Abstract
Dendritic cells (DC) have important functions in T cell immunity and T cell tolerance. Previously, it was believed that T cell unresponsiveness induced by immature DC (iDC) is caused by the absence of inflammatory signals in steady-state in vivo conditions and by the low expression levels of costimulatory molecules on iDC. However, a growing body of evidence now indicates that iDC can also actively maintain peripheral T cell tolerance by the induction and/or stimulation of regulatory T cell populations. In this study, we investigated the in vitro T cell stimulatory capacity of iDC and mature DC (mDC) and found that both DC types induced a significant increase in the number of transforming growth factor (TGF)-β and interleukin (IL)-10 double-positive CD4+ T cells within 1 week of autologous DC/T cell co-cultures. In iDC/T cell cultures, where antigen-specific T cell priming was significantly reduced as compared to mDC/T cell cultures, we demonstrated that the tolerogenic effect of iDC was mediated by soluble TGF-β and IL-10 secreted by CD4+CD25−FOXP3− T cells. In addition, the suppressive capacity of CD4+ T cells conditioned by iDC was transferable to already primed antigen-specific CD8+ T cell cultures. In contrast, addition of CD4+ T cells conditioned by mDC to primed antigen-specific CD8+ T cells resulted in enhanced CD8+ T cell responses, notwithstanding the presence of TGF-β+/IL-10+ T cells in the transferred fraction. In summary, we hypothesize that DC have an active role in inducing immunosuppressive cytokine-secreting regulatory T cells. We show that iDC-conditioned CD4+ T cells are globally immunosuppressive, while mDC induce globally immunostimulatory CD4+ T cells. Furthermore, TGF-β+/IL-10+ T cells are expanded by DC independent of their maturation status, but their suppressive function is dependent on immaturity of DC.
Keywords: dendritic cells; regulatory T cells; peripheral tolerance, transforming growth factor-β; interleukin-10
Introduction
Dendritic cells (DC), the professional antigen-presenting cells (APC) of the immune system, have important functions both in induction of T cell immunity and in maintaining peripheral tolerance [1, 2]. Currently, it is established that the main function of immature DC (iDC) in their in vivo steady-state condition is to capture and process antigens in the periphery and it is believed that this process is necessary to maintain peripheral self-tolerance to these antigens. Indeed, several reports have indicated that the presentation of antigens by iDC to T cells resulted in anergy or tolerance of the T cells [3–5]. Although the exact mechanisms are still poorly understood, it is now generally believed that the lack of a conclusive T cell mediated immune response after stimulation with iDC is caused by the absence of inflammatory danger signals in steady-state in vivo conditions and by the low expression levels of costimulatory molecules on iDC [6, 7]. In addition, it has also been demonstrated that iDC actively promote peripheral tolerance by the induction of interleukin (IL)-10-producing immunosuppressive regulatory T cells (Treg) [8–10], suggesting the importance of lack of DC maturation for T cell tolerance.
After encounter of a ‘danger’ signal (e.g. toll-like receptor [TLR] ligand), iDC mature [11] and migrate to the secondary lymphoid organs. Next, upon efficient antigen presentation to T cells, a robust immune response is induced in vivo. This maturation step is believed to be a crucial event to regulate DC function. A growing body of evidence now indicates that traditional DC maturation, as judged by surface marker expression (i.e. the upregulation of antigen-presenting and costimulatory molecules), can no longer be used to distinguish tolerogenic and immunogenic properties of DC. Although mainly iDC seem to trigger IL-10-producing Treg, several reports indicate that mature DC (mDC) are also able to induce IL-10-producing Treg in humans [12, 13].
In the present study, we examined some of the tolerogenic mechanisms of iDC. We compared the in vitro T cell stimulatory capacity of iDC and mDC in the absence and presence of MHC class I-restricted antigen at the cellular level. Our results indicate that the induction of an inefficient T cell immune response by autologous iDC in vitro (and possibly also in vivo) is not only a passive process (e.g. due to a low expression level of costimulatory molecules), but can also be manipulated in an active manner by the induction of suppressive cytokine-secreting T cells. On the other hand, mDC are capable of overruling T cell immunosuppression despite the co-activation of potential suppressor T cells.
