Abstract
Synaptic efficacy following long-term potentiation (LTP) and memory consolidation is associated with changes in the expression of immediate early genes (IEGs). These changes are often accompanied by increased expression of glial fibrillary acidic protein (GFAP). While the protein products of the majority of IEGs are mainly restricted to the cell body, Arg3.1/Arc product is rapidly delivered to dendrites, where it accumulates close to synaptic sites. Arg3.1/Arc protein was originally considered neurone specific; however, we have recently found Arg3.1/Arc immunoreactivity (Arg3.1/Arc-IR) within glial cells and demonstrated its increased expression after LTP in the hippocampal dentate gyrus (DG). Here, we have further investigated this novel finding, using electron microscopic immunocytochemistry to determine the localization and sub-cellular distribution of Arg3.1/Arc protein in GFAP positive glia (GFAP-IR) in the DG. Arg3.1/Arc labelling was seen prominently in GFAP-IR glial cell bodies and in large- and medium-sized glial filamentous processes. GFAP-labelled medium–small peri-synaptic glial profiles also displayed Arg3.1/Arc-IR; however, the very thin and distal glial filaments only displayed Arc-IR. Arc-IR was distributed throughout the cytoplasm, often associated with GFAP filaments, and along the plasma membrane of glial processes. Peri-synaptic glial Arg3.1/Arc-IR processes were apposed to pre- and/or post-synaptic profiles at asymmetric axospinous synapses. These data, taken with our earlier study which provided evidence for an increase in astrocytic Arg3.1/Arc-IR after the induction of LTP, suggest a role for glial Arg3.1/Arc in structural and synaptic plasticity which may be critical for the maintenance of cognitive functions.
Keywords: hippocampus, ultrastructure, glia, immediate early genes, Arc, plasticity
Introduction
Long-lasting changes in synaptic efficacy and plasticity following tetanic stimulation of the perforant path (long-term potentiation; LTP) are accompanied by the expression of immediate early genes (IEGs), including the activity-regulated cytoskeleton-associated gene Arc, also known as Arg3.1 in the dentate gyrus (DG) [1–4]. Injection of anti-sense oligonucleotides to Arg3.1/Arc mRNA and targeted deletion of the corresponding gene impair the maintenance of LTP and the consolidation of long-term memory [5, 6]. Arg3.1/Arc is also strongly up-regulated in both a cell type-specific and experience-dependent manner by behavioural experiences [5, 7–10]. While the products of the majority of IEGs are restricted to the cell body, newly synthesized Arg3.1/Arc mRNA in granule cells of the DG is preferentially localized to those regions of the dendritic tree that have been recently stimulated, where it is believed to play a role in synaptic plasticity and circuitry modification [4]. Particularly interesting is the fact that alterations and more specifically the reduction of Arg3.1/Arc protein results in the learning deficits and dysfunctional changes observed in Alzheimer's disease (AD) transgenic animal models [11, 12]. Even if there are limited data in humans, there is some evidence showing reduced Arg3.1/Arc expression in cortical areas and hippocampal CA1 [12, 13]; suggesting that the deficits observed in these mice might model the memory loss in the early stages of AD.
The redistribution of Arg3.1/Arc protein may be mediated by interaction with cytoskeletal proteins, such as microtubule-associated protein, MAP2 and actin [14]. In the DG a large number of synapses are enveloped by astroglia, due to the extension of their lamellate processes in the neuropil [15], allowing rapid and direct communication between glial cells and neurones, which is important for all neurophysiological processes including LTP, since it is well known that glial peri-synaptic processes change their shape in response to neuronal activity [15–19]. This glial process movement is mediated by the action of the cytoskeleton, in which actin is responsible for the protrusion of lamellipodia and/or extension of filopodia along neuronal surfaces [16, 20–22]. Furthermore, these astroglial processes show spontaneous motility at synaptic structures in situ, including both synaptic terminals and post-synaptic dendritic spines [17, 20].
Here, extending our previous observations on Arg3.1/Arc in glia in normal conditions and after LTP [1, 23], we have investigated the co-localization and sub-cellular distribution of Arc with glial fibrillary acidic protein (GFAP), which is the principal intermediate filament of mature astrocytes, and together with actin modulates astrocyte motility and shape providing structural stability to their processes [24]. Our data provide ultrastructural evidence for the presence of Arc in astrocytes in the DG. Furthermore, our results show that glial Arg3.1/Arc labelling is not restricted to cell bodies and medium-sized glial filamentous processes, but can also be found in medium-small peri-synaptic glial profiles. This targeting of Arg3.1/Arc protein to glial profiles suggests a possible role in input-specific synaptic function and plasticity, accounting for the maintenance of cognitive functions.
