and protein levels of VEGFRs determined by RT-PCR, qPCR and Western blot, respectively, in cultured normal and psoriatic epidermal keratinocytes. (A) VEGFR-1, band at 655bp; VEGFR-2, band at 777bp; VEGFR-3, band at 346bp; GAPDH at 223bp served as an internal control for mRNA. (B) Scatter plots for RT-PCR band intensity ratio of targeted VEGFR gene versus GAPDH using Gel-Pro Analyzer software V4.0 (Media Cybernetics, USA). Expression of VEGFRs mRNA is statistically significant different between LPK and other indicated cells (P <0.001). (C) Relative quantitation of VEGFRs expression levels in NHEK, NLPK, PLPK and LPK by real-time RT-PCR. The expression of VEGFRs was normalized to the endogenous control GAPDH. The relative fold change of VEGFRs in NLPK, PLPK and LPK was compared with NHEK. The level of VEGFRs in LPK is significantly higher than that in NHEK, PLPK and LPK (P <0.001). Bars: mean±SEM. (D) The anti–VEGFR-1, anti–VEGFR-2 and anti–VEGFR-3 antibodies show bands at about 180 and 200KD. GAPDH served as a loading control for protein normalization. (E) Scatter plot analysis of protein levels of VEGFRs versus GAPDH as determined by Western blot. Expression of VEGFRs protein is statistically significant different between LPK and other indicated cells (P < 0.001). NHEK, normal human epidermal ker-atinocytes (n= 11); NLPK, non-lesional psoriatic keratinocytes (n= 17); PLPK, perilesional psoriatic keratinocytes (n= 17); LPK, lesional psoriatic keratinocytes (n= 17); neg, negative control.
Protein level of VEGFRs was also examined in normal skin (n= 11) and, non-lesional, perilesional, lesional psoriatic skin (n= 17) from the same patient by Western blot. As shown in Figure 2D and E, a robust up-regulation of VEGFR-1, VEGFR-2 and VEGFR-3 protein was observed in lesional psoriatic skin compared to normal, non-lesional and perilesional psoriatic skin, paralleling the results obtained by RT-PCR (Fig. 2).
Calcium up-regulated expression of VEGF in non-lesional psoriatic keratinocytes in vitro
In non-lesional psoriatic epidermal keratinocytes, RT-PCR analysis identified the mRNA expression of four VEGF isoforms, including VEGF121, VEGF145, VEGF165 and VEGF189, corresponding to expected size at 350 bp, 446 bp, 482 bp and 536 bp respectively (Fig. 3A). Calcium-enhanced expression of VEGF121, VEGF165 and VEGF189 in a dose-dependent manner and maximally at 1.0 mM, while higher concentrations did not show more stimulation effect (Fig. 3A and B). Little or no difference was seen for the mRNA of VEGF145 after varies concentrations of calcium treatment (Fig. 3A and B). In parallel with mRNA expression, protein levels of VEGF121, VEGF165 and VEGF189, corresponding to 34 KD, 38 KD and 40 KD band respectively, were also peaked at a calcium concentration of 1.0 mM, especially for VEGF165 (Fig. 3C). Expression of VEGF165 protein was up-regulated by calcium more strongly than that of VEGF121 and VEGF189 (Fig. 3D).
3.
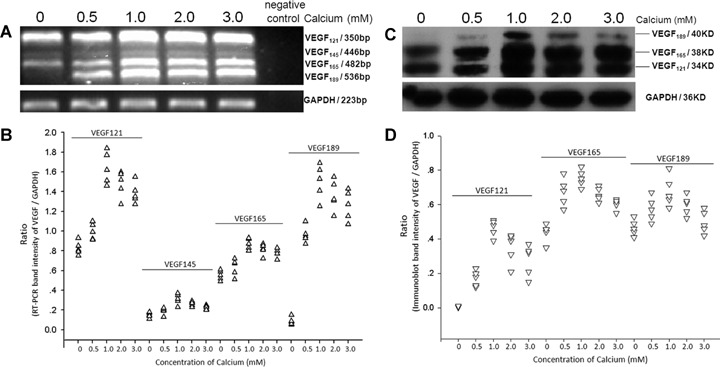
Expression of VEGF in non-lesional psoriatic keratinocytes after calcium treatment. Non-lesional psoriatic keratinocytes from five cases of psoriasis were incubated with calcium at concentrations of 0, 0.5, 1.0, 2.0 and 3.0 mM. (A) mRNA expression of VEGFafter calcium treatment; GAPDH served as an internal control.(B) Scatter plots for RT-PCR band intensity ratio of VEGF gene versus GAPDH.(C) Western blot results of VEGF; GAPDH served as a loading control for protein normalization.(D) Scatter plot analysis of protein level of VEGF versus GAPDH as determined by Western blot.