Materials and methods
Dendritic cell culture
Peripheral blood mononuclear cells (PBMC) were obtained from HLA-A*0201-positive human cytomegalovirus (CMV)-seropositive buffy coats provided by the Antwerp Blood Transfusion Center (Red Cross). PBMC were isolated by Ficoll-Hypaque gradient separation (LSM, ICN Biomedicals, Costa Mesa, CA, USA). Next, CD14+ monocytes were directly isolated by CD14 immunomagnetic bead selection (Miltenyi Biotec), according to the manufacturer's instructions and directly used for in vitro DC differentiation, while the CD14-depleted fraction, designated as peripheral blood lymphocytes (PBL), was cryopreserved and stored at −80°C for later use in DC/T cell co-cultures. CD14+ monocytes were differentiated for 6 days in Iscove's modified Dulbecco's Medium (IMDM; Cambrex, Verviers, Belgium) supplemented with L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), amphotericin B (Fungizone, 1.25 μg/ml), 2.5% human(h)AB serum (Sigma Aldrich, Bornem, Belgium), 100 ng/ml granulocyte-macrophage colony-stimulating factor (GM-CSF; Leucomax, Novartis Pharma, Basel, Switzerland) and 1000U/ml IL-4 (R&D Systems, Minneapolis, MN, USA). On day 6, DC cultures were either left for another 24 hrs [= immature (i)DC population] or matured for 24 hrs by adding the TLR3 ligand poly I: C (Invivogen, Paris, France) at a concentration of 6.5 μg/ml [= mature (m)DC population]. All immature and mature DC cultures were harvested on day 7 for use in different experiments. In one experimental setup0.5μg/ml soluble trimeric human CD40-ligand (sCD40L; kindly provided by Amgen, Thousand Oaks, CA, USA) was added for an additional 24 hrs until day 8 of DC culture.
Flow cytometry
Immunophenotyping of dendritic cells and stimulated T cells was done following previously described procedures [27]. For detection and characterization of expanded antigen-specific T cells, the following tetramers were used for direct immunofluorescence staining: an influenza matrix protein M1-tetramer-PE (a kind gift from Dr Pierre Van der Bruggen, Ludwig Institute for Cancer Research, Université Catholique de Louvain (UCL), Brussels, Belgium) and a CMV pp65-tetramer-PE (Orpegen Pharma, Heidelberg, Germany).
For intracellular characterization of stimulated T cells, the following murine anti-human monoclonal antibodies were used for direct immunofluorescence staining: TGF-β-PE (IQ Products, Groningen, the Netherlands) and IL-10-APC (Becton Dickinson, Erembodegem, Belgium). T cell staining with the anti-TGF-β and anti-IL-10 antibodies was done according to the following procedure: 5 × 106 T cells were washed and incubated for 16 hrs at 37°C in IMDM supplemented with 5% hAB serum in the presence of 2 μL brefeldin A/106 cells/ml (Golgistop, Becton Dickinson). Next, cells were stained for membrane markers (CD3, CD4, CD8 and CD25) for 30 min at 4°C, followed by fixation for 10 min at room temperature with a lyse/fix solution (Becton Dickinson) and permeabilization with Perm2 solution (Becton Dickinson) for 10 min at room temperature. After staining of cells with anti-TGF-β and anti-IL-10 antibodies for 1 hr at 4°C, flow cytometric analysis was done.
Labelled cells were analyzed on a FACScan (Becton Dickinson) or a LSR II (Becton Dickinson) flow cytometer. For analytical flow cytometry, at least 104 events with forward and side scatter properties of DC or lymphocytes with CD3, CD4 or CD8 staining were measured.