Materials and methods
Fixation and tissue processing
Male Sprague-Dawley rats (300–400 g; n= 3) were anaesthetized with an intra-peritoneal injection of sodium pento-barbital (100 mg/kg). The brains were fixed by perfusion through the aortic arch with 50 ml of 3.8% acrolein (TAAB, UK) in a solution of 2% paraformaldehyde and 0.1M phosphate buffer (PB) pH 7.4, followed by 250 ml of 2% paraformaldehyde. Brains were removed from the cranium and cut into 4–5 mm coronal slabs of tissue containing the entire rostrocaudal extent of the hippocampus. This tissue was then post-fixed for 30 min in 2% paraformaldehyde and sectioned at 40–50 (m on a vibrating microtome (VT1000, Leica, Milton Keynes, UK). To optimize detection of all Arc and GFAP-containing profiles and minimize methodological variability, sections through the dorsal hippocampus containing both hemispheres of all animals were processed at the same time, for combined immunoperoxidase and immunogold-silver labelling as described elsewhere [1, 25].
Antibodies
Two anti-Arg3.1/Arc anti-sera were used. (1) An Arg3.1/Arc polyclonal affinity-purified rabbit anti-serum raised against His6-Arg3.1/Arc recombinant protein comprising amino acids 130–396 (gift of Dr. D. Kuhl) and (2) A polyclonal affinity-purified goat anti-serum raised against a peptide mapping the N-terminus (amino acids 1–50) of rat Arc protein was obtained from Santa Cruz Biotechnology Inc. (E-19, sc-6382, Santa Cruz, CA, USA). The pattern of labelling obtained with both antibodies is consistent with the immunolabelling observed with our previous study as well as other Arg3.1/Arc antibodies (Fig. 1A and B) [1, 4].
1.
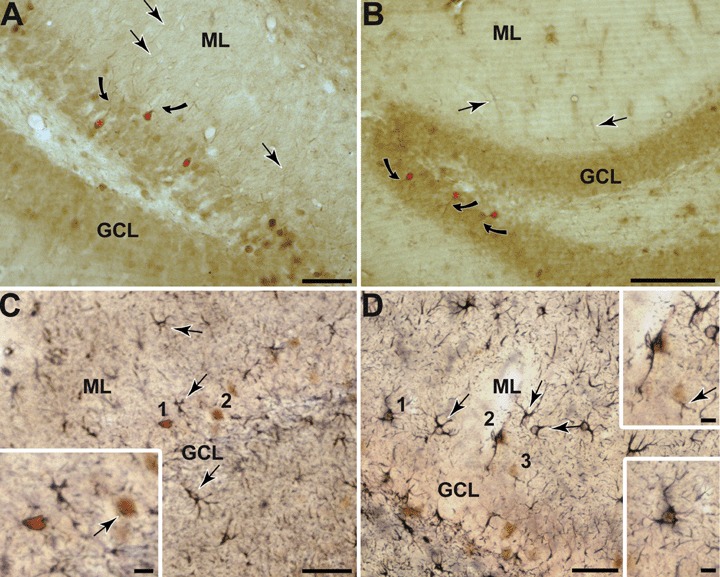
Photomicrographs showing Arc protein expression throughout the dentate gyrus in both, rat (A) and mice (B). Arc protein is present in the granule cells (red asterisks) as well as targeted to their proximal dendrites (curved arrows) and other scattered processes throughout the ML (arrows). C-D: Photomicrographs showing dentate gyrus GFAP and Arg3.1/Arc. Immunogold labelling for GFAP in round (C 1 and 2) and stellate cells (D 1-3) that also show brown peroxidase reaction product identifying Arg3.1/Arc (C and D boxed regions). Gold GFAP labelling is also seen in numerous cells and processes within the different layers of the DG (arrows), which is without Arg3.1/Arc peroxidase reaction product. Scale bars = 100 μm in A and B, 50 μm in C and D and 10 μm in insets.
