Abstract
We have previously reported that 8-epipuupehedione, a synthetic derivative of sesquiterpenes found in several kinds of sponges, is a potent inhibitor of angiogenesis. Here, we show that 8-epipuupehedione is also a potent anti- leukaemic compound, targeting three hallmarks of malignancy: proliferation, survival and extra-cellular matrix re-modelling. To fulfil this goal, we use the HL-60 promyeolocytic cells as our model system and the following experimental procedures: cell growth assay, Hoetsch staining, cell cycle analysis and DNA fragmentation, caspase 3 activity and zymographic assays. Our results show that this compound inhibits proliferation and has potent and specific pro-apoptotic effects on HL-60 promyelocytic cells, inducing their nuclei and DNA fragmentation, as well as caspase 3 activity activation. Furthermore, 8-epipuupehedione strongly inhibits matrix metalloproteinase-2 and urokinase production by HL-60 cells. These results suggest that 8-epipuupehedione could be an attractive drug for further evaluation in the treatment of leukemia.
Keywords: 8-epipuupehedione; promyelocytic leukaemia; apoptosis; matrix metalloproteinase-2; urokinase, angiogenesis
Introduction
Recent studies indicate that angiogenesis is important in the pathogenesis and progression of several forms of leukaemia, including acute promyelocytic, acute myeloid and chronic lymphocytic leukaemia [1–5]. Recently, the first anti-angiogenic compounds have been approved for the treatment of cancer and blindness, encouraging expectations in their therapeutical potential for the treatment of these and other angiogenesis-dependent diseases [6]. Since angiogenesis is a complex, multi-factorial process, the biosignalling pathways regulating the different steps of the process can be potential targets for the action of therapeutical agents [6–8].
We have reported previously that hyperforin behaves as a potent anti-angiogenic compound [9]. Afterwards, other groups have shown that hyperforin and other anti-angiogenic compounds are effective anti-leukaemic agents [10, 11]. We have also shown that puupehenone, a sesquiterpene produced by certain sponges, and related compounds inhibit angiogenesis [12]. In that study, we used bovine aortic endothelial cells (BAEC) as model system and a number of in vitro assays to dissect the effects of test compounds on the different steps of angiogenesis. We could conclude that the synthetic derivative 8-epipuupehedione was the most effective tested anti-angiogenic sesquiterpene, behaving as a potent inhibitor of the capability of endothelial cells to form capillary-like tubes and to remodel and invade extra-cellular matrix. Prompted by all these previous observations, the aim of the present communication was to test whether 8-epipuupehedione could have the potential to inhibit human leukaemia, using the HL-60 promyelocytic leukaemia cells as a model system.
Material and methods
Material and reagents
Cell culture media were purchased from Gibco (Grand Island, NY, USA) and Cambrex (Walkersville, MD, USA). Foetal bovine serum (FBS) was a product of Harlan-Seralab (Belton, U.K.). 8-Epipuupehedione was synthesized as previously reported [13], and provided to us by Instituto Biomar S.A. (León, Spain). Stock solution (10 mg/ml) was prepared in dimethyl sulfoxide (DMSO) and stored in aliquots at –20°C. In all the assays, the vehicle (DMSO) was at less than 1% (v/v) and controls with the vehicle alone were carried out in parallel. Supplements and other chemicals not listed in this section were obtained from Sigma-Aldrich. Plasticware for cell culture was supplied by NUNC (Roskilde, Denmark).
Cell cultures
Bovine aortic archs were isolated from calfs immediately after their sacrifice at the local slaughterhouse Famadesa (Málaga), transported to the lab immersed in PBS containing penicillin-streptomycin and amphotericin at standard cell culture concentrations, and used immediately upon arrival for isolation of primary BAEC by a collagenase treatment, as first described by Gospodarowicz et al.[14]. BAEC were grown in Dulbecco's modified Eagle's medium (DMEM) containing 1 g/l glucose, 10% FBS, 2 mM gluta-mine, 50 U/ml penicillin, 50 μg/ml streptomycin, 1.25 μg/ml amphotericin B. Human HL-60 promyelocytic cells, human colon carcinoma cell line HCT-116 and human fibrosarco-ma cell line HT-1080 were supplied by ATCC and grown in DMEM containing 4.5 g/l glucose (RPMI-1640 medium in the case of HL-60 cells), 10% FBS, 2 mM glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 1.25 μg/ml ampho-tericin B. Cell lines were maintained at 37°C and humidified 5% CO2 atmosphere.