Induction of MHC class I-restricted CMV pp65- or influenza M1 peptide-specific T cells
A CMV-specific HLA-A*0201-restricted peptide of the pp65 protein (amino acids (aa) 495-503, NLVPMVATV) and an influenza virus-specific HLA-A*0201-restricted matrix protein M1 peptide (M1; aa 58-66, GILGFVFTL) were used for activation of CMV pp65 and M1 peptide-specific T cells, respectively, as described previously [13]. For T cell activation experiments, PBL were cultured alone or with 5 × 105 autologous DC in the presence of CMV pp65 or influenza M1 peptide at a final concentration of 1 μg/ml culture medium. The experiments were performed in 24-well-plates in 2 ml IMDM supplemented with 5% hAB serum. In some experiments, the following neutralizing antibodies were added: 1 μg/ml anti-IL-10 antibody (Sigma) and 1 μg/ml anti-TGF-β antibody (R&D, Abingdon, UK). After 7 days of co-culture, T cells were analyzed for antigen specificity by means of tetramer staining and/or by interferon (IFN)-γ production following antigenic restimulation.
Suppression assay
CD4+ T cells, isolated by immunomagnetic bead selection (EasySep, Stem Cell Technologies, Meylan, France), were co-cultured with autologous DC populations in the absence of antigen. After 48 hrs of co-culture, CD4+ T cells were separated from stimulator DC by fluorescence-activated cell sorting (FACSVantage, Becton Dickinson) and tested for suppressive capacity. For this, sorted CD4+ T cells were added to 48 hrs-peptide-stimulated PBL cultures at a ratio of 1:10. On day 7 of culture, PBL were analyzed for antigen specificity by measuring IFN-γ production following antigenic restimulation.
Cytokine release assays
Quantitative detection of the cytokine expression profile of iDC and mDC was determined using a human Th1/Th2 multiplex fluorescent bead immunoassay(Bender MedSystems, Vienna, Austria), according to the manufacturer's instructions.
For detection of activated IFN-γ-producing CMV pp65 peptide-specific or influenza M1 peptide-specific T cells, cultured PBL were washed and stimulated for 18 hrs at 37°C with 1 μg/ml of CMV pp65 peptide or 1 μg/ml of influenza M1 peptide or left untreated as control. All experiments were done in quadruplicate. Collected supernatant samples were analyzed for secreted IFN-γ using a commercially available ELISA kit (Human IFN-γ Cytoset, Biosource, Nivelles, Belgium), according to the manufacturer's instructions.
In some experiments, supernatant samples of DC/T cell co-cultures were analyzed for the presence of immunosuppressive cytokines using a human TGF-β1 ELISA Set (BD Biosciences, San Diego, CA, USA) or an IL-10 EASIA (Biosource, Nivelles, Belgium), according to the manufacturer's instructions. The supernatants of the cultures were not acid-activated before the measurement was performed, in order to measure TGF-β in its active form only.
Statistics
The results are expressed as mean ± standard deviation. Comparisons were validated using paired Student's t-test. A P-value of ≤0.05 was considered as statistically significant.
Results
Characterization of monocyte-derived immature and mature dendritic cells
We cultured and characterized iDC and mDC as described previously [14, 15] with minor modifications. In order to address the functionality of DC, allogeneic T cell stimulatory capacity of both DC populations was assessed (Fig. 1A). Therefore, we co-cultured peripheral blood lymphocytes (PBL) with allogeneic iDC or mDC at a 4:1 ratio and used the level of interferon (IFN)-γ secreted as a measure for allogeneic T cell stimulatory capacity. Figure 1A demonstrates that mDC have a significantly higher ability to induce IFN-γ secretion by allogeneic PBL as compared to iDC. Next, the cytokine expression profile of iDC and mDC was determined using a multiplex fluorescent bead immunoassay (Fig. 1B). For this, cultured DC populations were harvested, washed and stimulated with soluble CD40 ligand (CD40L) for 24 hrs. IL-6, tumour necrosis factor (TNF)-α and IL-12 secretion was significantly increased in supernatant of mDC as compared to that of iDC.
1.
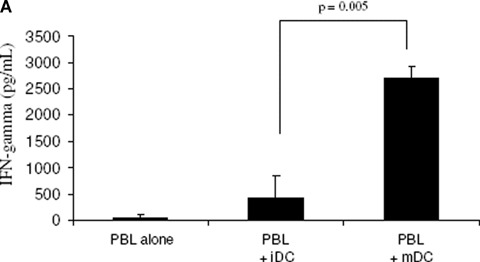
Characterization of in vitro differentiated monocyte-derived DC. (A) Allogeneic T cell stimulatory capacity. The results show the level of IFN-γ secreted in cell culture supernatant upon co-culture of iDC or mDC with allogeneic PBL. The level of IFN-γ secreted was used as a measure for allo-stimulatory capacity. The results from three independent PBMC donors are shown as mean ± standard deviation. (B) Cytokine secretion by iDC and mDC after a 24-hrs stimulation with CD40 ligand. Values are shown for IL-12, IL-6, and TNF-α (n= 3). The P-values indicated are calculated for the comparison between mDC and iDC.