A monoclonal mouse anti-serum generated against GFAP from pig spinal cord (Sigma-Aldrich Company Ltd., UK). Specificity was confirmed by western blot [24, 26]. To assess for non-specific background labelling or cross reactivity between antibodies derived from different host species, a series of control experiments were carried out. At both the light and electron microscopic level, omission of primary and/or secondary antibodies from the incubation cocktail resulted in a total absence of target labelling for immunoperoxidase and/or immunogold-silver labelling. Furthermore, Arg3.1/Arc -KO mice (n= 3; re-derived at NIMR Mill Hill, UK from animals generated by Dr. D. Kuhl) showed no immunoreactivity when processed with the Arc antibody. These primary antibodies are therefore regarded as specific to their designated targets.
Electron microscopic examination, nomenclature and analysis
For electron microscopy, ultrathin sections were cut and collected on copper mesh grids. These sections were counterstained with uranyl acetate and lead citrate [27] and examined with a JEOL-1010 electron microscope. Labelled profiles were classified as somata, dendrites, dendritic spines, unmyelinated axons, axon terminals and glia, according to their morphological features as defined by Peters et al.[28]. To determine the labelled profiles in electron micrographs, analysis was exclusively carried out on the most superficial portions of the tissue in contact with the embedding plastic to minimize artificial differences in labelling attributed to potential differences in penetration of reagents [29]. Regions chosen for this analysis were based on the presence of Arg3.1/ Arc and GFAP immunoreactivity and the morphological integrity of the tissue. The labelled profiles were assessed in a minimum of two non-consecutive single ultrathin sections obtained from nine coronal vibratome sections of three animals that were taken through the hippocampal DG at a level –2.8 mm/–4.8 mm posterior to Bregma, according to the rat brain atlas of Paxinos and Watson (1986) [30]. Adobe Photoshop and Illustrator (versions 7.0;Adobe Systems) software were utilized to build and label the composite illustrations.
Results
A diffuse distribution of Arg3.1/Arc immunoreactivity (Arg3.1/Arc-IR) was seen throughout the different layers of the DG, including some scattered Arg3.1/Arc-labelled cells in the granule cell layer (GCL), being equivalent in both rats and mice and in all analyzed animals (Fig. 1A and B), while GFAP-like immunoreactivity (GFAP-IR) in cell bodies was exclusively present in the molecular layer (ML; Fig. 1). Numerous GFAP-labelled processes were also visible crossing the GCL (Fig. 1C and D). Many of the GFAP-labelled cells had a stellate shape and multiple branched processes that are typical of astrocytes, while other GFAP-labelled cells resembled peri-vascular astrocytes (Fig. 1C and D). At light microscopy some of the GFAP-labelled cells also displayed somatic labelling for Arg3.1/Arc, especially in the outer layers of the GCL as well as throughout the ML (Fig. 1C and D).
Electron microscopy revealed and confirmed that Arg3.1/Arc immmunoperoxidase labelling was not only present in granule cells, their dendrites (Fig. 2C) and dendritic spines; but it also revealed prominent Arg3.1/Arc-IR in glial cell bodies and in many fila-mentous glial processes which also contained GFAP (Fig. 2). Arg3.1/Arc-IR was distributed throughout the cytoplasm of glial cells, often associated with immunogold positive GFAP filaments, and along the plasma membrane of the glial processes (Fig. 2A–C and F). Arg3.1/Arc labelling was present mainly in GFAP-IR large and medium-sized glial filamentous processes, but was also found in medium–small peri-synaptic glial profiles (Fig. 2A–C and F). These peri-synaptic glial Arg3.1/Arc-IR processes containing GFAP were apposed to pre-synaptic and/or post-synaptic profiles at asymmetric axospinous synapses (Fig. 2F and G). The very thin glial filaments displayed only Arg3.1/Arc-IR (Fig. 2F). GFAP-labelled glial processes were often peri-vascular and apposed the basal membrane of endothelial cells lining blood vessels showing that also in some cases displayed Arg3.1/Arc-IR (Fig. 2D and E).
2.