Cell growth assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Chemical Co., St. Louis, MO) dye reduction assay in 96-well microplates was used. The assay is dependent on the reduction of MTT by mitochon-drial dehydrogenases of viable cell to a blue formazan product, which can be measured spectrophotometrically. HL-60 cells (2 x 103 cells in a total volume of 100 μl of complete medium) were incubated in each well with 1:1 serial dilutions of 8-epipuupehedione, down to concentrations in the submicromolar range. After 3 days of incubation (37°C, 5% CO2 in a humid atmosphere), 10 μl of MTT (5 mg/ml in phosphate-buffered saline [PBS] were added to each well and the plate was incubated for a further 4 h (37°C). The resulting formazan was dissolved in 150 μl of 0.04 N HCl/2-propanol and read at 550 nm. All determinations were carried out in quadruplicate and four independent experimetns were carried out. IC50 values were calculated as those concentrations of 8-epipuupehedione yielding a 50% cell survival.
Hoechst staining
For the study of nuclear morphologic changes induced by 8-epipuupehedione, adherent BAEC and HT-1080 cells were seeded on coverslips, grown to sub-confluency and treated with 8-epipuupehedione for the indicated time. Non-adherent HL60 cells were treated with 8-epipuupehedione and a total of 5 x 105 cells were attached to slides using a Hettich cytospin. After fixation with formalin solution, cells were stained with Hoechst 1 μg/ml in PBS. Coverslips were mounted on slides using Dakocytomation Fluorescent Mounting Medium (Dako) and observed under a fluorescence microscope (Leica, TCS-NT).
Cell cycle analysis
After treatment, cells were harvested and centrifuged. Pellets were washed with PBS and suspended in 250 μl of ice-cold PBS. For fixation, 70% ice-cold ethanol was added while continuous gentle vortexing and samples were maintained on ice for 1 hr. Finally, cells were centrifuged and washed twice with PBS, suspended in 1 ml propidium iodide staining solution (40 μg/ml propidium iodide and 0.1 mg/ml RNase-A in PBS) and incubated for 1 hr at 37°C protected from light. Percentage of subG1, G1, S and G2/M cells was determined using a FACScan flow cytometer (BD).
DNA fragmentation assay
For DNA internucleosomal fragmentation analysis, BAEC and HL-60 cells were treated with 8-epipuupehedione for 14 hrs. Afterwards, cells were harvested and centrifuged, and pellets were frozen in liquid nitrogen. Internucleosomal fragmented DNA was isolated and visualized by agarose gel electrophoresis, as described by us elsewhere [15].
Caspase-3 activity assay
After treatments, BAEC and HL-60 cells were harvested. A total of 5 x 105 cells were suspended in 25 μL PBS and snap-frozen in wells of a 96-well opaque microtiter plate on liquid nitrogen. After thawing on ice, 50 μl of assay buffer (100 mM HEPES pH 7.2, 10% sucrose, 0.1% CHAPS) containing 50 μM of the selective fluorogenic substrate Ac-DEVD-AMC was added per well. Cleavage of the fluoro-genic substrate at 37°C was measured for 30 min using a Fluoroscan II microplate reader (Thermo Electron Co., Waltham, MA, USA) at the excitation/emission wave-lengths 355/460 nm. Slopes of the fluorescence curves obtained were used as activity values and were represented as fold increases, taking control values as 1. Data from duplicate samples were used in each experiment.