Autologous T cell stimulatory capacity of monocyte-derived immature dendritic cells
In order to determine the capacity of iDC to stimulate autologous antigen-specific CD8+ T cells, we co-cultured PBL for 7 days with a MHC class I-restricted cytomegalovirus (CMV) pp65 peptide or influenza M1 peptide with or without autologous iDC or mDC at a 10:1 ratio. Next, cultured PBL were analyzed for the presence and activation of antigen-specific CD8+ T cells by CMV pp65 peptide-specific or influenza M1 peptide-specific T cell tetramer staining and by the level of antigen-specific IFN-γ secretion. Following restimulation of cultured PBL with CMV pp65 peptide, a significantly lower amount of IFN-γ was produced by PBL co-cultured with iDC as compared to cultures without iDC (Fig. 2A). In addition, Figures 2B and 2C demonstrate a significantly lower number of influenza M1 (n= 7) peptide-specific CD8+ T cells in T cell cultures after co-culture of PBL with iDC as compared to cultures without iDC. Similar results were obtained when PBL were cultured with CMV pp65 pep-tide with or without iDC (n= 2, data not shown).
2.
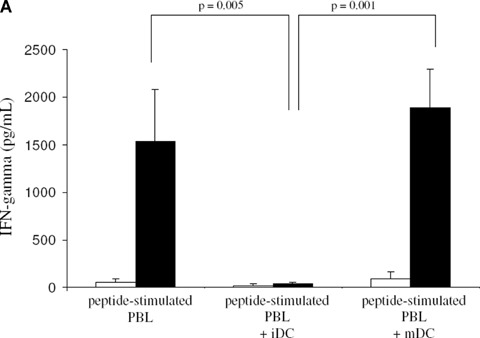
T cell stimulatory capacity of iDC and mDC.(A) Autologous T cell stimulatory capacity. PBL stimulated with CMV pp65 peptide with or without iDC or mDC were restimulated with CMV pp65 peptide after 7 days of initial co-culture (black bars). Controls (open bars) represent non-restimulated PBL. The level of IFN-γ secreted was used as a measure for autologous T cell stimulatory capacity. The results are shown as mean ± standard deviation of data obtained from four individual PBMC donors.(B) Tetramer staining results show the percentage of influenza M1 peptide-specific T cells within the CD8+ T cell population from unstimulated PBL, from PBL stimulated with influenza M1 peptide alone and from PBL co-cultured with iDC or mDC in the presence of influenza M1 peptide. The results shown are obtained from five independent donors.(C) Representative example showing tetramer staining of influenza M1 peptide-specific T cells within the CD3+ T cell population from unstimulated PBL, PBL stimulated with influenza M1 peptide alone and from PBL co-cultured with iDC or mDC in the presence of influenza M1 peptide.
Monocyte-derived immature and mature dendritic cells increase the number of TGF-β and IL-10 double-positive CD4+ T cells during co-culture with autologous lymphocytes
In order to understand the low level of autologous T cell activation after co-culture with iDC, we analyzed autologous DC/T cell co-cultures for suppressive T cell populations. For this, PBL were stimulated with CMV pp65 peptide with or without autologous iDC or mDC at a 10:1 ratio. After 6 days of co-culture, cultured cells were characterized by multiparametric flow cytometry in order to determine expression of intracellular immunosuppressive cytokines (Table 1 and Fig. 3). The results show a significant increase of TGF-β and IL-10 double-positive CD4+ T cells in T cell cultures after co-culture with both autologous iDC and mDC. Further analysis confirmed that the majority of TGF-β- and IL-10-expressing cells are positive for both cytokines (>70% within the TGF-β-and/or IL-10-producing cells) and these cells are negative for CD25, CD69, intracellular CTLA-4 and FOXP3 (data not shown), compatible with the phenotype described for adaptive regulatory T cells.