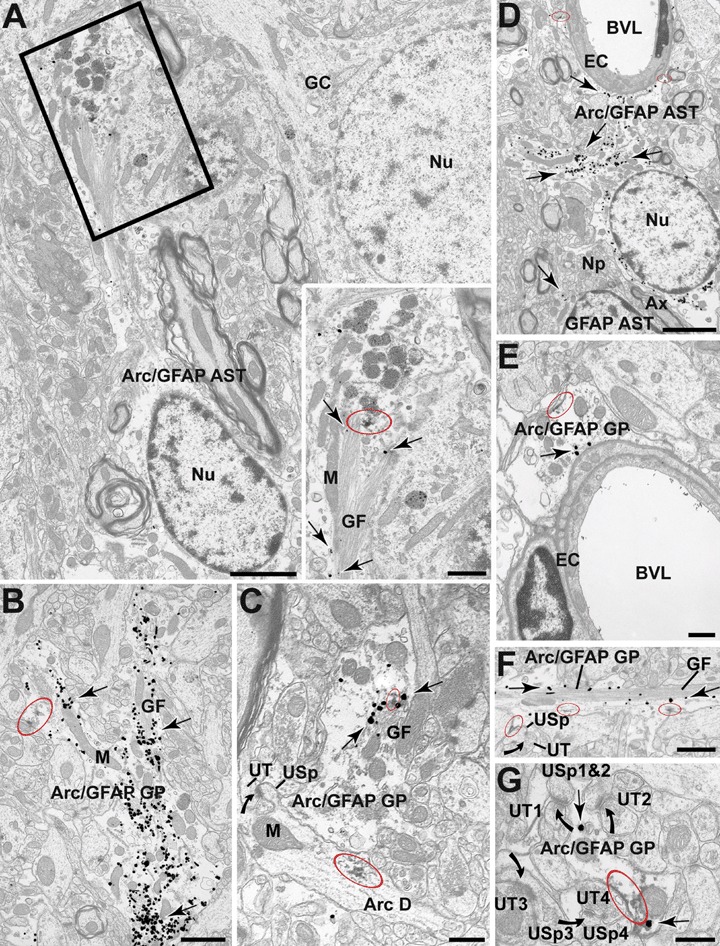
Electron micrographs from the DG showing immunoperoxidase localization of Arg3.1/Arc (red ellipses) within immunogold GFAP positive glial cell bodies (Arc/GFAP AST) and glial processes (Arc/GFAP GP; arrows). A: GFAP positive astrocytes located in the outer layers of the GC layer showing Arg3.1/Arc peroxidase reaction product in close association with the glial filaments (GF; boxed region). B and C: Arg3.1/Arc immunoperoxidase labelling within medium-sized GFAP positive glial profiles (arrows) within the ML directly associated with the plasma membrane as well as with the glial filaments (GF). In (C) we can also observed the typical dendritic presence of peroxidase-labelled Arg3.1/Arc (Arc D) which gives rise to an unlabelled spine (USp) that receives an asymmetric synapse from an unlabelled axon terminal (UT). D and E: Arg3.1/Arc Peroxidase reaction product within the cytoplasm of glial processes apposed to the basal membrane of endothelial cells (EC) lining the blood vessel lumen (BVL). Peroxidase labelling for Arg3.1/Arc appears within the cytoplasm and along discrete segments of the plasma membrane. In D the Arg3.1/Arc-labelled glial processes extending towards the BVL are proximal to the astrocytic cell body. We can also see that the Arc/GFAP astrocyte is in the vicinity of a single labelled GFAP astrocyte but separated by abundant neuropil (NP) and a myelinated axon (Ax). F and G: Peroxidase labelling for Arg3.1/Arc within the cytoplasm and along discrete segments of the plasma membrane of peri-synaptic glial profiles. The glial processes appose unlabelled dendritic spines (USp). All spines receive perforated (as in F) and unperforated asymmetric synapses (in G; curved arrows) from unlabelled terminal (UT). GC = granule cell, GP = glial profile, M = mitochondrion, Nu = nucleus. Scale bars = 2 μm in A–D, 1 μm in B–F, 0.5 μm in C–E, 0.3 μm in G and 0.8 μm in boxed region.
Discussion
The present study has used dual labelling immunocytochemistry at electron microscope level to demonstrate the distribution of Arg3.1/Arc protein in mature GFAP reactive astrocytes throughout the DG. In previous studies we have shown that there are no qualitative differences in the sub-cellular distribution of Arg3.1/Arc-IR with either immunoperoxidase labelling or immunogold with silver intensification [1, 23]. Each method has advantages and limitations. Immunogold is less sensitive than immunoperoxidase but allows selective sub-cellular localization [31]; yet immunoperoxidase facilitates analysis of labelled structures, which was the reason we labeled Arg3.1/Arc with peroxidase and GFAP with immunogold. The presence of immunoreactive profiles is markedly influenced by the penetration of anti-sera and immunoreagents as well as the depth of the en face thin section. Thus, our study of Arg3.1/Arc-GFAP co-localization was made exclusively at the tissue-Epon interface, where greater densities of immunolabelled profiles are seen [29].