Calcium up-regulated expression of VEGFRs in non-lesional psoriatic keratinocytes independent of up-regulation of VEGF in vitro
In a dose-dependent manner, calcium regulated the expression of VEGFRs in a parabolic pattern: calcium maximally enhanced the expression of VEGFRs mRNA at a concentration of 1.0mM, whereas higher concentrations (≥ 2.0 mM) gradually restored the mRNA expression of VEGFRs to basal levels (Fig. 4A and B). In parallel with mRNA expression, protein levels of VEGFR-1 and VEGFR-3 were also peaked at a calcium concentration of 1.0 mM, while VEGFR-2 was peaked at 0.5 mM and maintained at the same level at 1.0 mM, then restored to basal levels (Fig. 4C).
4.
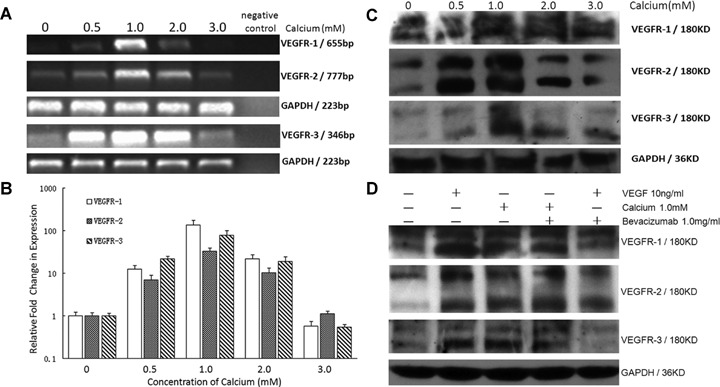
Effect of calcium and VEGF165 on expression of VEGFRs in non-lesional psoriatic keratinocytes.(A) Calcium regulates mRNA level of VEGFRs. Non-lesional psoriatic keratinocytes (n= 5) were incubated with calcium at concentrations of 0, 0.5, 1.0, 2.0 and 3.0 mM. GAPDH served as internal control for mRNA.(B) Relative quantitation of VEGFRs mRNA by real-time RT-PCR. The expression of VEGFRs was normalized to the endogenous control GAPDH. The relative fold change of VEGFRs in non-lesional psoriatic keratinocytes incubated with calcium was compared to that without calcium. The mRNA level of VEGFRs in NLPK treated with calcium at 1.0 mM is significantly higher than that in other indicated concentration (P <0.001). Bars: mean±SEM. (C) Bands at 180 KD and 200 KD are detected for VEGFR-1, VEGFR-2 and VEGFR-3. GAPDH served as a loading control.(D) Effect of VEGF165 and calcium with or without bevacizumab on protein levels of VEGFRs in non-lesional psoriatic keratinocytes. Non-lesional psoriatic keratinocytes (n= 3) were exposed to VEGF at 10ng/ml with or without 1.0mg/ml bevacizumab, and calcium at 1.0mM with or without 1.0mg/ml bevacizumab. Bands at 180 KD and 200 KD are detected for VEGFR-1, VEGFR-2 and VEGFR-3. GAPDH served as a loading control.