Zymographic assays
To prepare conditioned media and cell extracts, HL/60 cells were grown in 6-well plates. After 3 days of culture, cells were washed twice with PBS and suspended in 1.5 ml of fresh complete culture medium containing 200 KIU of aprotinin/mL. Additionally, some wells received 4 μM 8-epipuu-pehedione. After 24 hrs of incubation, conditioned media were collected and concentrated 25× by centrifugation in Amicon Ultra concentration tubes (Millipore). The cells were washed twice with PBS and harvested by scrapping into 0.5 ml of 0.2% Triton X-100 in 0.1 M Tris/HCl containing 200 KIU of Trasylol/mL. Cell lysates were centrifuged at 1000×g and 4°C for 20 min. Afterwards, the supernatants were collected and used for zymography. Duplicates were used to determine cell number with a Coulter counter.
Assays of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor (PAI) activities in gel were carried out as previously explained [16]. Aliquots of cell extracts normalized for equal cell numbers were subjected to sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) at 4°C under non-reducing conditions, with 5% stacking gel and 10% resolving gel. Gels were washed for 10 min twice with 50 mM Tris/HCl, pH 7.4, supplemented with 2% Triton X-100 and twice with 50 mM Tris/HCl, pH 7.4 and laid over a substrate gel prepared with agar (0.8%), plasminogen (40 μg/ml) and skimmed milk (1.5% in PBS). Gels were incubated under a moist atmosphere overnight at 4°C and then incubated at 37°C. After 4–8 hrs, bands of proteolysis due to uPA activity were photographed under dark field. Extended incubation revealed PAI bands of activity as white bands over a dark ground.
The gelatinolytic activity of matrix metalloproteinase-2 (MMP-2) delivered to the conditioned media was detected in gelatinograms as previously described [17]. Aliquots of concentrated conditioned media normalized for equal cell numbers were subjected to non-reducing SDS/PAGE as above but with gelatin (1 mg/ml) added to the 10% resolving gel. After electrophoresis, gels were washed twice with 50 mM Tris/HCl, pH 7.4, supplemented with 2% Triton X-100, and twice with 50 mM Tris/HCl, pH 7.4. Each wash with continuous shaking lasted 10 min. After the washes, the gels were incubated overnight at 37°C and immersed in a substrate buffer (50 mM Tris/HCl, pH 7.4, supplemented with 1% Triton X-100, 5 mM CaCl2, and 0.02% Na3N). In some experiments, 4 μM 8-epipuupehedione was added to the substrate buffer. Finally, the gels were stained with Commassie blue R-250 and the bands of gelatinase activity could be detected as non-stained bands in a dark, stained background.
Results and discussion
Firstly, we wanted to test the effect of 8-epipuupehe-dione on HL-60 growth.Figure 1A shows that, in fact, this is a potent inhibitor of HL-60 cell growth, yielding an IC50 value of 2.4±0.8 μM (means±S.D.of four independent experiments, each with quadruplicate samples), which is at least an order of magnitude lower than those obtained with human umbilical vascular endothelial cells (own unpublished results) and BAEC, as well as with a number of different kinds of human cancer cells, including lung and breast carcinoma, colon and pancreatic adenocarcinoma and glioblas-toma cells [12]. This remarkable differential behaviour points to a certain specificity of the effects of 8-epipu-upehedione on HL-60 promyelocytic leukaemia cells.
1.