1.
Intracellular TGF-β and IL-10 staining of PBL stimulated with CMV pp65 peptide with or without iDC or mDC
| % TGF-β+ within CD3+CD4+CD25− | % IL-10+ within CD3+CD4+CD25− | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Peptide-stimulated PBL | Peptide-stimulated PBL + iDC | Peptide-stimulated PBL + mDC | Peptide-stimulated PBL | Peptide-stimulated PBL + iDC | Peptide-stimulated PBL + mDC | |||||
| 1 | 0.77 | 1.28 | ND* | ND | ND | ND | |||||
| 2 | 0.44 | 1.49 | ND | ND | ND | ND | |||||
| 3 | 1.07 | 3.07 | 2.14 | 1.25 | 3.5 | 2.56 | |||||
| 4 | 0.98 | 2.73 | 2.1 | 0.94 | 2.69 | 2.33 | |||||
| Fold increase (mean)† | 2.68 | 2.07 | 2.83 | 2.26 | |||||||
| P-value‡ | 0.015 | 0.01 | 0.04 | 0.01 | |||||||
ND, not done.
The indicated values represent the mean fold increase of TGF-β+ or IL-10+ CD3+CD4+CD25− T cells within autologous peptide-stimulated DC/T cell cultures as compared to peptide-stimulated PBL alone.
The P-values indicated are calculated for CMV pp65 peptide-stimulated PBL versus PBL co-cultured with iDC or mDC in the presence of CMV pp65 peptide.
3.
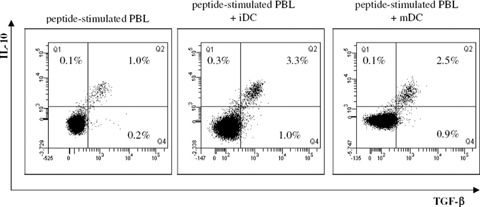
Flow cytometric analysis of immunosuppressive cytokine-expressing regulatory T cells. Representative example of intracellular TGF-β and IL-10 staining of PBL stimulated for 6 days with CMV pp65 peptide with or without iDC or mDC. Dot plots are gated on CD3+CD4+CD25− T cells and show TGF-β staining (x-axis) versus IL-10 staining (y-axis). The indicated percentages are within the CD3+CD4+CD25− T cell population.
Soluble factors in iDC/T cell co-culture supernatant suppress IFN-γ production by autologous T cells following antigenic stimulation
The results described above suggest that the low level of autologous T cell activation after co-culture with iDC might be due to the activation of TGF-β/IL-10-producing T cells by iDC. In order to test this hypothesis, we analyzed iDC/T cell co-culture supernatant samples for the presence of immunosuppressive cytokines. For this, PBL were stimulated with CMV pp65 peptide with or without iDC. After 7 days of co-culture, analysis of culture supernatant samples performed with a TGF-β ELISA revealed a significantly higher amount of TGF-β in the supernatant of the iDC/T cell co-cultures as compared to the T cell cultures stimulated with peptide alone (Fig. 4A). In contrast, no IL-10 could be detected by ELISA (data not shown). In order to demonstrate that secreted factors (e.g. TGF-β and/or IL-10) suppress T cell activation, the following experiments were done. First, when antigenic restimulation of CMV pp65 peptide-stimulated PBL was done in the presence of day 7 iDC/T cell co-culture supernatant, the IFN-γ response observed was significantly inhibited (Fig. 4B). Second, when PBL were co-cultured with iDC in the presence of CMV pp65 peptide and with an anti-TGF-β and/or anti-IL-10 neutralizing antibody, the level of autologous T cell activation after co-culture with iDC increased. Table 2 demonstrates a significant increase of CMV pp65 peptide-specific IFN-γ production by PBL stimulated with iDC upon addition of anti-TGF-β and anti-IL-10 neutralizing antibodies during initial iDC/T cell co-culture. Moreover, both neutralizing antibodies seem to be working cooperatively: there is only significant increase in antigen-specific IFN-γ secretion when both antibodies are used together. This strongly suggests that both TGF-β and IL-10 are needed to mediate the immunosuppressive effect induced by Treg conditioned by iDC.