In our previous work, we have shown that at a concentration of 10 ng/ml VEGF165 maximally enhances the expression of VEGFR-2 mRNA and protein in HaCaT cells [16]. Our present data shows that VEGF165 at 10 ng/ml also markedly increases expression of VEGFR-1, VEGFR-2 and VEGFR-3 in non-lesional psoriatic keratinocytes (Fig. 4D). Exposure of non-lesional psoriatic keratinocytes to bevacizumab at concentration of 1.0 mg/ml moderately to mostly reduced the amount of VEGF-induced VEGFR-1, VEGFR-2 and VEGFR-3 protein synthesis (Fig. 4D). However, no relevant inhibition of overexpression of VEGFRs was seen when non-lesional psoriatic keratinocytes were treated with 1.0 mM calcium addition with 1.0 mg/ml bevacizumab (Fig. 4D).
Discussion
VEGFRs are expressed in endothelial cells [4, 12, 25], but are also expressed in a variety of other cells and tissues [26]. Recently, VEGFRs were detected in normal human epidermal keratinocytes at both mRNA and protein levels [14,15]. Moreover, through the VEGFR-2 pathway, VEGF enhanced the proliferation and migration of keratinocytes [15,16].
The expression of VEGFR-1 and VEGFR-2 in psoriasis was noted by one previous study [5], which demonstrated that VEGFR-1 and VEGFR-2 were overexpressed by PDMECs in psoriasis lesional skin through in situ hybridization (ISH). Although the authors did not mention expression of VEGFR-1 and VEGFR-2 in psoriatic epidermis, we could clearly observe that the ISH signals for VEGFR-2 in their photo (Fig. 1D in ref. 5) not only strongly labeled the PDMECs, but also dispersively labeled the lesional psoriatic epidermal keratinocytes.
To elucidate whether VEGFRs are aberrantly expressed by psoriatic epidermal keratinocytes, we compared psoriatic skin samples, including non-lesional, perilesional and lesional specimens, to normal skin samples in vivo and in vitro. Immunofluorescence showed that VEGFRs were intensely and uniformly distributed in the basal, spinous and granular stratum and PDMECs in non-lesional, perilesional and lesional epidermis (Fig. 1). However, in normal epidermis, VEGFR-1 and VEGFR-2 were preferentially distributed in the basal and suprabasal stratum. Given the probable role of VEGF as an epidermal mitogen, the restriction of VEGFR-1 and VEGFR-2 expression to the basal epidermal compartment may largely limit proliferation to basal and suprabasal keratinocytes in normal skin. However, in psoriatic epidermis, VEGFR-1 and VEGFR-2 are distributed in the whole viable epidermis, which may indicate that in psoriasis, the regenerative potency is no longer limited to the basal and suprabasal keratinocytes, but expands to the whole viable epidermis, including the parakeratotic keratinocytes (Fig. 1). Moreover, it is noteworthy that no VEGFRs were detected in stratum corneum in normal, non-lesional and perilesional samples. However, in parakeratotic stratum corneum of lesional psoriatic epidermis, VEGFRs were demonstrated. It is well known that the parakeratosis (nuclei still present in the thickened cornified layer) is due to altered differentiation of keratinocytes [2], therefore, it is intriguing to speculate that expression of VEGFRs may correlate with keratinocyte differentiation.
In contrast to VEGFR-1 and VEGFR-2, no obvious changes were observed for the distribution pattern of VEGFR-3 between normal and psoriatic viable epidermal layers. In adult tissues, VEGFR-3 expression was detected in normal adult tissues mainly in the lymphatic endothelium [27–30]. Blocking VEGFR-3 signaling by using a soluble VEGFR-3 protein caused regression of developing lymphatic vessels by inducing apoptosis of endothelial cell [31]. Therefore, the roles of VEGFR-3 in the epidermis and the bioactivities of keratinocytes need to be further explored.
Consistent with these immunofluorescent findings, the enhanced expression of VEGFRs in the psoriatic keratinocytes was further displayed at the mRNA and protein levels in vitro. Our findings suggested a significantly increase of VEGFRs in hyperplastic lesional psoriatic keratinocytes compared with uninvolved skin from the same individuals and normal controls. The marked differences of mRNA and protein levels of VEGFRs between lesional and uninvolved psoriatic keratinocytes indicate that a close correlation may exist between VEGFRs and keratinocyte activity in psoriasis.