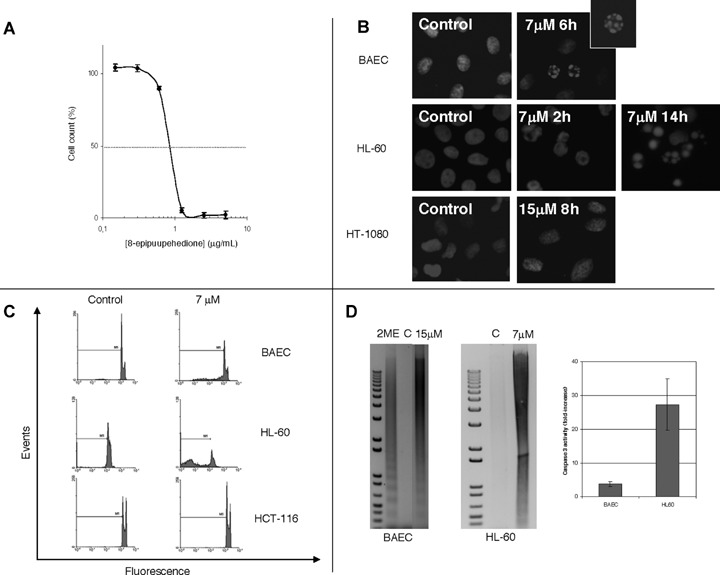
8-epipuupehedione inhibits HL-60 cell proliferation (A) and induces endothelial bovine aortic endothelial cells (BAEC) and leukaemia (HL-60), but not tumour (HT-1080 and HCT-116) cell apoptosis (B, C and D), by activation of caspase activity (D). For the study of the dose-dependent effect of 8-epipuupehedione on the in vitro growth of HL60 cells, the MTT dye reduction assay was used (A). Cell proliferation is represented as a percentage of control-cells growth. Each point represents the mean of quadruplicates; SD values were always lower than 10% of the mean values and are omitted for clarity. A typical curve is represented out of four independent experiments. For the study of nuclear morphologic changes induced by 8-epipuupehedione (B), treatments were carried out for the indicated times and Hoetsch staining was carried out as described in Material and methods section. For cell cycle analyses (C), BAEC, HL-60 and HCT-116 cells were treated with 8-epipuupehedione (7 μM) for 14 hrs, fixed with 70% ice-cold ethanol, stained with propidium iodide and submitted to flow cytometry, as described in Material and methods section. Sub-G1 area is indicated as M1. For DNA internucleosomal fragmentation and caspase-3 activation analyses (D), BAEC and HL-60 cells were treated with 8-epipuupehedione for 14 hrs. For DNA fragmentation assay, cells were harvested and centrifuged and pellets were frozen in liquid nitrogen. Internucleosomal fragmented DNA was isolated and visualized by agarose gel electrophoresis. BAE cells treated with 2-methoxiestradiol (2ME) for 24 hrs were used as positive internal control. C, control, untreated cells. Caspase-3 activity assay was carried out as described in the Material and methods section. Data are represented as fold-increases of control values. Data from duplicate samples were used in each experiment.
To test whether 8-epipuupehedione affected survival of HL-60 cells in a cell type-specific manner, we carried out several assays to analysis apoptosis. Direct Hoetsch staining of cell nuclei revealed that both endothelial (BAEC) and leukaemia (HL-60) cell cultures, but not tumor (HT-1080 human fibrosarco-ma) cell cultures, present a subpopulation of apop-totic cells, exhibiting fragmented nuclei (Fig. 1B). Furthermore, FACS analysis of propidium iodide stained cells revealed an increase in sub-G1 populations (characteristic of cells entering apoptosis) of both BAEC and HL-60 cells, but not in those of HCT-116 human colon adenocarcinoma cells treated with 7 μM 8-epipuupehedione (Fig. 1C). Results in Fig. 1B and C together reinforce the idea that 8-epipuupehe-dione is specific for endothelial an leukaemic cells but not for other kinds of cells. Furthermore, induction of DNA fragmentation and activation of the efector caspase 3 of BAEC and HL-60 by 8-epipuupehe-dione (Fig. 1D) confirm that this compound is able to induce specific apoptosis in these cells. On the other hand, since the activation of caspase 3 by 8-epipuu-pehedione in HL-60 cells is 7-fold that produced in BAEC cells, it seems that the proapoptotic effect of this compound is more intense for leukaemic than for endothelial cells.