Arg3.1/Arc has always been considered to be neurone specific [4, 32]. Our data provide the first direct in situ evidence for the presence of Arg3.1/Arc in mature reactive GFAP positive astrocytes in the DG, extending our recent observations of LTP-associated changes of Arc distribution in glial processes, which was based on morphological criteria alone [1]. We have found a generalized co-expression of Arg3.1/Arc and GFAP in all the sub-divisions of the DG which suggests that expression of Arg3.1/Arc protein in mature glia may be strategically located to modulate synaptic function in a context of glial-neuronal communication [15, 17, 33, 34], as shown by their peri-synaptic location. Astrocytes are involved in several forms of plasticity, including hippocampal axonal sprouting [35]. The astrocytic coverage of neurones could modify synaptic activity by controlling neuro-transmitter levels [35, 36]; localization of vesicular glutamate transporters in astrocytic processes near neuronal terminals and/or dendrites provides further anatomical evidence for such a role [35, 37]. Furthermore, astroglia may actively signal to neurones through the release of gliotransmitters, including glutamate [19, 34].
Plasticity in neuronal-glial networks is directly related to the motility of the cytoskeleton, such as actin, and actin-associated proteins like ezrin, radex-in and moesin (ERM proteins) [15]. Recently it has been suggested that ERM proteins appear not only in glia but also in neurones undergoing development; our data suggest that Arg3.1/Arc may also have a role in these peri-synaptic glial processes. In addition, actin filaments are the only cytoskeleton components in the peripheral processes which are devoid of GFAP [15], a finding that is confirmed by our results showing only Arg3.1/Arc-IR in very fine cellular processes. Astrocytic modifications appear in response to various CNS insults including those associated with ageing [35, 38]. Reactive astrocytes up-regulate the intermediate filament GFAP, as well as F-actin and α-actinin [35, 38]. F-actin filaments are known to co-precipitate with Arg3.1/Arc protein in neurones, so the presence of Arg3.1/Arc and the up regulation of F-actin filaments in astroglia could reflect a functional role. Finally, we have also shown Arg3.1/Arc-IR in GFAP-labelled peri-vascular glial and medium, medium–small processes, that contribute to the regulation of local blood flow. This is in agreement with the recent demonstration that neuronal stimulation leading to glutamate release activates peri-vascular glial processes inducing a dilatation of the blood vessels by stimulation of glial glutamate receptors [37, 39].
In conclusion, the present study demonstrates the presence of Arg3.1/Arc in GFAP-expressing glial profiles throughout the molecular layer of the DG. This expression may reflect a role for Arg3.1/Arc in structural and synaptic plasticity as well as in synaptic function. Arg3.1/Arc active role in synaptic function and LTP [1] through glia-neuron interaction contributes to the growing evidence that Arg3.1/Arc is critical for normal memory function and that their dysregulation might account for the memory loss observed in the early stages of AD [11–13]. Thus the maintenance of Arg3.1/Arc glia-neurone presence and association could be a new therapeutic cue for AD and other memory related disorders.
Acknowledgments
The authors would like to thank Dr. D. Kuhl for kindly providing the Arg3.1/Arc anti-serum and KO mice. This work was supported by BBSRC grant 10/1505/13.