Calcium has been shown to regulate the proliferation of epidermal keratinocytes in vitro[19,20,32]. The rise in free calcium after an increase in extracellular calcium concentration appears to be a prerequisite for keratinocyte differentiation [19,32–34]. Increased calmodulin, a calcium-binding protein, levels are associated with epidermal hyperproliferation and/or with the state of differentiation [17]. Psoriatic plaques contain 2–3 times more calmodulin than the skin of normal controls. Adjacent uninvolved psoriatic skin also has significantly elevated calmodulin levels [17]. In calcium-induced dissociation of retinal pigment epithelium cells, the expression of VEGF121 and VEGF165 was up-regulated [35]. Calcium channel blocker benidipine suppresses expression of VEGF [36], which was both expressed and secreted by epidermal keratinocytes [37]. Several other studies have demonstrated that VEGF expression is increased in lesional psoriatic skin, and that plasma levels of circulating VEGF protein is significantly elevated in psoriatic patients [9] and has a significant impact in psoriasis [38]. Therefore, we studied the effect of calcium on expression of VEGF splice variants, and the effect of calcium and VEGF165 on the expression of VEGFRs in the non-lesional psoriatic keratinocytes, which have important cellular or genetic differences with skin of normal individuals [3,9], to investigate the possible contributors for over-expression of VEGFRs in psoriasis.
Exogenous calcium up-regulated the mRNA and protein levels of VEGFRs in a dose-dependent manner and peaked at concentrations of 0.5∼1.0 mM in non-lesional psoriatic keratinocytes (Fig. 4). Furthermore, calcium also enhanced mRNA and protein of VEGF121, VEGF189 and especially, VEGF165 in a dose-dependent manner and peaked at 1.0 mM. Therefore, it is appealing to conclude that calcium blockers may be effective in the therapy of psoriasis, although the calcium blockers did not decrease the concentrations of VEGF and its receptor VEGFR-1 in systemic sclerosis, a VEGF/VEGFR axis defective disease [38]. Further investigations should be done to confirm this.
We simultaneously determined the protein levels of VEGFRs in non-lesional psoriatic keratinocytes after being stimulated by VEGF165 at a concentration of 10 ng/ml, which enhanced the protein levels of VEGFR-1, VEGFR-2 and VEGFR-3. Since exogenous VEGF could significantly enhance expression of VEGFRs in vitro, a relative abundance of VEGF present in lesional psoriatic skin might increase expression of VEGFRs in vivo.
Both calcium and VEGF could upregulate expression of VEGFRs, and calcium also could up-regulate the expression of VEGF. So, is it reasonable that, calcium-enhanced expression of VEGFRs indirectly through enhanced expression of VEGF?
To test this hypothesis, a targeted, recombinant humanized neutralizing monoclonal IgG1 antibody that binds to and inhibits the biologic activity of human VEGF in in vitro and in vivo assay systems [39], bevacizumab, was included in the study. Bevacizumab alleviated VEGF-induced up-regulation of VEGFRs moderately to mostly. However, it did not inhibit calcium-induced overexpression of VEGFRs in cultured non-lesional psoriatic keratinocytes. Based on these results we draw two conclusions:(i) calcium enhances expression of VEGFRs completely independent of VEGF and;(ii) the concentration of calcium-induced VEGF is not enough to up-regulate expression of VEGFRs, that is, calcium enhances expression of VEGFRs mainly independent of VEGF. However, the precise mechanism of calcium- and VEGF-enhanced expression of VEGFRs is still unknown. An autocrine loop formed by VEGF and its receptors may have an impact on the regulation of keratinocyte survival in psoriasis.
In summary, this study demonstrates overexpres-sion of VEGFR-1, VEGFR-2 and VEGFR-3 in psoriatic epidermis in vivo and in vitro. We also demonstrate that both exogenous VEGF165 and calcium can enhance expression of VEGFRs in non-lesional psoriatic keratinocytes. Although calcium also upregulate expression of VEGF isoforms including VEGF165 at mRNA and protein levels, blockade of VEGF activity can not inhibit calcium-induced up-regulation of protein levels of VEGFRs. Therefore, calcium may enhance expression of VEGFRs independent of VEGF. The additional efforts in VEGF/VEGFRs research will certainly provide invaluable clues to facilitate the development of novel molecular targets for the treatment of psoriasis [38] and their associated biological markers for predicting response. Monoclonal antibodies against VEGFRs may attenuate the development and maintenance of psoriatic lesion.