The anti-angiogenic phloroglucinol hyperforin has been previously shown to induce leukaemia cell apoptosis and inhibition of matrix metalloproteinase-9 (MMP-9) secretion [10]. MMP-9, along with MMP-2 and urokinase are key extracellular matrix re-model-ling enzymes related with the invasive potential of endothelial and tumour cells. We have previously found that 8-epipuupehedione had no effect on BAEC MMP-2 production and secretion, but strongly inhibited urokinase production [12]. Therefore, we wanted to test the effects of 8-epipuupehedione pretreatment on HL-60 extracellular matrix degrading enzymes. Under the used culture conditions, in our hands HL-60 cells produce very low levels of MMP-9 but high levels of urokinase and levels of MMP-2 detectable in concentrated conditioned media. Our results with gelatin zymography show that 4 μM 8-epipuupehedione completely inhibits MMP-2 secretion by HL-60 cells, but does not inhibit its activity directly when added to zymography substrate buffer (Fig. 2A and B). Once again, these results point to a certain specificity of the effects of 8-epipuupehe-dione for leukaemia cells, since we have previously shown that this compound has no relevant effects on the production of MMP-2 by endothelial cells [12]. On the other hand, plasminogen zymography shows that 4 μM 8-epipuupehedione strongly inhibits urokinase production (Fig. 2C). Furthermore, 4 μM 8-epipuupe-hedione strongly induces the production of PAI -2 (the specific endogenous inhibitor of urokinase) by HL-60 cells (Fig. 2D), suggesting that this compound is able to shift the proteolytic balance towards anti-proteolysis [17]. These results are in agreement with the previous observation that the induction of HL-60 cell apoptosis down-regulates the expression/release of matrix metalloproteinases, thus reducing its invasive potential [18].
2.
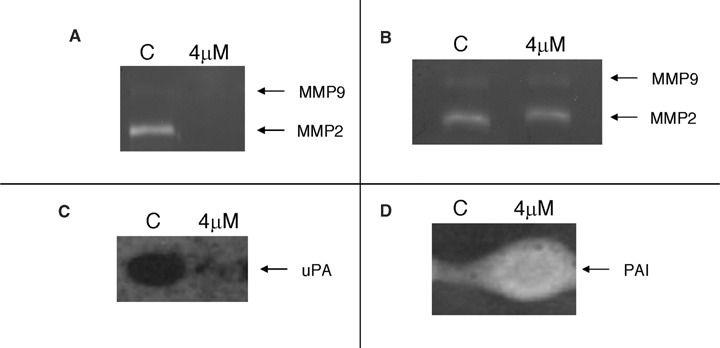
8-epipuupehedione inhibits the production of MMP-2 (A) but not MMP-2 activity (B) and urokinase (C) and increases plasminogen activator inhibitor (PAI) levels (D) produced by HL-60 cells. Cells were treated for 24 hrs with 4 μM 8-epipuupehedione in serum-free culture medium containing aprotinin. After incubation, conditioned media were collected and concentrated 25x and cell extracts were obtained. For zymographic assays (carried out as described in the Material and methods section), conditioned media (for gelatinolytic asays) and cell extracts (for urokinase detection by plasminogen zymography) were normalized for equal cellular density.
In conclusion, our results demonstrate that 8-epipuupehedione exhibits potent and specific pro-apoptotic effects and inhibits the extracellular matrix re-modelling potential of HL-60 promyelocytic leukaemia cells. Consequently, through its anti-leukaemic and anti-angiogneic properties, this compound can be regarded as a drug of great interest for the development of new therapeutical strategies for the treatment of promyelocytic leukaemia. Furthermore, its potential use against other kind of leukaemia deserves to be studied in the near future.
Acknowledgments
Thanks are due to Instituto Biomar S.A. (León, Spain) for providing us the compound. Authors are indebted to Auxiliadora López Jiménez for her excellent technical assistance. Our experimental work is supported by grants CTQ2006–15279-C03–03/BQU, SAF2005–01812 and PTR95–0904 (Spanish Ministry of Education and Science), Fundación Ramón Areces and funds from group CVI-267.