References
- 1.Rodríguez JJ, Davies HA, Silva AT, De Souza IEJ, Peddie CJ, Colyer FM, Fine A, Errington ML, Bliss TVP, Stewart MG. Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. Eur J Neurosci. 2005;21:2384–96. doi: 10.1111/j.1460-9568.2005.04068.x. [DOI] [PubMed] [Google Scholar]
- 2.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci. 1995;92:5734–8. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated potein that is enriched in neuronal dendrites. Neuron. 1995;14:433–45. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 4.Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci. 2001;98:7062–8. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bösl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg 3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–44. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific induction of the immediate early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999;2:1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 8.Kelly MP, Deadwyler S. Acquisition of a novel behavior induces higher levels of Arc mRNA than does overtrained performance. Neuroscience. 2002;110:617–26. doi: 10.1016/s0306-4522(01)00605-4. [DOI] [PubMed] [Google Scholar]
- 9.Kelly MP, Deadwyler S. Experience-dependent regulation of the immediate-early gene Arc differs across brain regions. J Neurosci. 2003;23:6443–51. doi: 10.1523/JNEUROSCI.23-16-06443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montag-Sallaz M, Montag D. Learning-induced arg 3.1/arc mRNA expression in the mouse brain. Learning Memory. 2003;10:99–107. doi: 10.1101/lm.53403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palop JJ, Chin J, Bien-Ly N, Massaro C, Yeung BZ, Yu G-Q, Mucke L. Vulnerability of dentate granule cells to disruption of Arc expression in human amyloid precursor protein transgenic mice. J Neurosci. 2005;19:9686–93. doi: 10.1523/JNEUROSCI.2829-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickey CA, Loring JF, Montgomery J, Gordon MN, Eastman PS, Morgan D. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J Neurosci. 2003;23:5219–26. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginsberg SD, Hemby SE, Lee VM, Eberwine JH, Trojanowski JQ. Expression profile of transcripts in Alzheimer's disease tangle-bearing CA1 neurons. Ann Neurol. 2000;48:77–87. [PubMed] [Google Scholar]
- 14.Fujimoto T, Tanaka H, Kumamaru E, Okamura K, Miki N. Arc interacts with microtubules/microtubule associated protein 2 and attenuates microtubule associated protein 2 immunoreactivity in the dendrites. J Neurosci Res. 2004;76:51–63. doi: 10.1002/jnr.20056. [DOI] [PubMed] [Google Scholar]
- 15.Derouiche A, Frotscher M. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia. 2001;36:330–41. doi: 10.1002/glia.1120. [DOI] [PubMed] [Google Scholar]
- 16.Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006;26:8881–91. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opinion Neurobiol. 2005;15:542–8. doi: 10.1016/j.conb.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–40. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 20.Hirrlinger J, Hülsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–9. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- 21.Lepekin EA, Eliasson C, Berthold C-E, Berezin V, Bock E, Pekny M. Intermediate filaments regulate astrocyte motility. J Neurochem. 2001;79:617–25. doi: 10.1046/j.1471-4159.2001.00595.x. [DOI] [PubMed] [Google Scholar]
- 22.Mitchinson TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–9. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 23.Rodríguez JJ, Davies HA, Errington ML, Kuhl D, Bliss TVP, Stewart MG. Expression of Arg3.1/Arc in hippocampal dentate gyrus astrocytes: ultrastructural evidence and co-localization with glial fibrillary acidic protein. FENS Forum Abstracts. 2006:130. doi: 10.1111/j.1582-4934.2007.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–51. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 25.Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labelling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Meth. 1990;33:113–27. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. 3. New York: Oxford University Press; 1991. [Google Scholar]
- 29.Pickel VM, Johnson E, Carson M, Chan J. Ultrastructure of spared dopamine terminals in caudate-putamen nuclei of adult rats neonatally treated with intranigral 6-hydroxydopamine. Dev Brain Res. 1992;70:75–86. doi: 10.1016/0165-3806(92)90105-6. [DOI] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5. New York: Elsevier Academic Press; 2005. [Google Scholar]
- 31.Leranth C, Pickel VM. Electron microscopic pre-embedding double immunostaining methods. In: Heimer L, Zaborsky L, editors. Tract-tracing methods, recent progress. New York: Plenum Publishing; 1989. pp. 129–72. [Google Scholar]
- 32.Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Saphiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Verkhratsky A. Patching the glia reveals the functional organisation of the brain. Pflugers Arch. 2006;453:411–20. doi: 10.1007/s00424-006-0099-9. [DOI] [PubMed] [Google Scholar]
- 34.Verkhratsky A, Toescu EC. Neuronal-glial networks as substrate for CNS integration. J Cell Mol Med. 2006;10:826–36. doi: 10.1111/j.1582-4934.2006.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 35.Finch CE. Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging. 2003;24:S123–7. doi: 10.1016/s0197-4580(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 36.Araque A, Carmignoto G, Haydon PG. Dynamic signalling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 37.Volterra A, Steinhäuser C. Glial modulation of synaptic transmission in the hippocampus. Glia. 2004;47:249–57. doi: 10.1002/glia.20080. [DOI] [PubMed] [Google Scholar]
- 38.Abd-El-Basset EM, Fedoroff S. Upregulation of F-actin and α-actinin in reactive astrocytes. J Neurosci Res. 1997;49:608–16. doi: 10.1002/(SICI)1097-4547(19970901)49:5<608::AID-JNR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]