4.
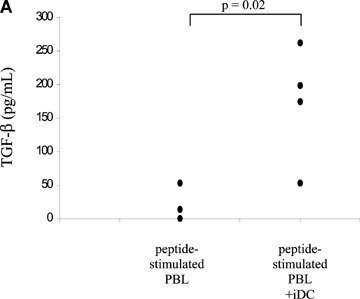
Soluble factors in iDC/T cell co-culture supernatant suppress IFN-γ production by T cells following antigenic stimulation. (A) TGF-β concentration in supernatants of CMV pp65 peptide-stimulated PBL cultured with and without iDC. The results are shown as mean values of four independent PBMC donors. Background TGF-β concentration from non-conditioned culture medium was subtracted from the culture values. (B) Inhibitory effect of iDC/T cell co-culture supernatant on antigen-specific IFN-γ secretion after restimulation of CMV pp65 peptide-stimulated PBL with CMV pp65 peptide (black bars). Triple experiments shown as mean ± standard deviation for one representative experiment of three independent donors (P= 0.01). Controls (open bars) represent non-restimulated PBL.
2.
Effect of anti-TGF-β and anti-IL10 neutralizing antibodies during co-culture of PBL with iDC in the presence of CMV pp65 peptide on the antigen-specific IFN-γ response after restimulation with CMV pp65 peptide
| IFN-gamma (pg/ml) | |||||
|---|---|---|---|---|---|
| Donor | Peptide-stimulated PBL + iDC | Peptide-stimulated PBL + iDC + anti-TGF-β | Peptide-stimulated PBL + iDC + anti-IL-10 | Peptide-stimulated PBL + iDC + anti-TGF-β+ anti-IL-10 | |
| 1 | 1± 1 | 35 ± 3 | 62 ± 18 | 170 ± 43 | |
| 2 | 156 ± 28 | 561 ± 73 | 1120 ± 152 | 1735 ± 148 | |
| 3 | 790 ± 224 | 923± 118 | 2100 ± 14 | 1842 ± 81 | |
| 4 | 350 ± 94 | 443 ± 105 | 608 ± 92 | 1061 ± 123 | |
| P-value* | 0.07 | 0.06 | 0.03 | ||
The P-values indicated are calculated for peptide-stimulated PBL + iDC versus peptide-stimulated PBL + iDC supplemented with anti-TGF-β or anti-IL-10 or anti-TGF-β+ anti-IL-10 during initial PBL co-culture.
CD4+ T cells conditioned by iDC, but not by mDC, inhibit efficient activation of IFN-γ-producing antigen-specific T cells
As demonstrated above, the low level of autologous T cell stimulation observed using iDC is mediated, at least for a major part, by the induction of TGF-β+/IL10+ suppressive T cells (Table 2). To investigate this hypothesis, we designed the following experimental setup (Fig. 5). Purified CD4+ T cells were co-cultured for 48 hrs with iDC or mDC in the absence of antigen. These conditioned CD4+ T cells were then separated from stimulator DC populations by flow cytometric cell sorting and added at a 1:10 ratio to PBL cultures primed for 2 days with CMV pp65 peptide. Next, following 7 days of co-culture, cultured PBL were restimulated with CMV pp65 pep-tide in order to determine the level of antigen-specific IFN-γ secretion. Figure 5B demonstrates a significant decrease of the antigen-specific IFN-γ immune response when CD4+ T cells conditioned by iDC, but not control CD4+ T cells, were added to PBL stimulated with peptide alone. Interestingly, when CD4+ T cells conditioned by mDC were added to PBL stimulated with CMV pp65 peptide, a significant increase of the antigen-specific IFN-γ immune response was observed as compared to PBL stimulated with peptide alone.
5.