Acknowledgments
The authors thank Professor Mark R Pittelkow and Dr. Yong-Gang Yao for their helpful discussion. The authors also thank Prof.Ming-Hai Wang for his critical reading. This work was supported by grant (no. 30471565) from the National Natural Science Foundation of China (NSFC).
References
- 1.Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 2.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 3.Pittelkow MR. Psoriasis: more than skin deep. Nat Med. 2005;11:17–8. doi: 10.1038/nm0105-17. [DOI] [PubMed] [Google Scholar]
- 4.Detmar M, Brown LF, Claffey KP, Yeo KT, Kocher O, Jackman RW, Berse B, Dvorak HF. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J Exp Med. 1994;180:1141–6. doi: 10.1084/jem.180.3.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhushan M, McLaughlin B, Weiss JB, Griffiths CE. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br J Dermatol. 1999;141:1054–60. doi: 10.1046/j.1365-2133.1999.03205.x. [DOI] [PubMed] [Google Scholar]
- 6.Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 7.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS. Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood. 2003;102:161–8. doi: 10.1182/blood-2002-12-3793. [DOI] [PubMed] [Google Scholar]
- 8.Detmar M. Evidence for vascular endothelial growth factor (VEGF) as a modifier gene in psoriasis. J Invest Dermatol. 2004;122:xiv—xv. doi: 10.1046/j.0022-202X.2003.22140.x. [DOI] [PubMed] [Google Scholar]
- 9.Young HS, Summers AM, Bhushan M, Brenchley PE, Griffiths CE. Single-nucleotide polymorphisms of vascular endothelial growth factor in psoriasis of early onset. J Invest Dermatol. 2004;122:209–15. doi: 10.1046/j.0022-202X.2003.22107.x. [DOI] [PubMed] [Google Scholar]
- 10.Stacker SA, Achen MA. The vascular endothelial growth factor family: Signalling for vascular development. Growth Factors. 1999;17:1–11. doi: 10.3109/08977199909001058. [DOI] [PubMed] [Google Scholar]
- 11.Veikkola T, Alitalo K. VEGFs, receptors and angio-genesis. Semin Cancer Biol. 1999;9:211–20. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 12.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 13.Gu C, Rodriguez RE, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R, DiPietro LA. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol. 2005;167:1257–66. doi: 10.1016/S0002-9440(10)61213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man XY, Yang XH, Cai SQ, Yao YG, Zheng M. Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Mol Med. 2006;12:127–36. doi: 10.2119/2006-00024.Man. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XH, Man XY, Cai SQ, Yao YG, Bu ZY, Zheng M. Expression of VEGFR-2 on HaCaT cells is regulated by VEGF and plays an active role in mediating VEGF induced effects. Biochem Biophys Res Commun. 2006;349:31–8. doi: 10.1016/j.bbrc.2006.07.213. [DOI] [PubMed] [Google Scholar]
- 17.Fairley JA, Marcelo CL, Hogan VA, Voorhees JJ. Increased calmodulin levels in psoriasis and low Ca++ regulated mouse epidermal keratinocyte cultures. J Invest Dermatol. 1985;84:195–8. doi: 10.1111/1523-1747.ep12264823. [DOI] [PubMed] [Google Scholar]
- 18.Pillai S, Menon GK, Bikle DD, Elias PM. Localization and quantitation of calcium pools and calcium binding sites in cultured human keratinocytes. J Cell Physiol. 1993;154:101–12. doi: 10.1002/jcp.1041540113. [DOI] [PubMed] [Google Scholar]
- 19.Hennings H, Michael D, Cheng C, Steinert PM, Holbrook K, Yuspa S. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–54. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 20.Boyce ST, Ham RG. Calcium-regulated differentiation in normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial cultures. J Invest Dermatol. 1983;81:33–40. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 21.Bruno M, Dominique B, Michel B, Caroline D, Rainer S. Influence of calcium on the proteolytic degradation of the calmodulin-like skin protein (calmodulin-like protein 5) in psoriatic epidermis. Exp Dermatol. 2006;15:469–77. doi: 10.1111/j.1600-0625.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 22.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardones AR, Banez LL. VEGF inhibitors in cancer therapy. Curr Pharm Des. 2006;12:387–94. doi: 10.2174/138161206775201910. [DOI] [PubMed] [Google Scholar]