References
- 1.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–13. [PubMed] [Google Scholar]
- 2.Kini AR, Peterson LA, Tallman MS, Lingen MW. Angiogenesis in acute promyelocytic leukemia: induction by vascular endothelial growth factor and inhibition by all-trans retinoic acid. Blood. 2001;97:3919–24. doi: 10.1182/blood.v97.12.3919. [DOI] [PubMed] [Google Scholar]
- 3.Molica S, Vacca A, Levato D, Merchionne E, Ribatti D. Angiogenesis in acute and chronic lym-phocytic leukemia. Leuk Res. 2004;28:1239–40. doi: 10.1016/j.leukres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Kini AR. Angiogenesis in leukemia and lymphoma. Cancer Treat Res. 2004;121:221–38. doi: 10.1007/1-4020-7920-6_9. [DOI] [PubMed] [Google Scholar]
- 5.Letilovic T, Vrhovac R, Verstovsek S, Jaksic B, Ferrajoli A. Role of angiogenesis in chronic lympho-cytic leukemia. Cancer. 2006;107:925–34. doi: 10.1002/cncr.22086. [DOI] [PubMed] [Google Scholar]
- 6.Quesada AR, Muñoz-Chápuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 2006;26:483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Chápuli R, Quesada AR, Medina MA. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci. 2004;61:2224–43. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina MA, Muñoz-Chápuli R, Quesada AR. Challenges of antiangiogenic cancer therapy: trials and errors, and renewed hope. J Cell Mol Med. 2007;11:374–82. doi: 10.1111/j.1582-4934.2007.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-Poveda B, Quesada AR, Medina MA. Hyperforin, a bioactive compound of St. John's wort, is a new inhibitor of angiogenesis targeting several key steps of the process. Int J Cancer. 2005;117:775–80. doi: 10.1002/ijc.21246. [DOI] [PubMed] [Google Scholar]
- 10.Quiney C, Billard C, Mirshahi O, Fourneron JD, Kolb JP. Hyperforin inhibits MMP-9 secretion by B-CLL cells and microtubule formation by endothelial cells. Leukemia. 2006;20:583–9. doi: 10.1038/sj.leu.2404134. [DOI] [PubMed] [Google Scholar]
- 11.Alimoghaddam K, Shariftabrizi A, Tavangar SM, Sanaat Z, Rostami S, Jahani M, Ghavamzadeh A. Anti-leukemic and anti-angiogenesis efficacy of arsenic trioxide in new cases of acute promyeolocyt-ic leukemia. Leuk Lymphoma. 2006;47:81–8. doi: 10.1080/10428190500300373. [DOI] [PubMed] [Google Scholar]
- 12.Castro ME, González-Iriarte M, Barrero AF, Salvador-Tormo N, Muñoz-Chápuli R, Medina MA, Quesada AR. Study of puupehenone and related compounds as inhibitors of angiogenesis. Int J Cancer. 2004;110:31–8. doi: 10.1002/ijc.20068. [DOI] [PubMed] [Google Scholar]
- 13.Barrero AF, Álvarez-Miranda EJ, Chahboun R, Cortés M, Armstrong V. Synthesis and antitumor activity of puupehedione and related compouns. Tetrahedron. 1999;55:15181–95. [Google Scholar]
- 14.Gospodarowicz D, Moran J, Braun D, Birdwell C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc Natl Acad Sci USA. 1976;73:4120–4. doi: 10.1073/pnas.73.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fajardo I, Urdiales JL, Sánchez-Jiménez F, Medina MA. An experiment on apoptosis induced by polyamine adducts produced in the presence of serum amine oxidase. Biochem Educ. 2000;28:110–2. [PubMed] [Google Scholar]
- 16.García de Vea R, Schweigerer L, Medina MA. Modulation of the proteolytic balance plasminogen activator/plasminogen activator inhibitor by enhanced N-myc oncogene expression or application of genistein. Eur J Cancer. 1998;34:1736–40. doi: 10.1016/s0959-8049(98)00285-8. [DOI] [PubMed] [Google Scholar]
- 17.Fajardo I, Quesada AR, Núñez de Castro I, Sánchez-Jiménez F, Medina MA. A comparative study of the effects of genistein and 2-methoxyestra-diol on the proteolytic balance and tumour cell proliferation. Br J Cancer. 1999;80:17–24. doi: 10.1038/sj.bjc.6690315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gazzanelli G, Luchetti F, Burattini S, Mannello F, Falcieri E, Papa S. Matrix metalloproteinases expression in HL-60 promyelocytic leukemia cells during apoptosis. Apoptosis. 2000;5:1656–72. doi: 10.1023/a:1009688831531. [DOI] [PubMed] [Google Scholar]