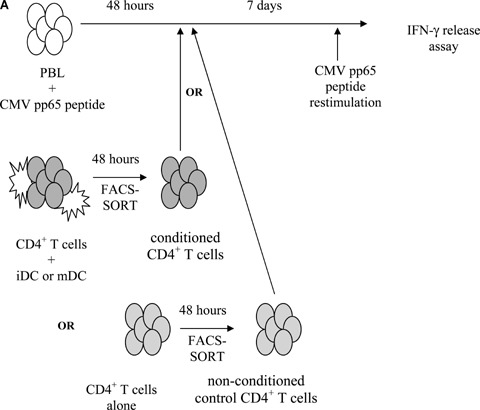
T cell suppression assay. (A) Experimental design. (B) Effect of CD4+ T cells conditioned by iDC or mDC during PBL stimulation with CMV pp65 peptide and of non-conditioned control CD4+ T cells on antigen-specific IFN-γ response after restimulation with CMV pp65 peptide. The level of IFN-γ secreted was used as a measure for autologous T cell activation. The results obtained from four independent experiments performed with different PBMC donors are represented as relative to the IFN-γ response from CMV pp65 peptide-stimulated PBL cultures, which was normalized to 100%.
Discussion
In this study, we demonstrate that both immature and mature DC actively induce TGF-β/IL-10 double-positive CD4+CD25−FOXP3− adaptive regulatory T cells during in vitro co-culture with T cells. The inefficient T cell stimulation when performed with iDC as stimulator cells was mediated by soluble factors, as evidenced by iDC/T cell culture supernatant and could be attributed to the direct effects of TGF-β and IL-10, as demonstrated by neutralizing antibodies to both cytokines. In addition, the suppressive capacity of CD4+ T cells conditioned by iDC was shown to be transferable when these CD4+ T cells were added to an already primed T cell response.
Currently, two adaptive Treg populations have been characterized, based on the expression of different immunosuppressive cytokines: CD4+ regulatory T cells type 1 (Tr1 cells) and CD4+ T helper 3 regulatory T cells (Th3 cells). Tr1 cells express high levels of IL-10, moderate levels of IL-5, IFN-γ and TGF-β and are negative for IL-2 and IL-4 [16, 17]. Th3 cells express high levels of TGF-β[18, 19]. Tr1 and Th3 cells have been shown to originate from naive resting T cells after stimulation with DC [20, 21], dependent on DC type and activation state. In agreement with others [9, 10], we show that adaptive Treg are induced after stimulation with iDC. However, and in contrast to previous studies [9, 10] where multiple stimulations with allogeneic iDC were needed for induction of adaptive Treg, in our hands an adaptive Treg population was induced after one stimulation with autologous iDC. In addition, we have shown here at the single cell level, as far as we know for the first time, that the majority (> 70%) of these immunosuppressive cytokine-producing adaptive Treg are a single population co-expressing IL-10 and TGF-β. Moreover, using neutralizing antibodies against immunosuppressive cytokines, we confirm that both cytokines were secreted by these T cells, although only TGF-β was detectable by means of ELISA and that both cytokines were needed to mediate the immunosuppressive effect.
In our hands, mature DC also induce cells with an adaptive Treg phenotype (TGF-β+IL-10+ CD4+CD25−FOXP3−) as well as with a naturally occurring Treg phenotype (CD4+CD25+FOXP3+ T cells, data not shown), compatible with other recent studies [22, 23]. However, and in contrast to these studies, in our hands, DC maturation performed with TLR ligation seems to impair the inhibitory function of suppressor T cells conditioned by mature DC (mDC). The reason for this difference is not certain at present, but could lie in the difference of TLR ligands used [the TLR3 ligand poly I:C in this study versus the TLR2/4 ligand lipopolysaccharide (LPS) in the studies mentioned above] and in the assay used [autologous antigen-primed DC/T cell co-cultures in this study versus allogeneic mixed leukocyte reactions in the studies mentioned above]. Currently, this subject is under intensive investigation in our laboratory.