- 24.Luthra S, Narayanan R, Marques LE, Chwa M, Kim DW, Dong J, Seigel GM, Neekhra A, Gramajo AL, Brown DJ, Kenney MC, Kuppermann BD. Evaluation of in vitro effects of bevacizumab (Avastin) on retinal pigment epithelial, neurosensory retinal, and microvascular endothelial cells. Retina. 2006;26:512–8. doi: 10.1097/01.iae.0000222547.35820.52. [DOI] [PubMed] [Google Scholar]
- 25.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Dvorak HF, Senger GR. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993;143:1255–62. [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 27.Kaipainen A, Korhonen J, Mustonen T, Van Hinsbergh VM, Fang GH, Dumont D, Senger DR. Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–70. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science. 1998;282:946–9. doi: 10.1126/science.282.5390.946. [DOI] [PubMed] [Google Scholar]
- 29.Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, Matikainen MT, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K. Lymphatic endothelium and Kaposi's sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58:1599–604. [PubMed] [Google Scholar]
- 30.Partanen TA, Arola J, Saaristo A, Jussila L, Ora A, Miettinen M, Achen MG, Alitalo K. VEGF-C and VEGF-D expression in neuroendocrine cells and their receptor, VEGFR-3, in fenestrated blood vessels in human tissues. FASEB J. 2000;14:2087–96. doi: 10.1096/fj.99-1049com. [DOI] [PubMed] [Google Scholar]
- 31.Mäkinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 32.Karvonen SL, Korkiamaki T, Yla-Outinen H, Nissinen M, Teerikangas H, Pummi K, Karvonen J, Peltonen J. Psoriasis and altered calcium metabolism: downregulated capacitative calcium influx and defective calcium-mediated cell signaling in cultured psoriatic keratinocytes. J Invest Dermatol. 2000;114:693–700. doi: 10.1046/j.1523-1747.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- 33.Sharpe GR, Gillespie JI, Greenwell JR. An increase in intracellular free calcium is an early event during differentiation of cultured human keratinocytes. FEBS Lett. 1989;254:25–8. doi: 10.1016/0014-5793(89)81002-6. [DOI] [PubMed] [Google Scholar]
- 34.Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of calcium receptor. J Clin Invest. 1996;97:1085–93. doi: 10.1172/JCI118501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang XF, Cui JZ, Prasad SS, Matsubara JA. Altered gene expression of angiogenic factors induced by calcium-mediated dissociation of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:1508–15. doi: 10.1167/iovs.04-0951. [DOI] [PubMed] [Google Scholar]
- 36.Jesmin S, Sakuma I, Hattori Y, Fujii S, Kitabatake A. Long-acting calcium channel blocker benidipine suppresses expression of angiogenic growth factors and prevents cardiac remodelling in a Type II diabetic rat model. Diabetologia. 2002;45:402–15. doi: 10.1007/s00125-001-0765-6. [DOI] [PubMed] [Google Scholar]
- 37.Diaz BV, Lenoir MC, Ladoux A, Frelin C, Demarchez M, Michel S. Regulation of vascular endothelial growth factor expression in human ker-atinocytes by retinoids. J Biol Chem. 2000;275:642–50. doi: 10.1074/jbc.275.1.642. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho JF, Blank M, Shoenfeld Y. Vascular endothelial growth factor (VEGF) in autoimmune diseases. J Clin Immunol. 2007;27:246–56. doi: 10.1007/s10875-007-9083-1. [DOI] [PubMed] [Google Scholar]
- 39.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]