Our results confirm that iDC are capable of maintaining peripheral tolerance by suppressing allogene-ic and autologous T cell responses both at the level of IFN-γ secretion by antigen-specific T cells and by reducing expansion of antigen-specific T cells. In this study, we also demonstrated that immature DC do not secrete significant amounts of IL-6 and IL-12 as compared to mature DC. It has been shown that both cytokines can restore proliferation and cytokine expression by effector T cells in the presence of regulatory T cells [24–26]. This feature of mDC is likely to play a role in the efficient T cell activation observed and in the apparent refractoriness to regulatory T cells. In an attempt to reverse the tolerogenic properties of iDC, we added IL-6 and/or IL-12 during T cell activation experiments with iDC. However, in our hands, induction of T cell immunity by iDC was not improved (data not shown). This is consistent with the observations of Medzhitov et al. [24], who showed that IL-6 is an important but not sufficient factor to reverse suppression by Treg, emphasizing the possible role of other, yet unidentified, factors.
Based on these results, we conclude that DC have a complementary role in inducing both regulatory T cells and effector T cells, where the final result of antigen-specific T cell activation will depend on the activation state of the DC. Our results warrant further investigation into the interplay of regulatory T cell subsets and the mechanisms involved in this inhibition of antigen-specific T cell activation.
Acknowledgments
This work was supported by grants no. G.0456.03, G.0313.01 and WO.012.02 of the Fund for Scientific Research, Flanders, Belgium (FWO-Vlaanderen), by grants of the Fortis Bank Verzekeringen – financed Cancer Research, by research grants of the Foundation against Cancer (Belgische Federatie tegen Kanker, now Stichting tegen Kanker) and by grant no. 802 of the Antwerp University Concerted Research Action (BOF-GOA). VFIVT and PP are postdoctoral fellows of the Fund for Scientific Research (FWO), Vlaanderen. NC held a PhD fellowship of the Flemish Institute for Science and Technology (IWT) and ELJMS holds a PhD fellowship of the FWO, Vlaanderen.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Moser M. Dendritic cells in immunity and tolerance-do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/s1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 3.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–79. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–16. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz RH, Mueller DL, Jenkins MK, Quill H. T-cell clonal anergy. Cold Spring Harb Symp Quant Biol. 1989;54:605–10. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 8.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 9.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–9. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 11.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 12.Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autol-ogous dendritic cells. Eur J Immunol. 2004;34:762–72. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 13.Lundqvist A, Palmborg A, Pavlenko M, Levitskaya J, Pisa P. Mature dendritic cells induce tumor-specific type 1 regulatory T cells. J Immunother. 2005;28:229–35. doi: 10.1097/01.cji.0000158854.15664.c2. [DOI] [PubMed] [Google Scholar]
- 14.Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Ponsaerts P, Van Den Bosch G, Cools N, Van Driessche A, Nijs G, Lenjou M, Lardon F, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN, Van Tendeloo VF. Messenger RNA electroporation of human monocytes, followed by rapid in vitro differentiation, leads to highly stimulatory antigen-loaded mature dendritic cells. J Immunol. 2002;169:1669–75. doi: 10.4049/jimmunol.169.4.1669. [DOI] [PubMed] [Google Scholar]
- 16.Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–60. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 18.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178:179–85. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 19.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. II; TGF-beta-transgenic Th3 cells resue IL-2-deficient mice from autoimmunity. J Immunol. 2007;178:172–8. doi: 10.4049/jimmunol.178.1.172. [DOI] [PubMed] [Google Scholar]
- 20.Enk AH. Dendritic cells in tolerance induction. Immunol Lett. 2005;99:8–11. doi: 10.1016/j.imlet.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki S, Patel M, Harper A, Bonito A, Fukuyama H, Pack M, Tarbell KV, Talmor M, Ravetch JV, Inaba K, Steinman RM. Effective expansion of alloantigen-specific Foxp3+ CD25+ CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc Natl Acad Sci USA. 2006;103:2758–63. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autol-ogous dendritic cells. Eur J Immunol. 2004;34:762–72. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–6. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 26.King IL, Segal BM. Cutting edge: IL-12 induces CD4+ CD25− T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–5. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 27.Ponsaerts P, Van Tendeloo VF, Cools N, Van Driessche A, Lardon F, Nijs G, Lenjou M, Mertens G, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. mRNA-electroporated mature den-dritic cells retain transgene expression, phenotypical properties and stimulatory capacity after cryopreser-vation. Leukemia. 2002;16:1324–30. doi: 10.1038/sj.leu.2402511. [DOI] [PubMed] [Google Scholar]


