Abstract
Arterial calcifications as found with various imaging techniques, like plain X-ray, computed tomography or ultrasound are associated with increased cardiovascular risk. The prevalence of arterial calcification increases with age and is stimulated by several common cardiovascular risk factors. In this review, the clinical importance of arterial calcification and the currently known proteins involved are discussed. Arterial calcification is the result of a complex interplay between stimulating (bone morphogenetic protein type 2 [BMP-2], RANKL) and inhibitory (matrix Gla protein, BMP-7, osteoprotegerin, fetuin-A, osteopontin) proteins. Vascular calcification is especially prevalent and related to adverse outcome in patients with renal insufficiency and diabetes mellitus. We address the special circumstances and mechanisms in these patient groups. Treatment and prevention of arterial calcification is possible by the use of specific drugs. However, it remains to be proven that reduction of vascular calcification in itself leads to a reduced cardiovascular risk.
Keywords: arterial calcification, proteins, cardiovascular risk, renal insufficiency, diabetes mellitus
Introduction
Arterial calcification is often seen in studies using various imaging techniques. Although radiological techniques to visualize calcification are emerging, observing calcification is usually a coincidence on images performed for indications other than detecting calcified arteries. These calcifications are not as innocent as doctors tend to believe. They usually harbour an increased vascular risk [1]. Deposits of calcium in the vascular wall can be detected with X-ray. When vascular structures on X-ray images of the chest, the abdomen or extremities have similar density compared to bone, calcification is diagnosed. These images, however, are not valid for quantification of calcium. Using computed tomography (CT), the density of pixels is expressed as Hounsfield units and calcium is usually suspected when a pixel exceeds 130 Hounsfield units. At least in the coronary arteries, this technique is currently the only validated technique for quantification of vascular calcification [2]. Calcification of the vascular wall has been shown to predict increased cardiovascular risk, independent of the classical cardiovascular risk factors [3–6].
Vascular calcification consists of calcium salt precipitates, mostly in apatite form, similar to the hydroxyapatite found in bone. Several risk factors which are associated with the presence or progression of vascular calcification have been identified [3, 7]. In the past vascular calcification was seen as an inert end-point of atherosclerosis, however, recently it has become clear that it is a actively regulated process already occurring in the early stages of atherosclerotic lesions [8–10].
Many new pathways regulating calcification are being discovered. Recent research suggests important roles for activation of receptor tyrosine kinase A×1, transglutaminase pathways or stimulation of the calcium receptor on vascular smooth muscle cells (VSMCs) [11–13]. In this review, we will focus on the currently known proteins involved in arterial calcification. These findings advance our understanding of the arterial calcification process and provide us with possible future interventions.
Prevalence of calcifications and relation to cardiovascular outcome
Calcification of the arteries is usually detected with plain X-ray or CT. Prevalence is highly dependent upon the studied population. In a population-based cohort of over 100,000 men and women (mean age 47 years; 30–89 years range), who had a chest X-ray for screening purposes, prevalence of calcification in the aorta was 1.9% in male patients and 2.6% in female patients [3]. Calcification results in an increase of aortic stiffness and hence contributes to systolic hypertension and left ventricular hypertrophy, coronary insufficiency, ischemia and congestive heart failure [14–17]. Although calcification of the thoracic aorta commonly increases with age, the presence of calcifications increases the risk for cardiovascular events independently of age [3]. Most of the risk factors associated with calcification of the aortic arch are also well known cardiovascular risk factors (Table 1). Despite this similarity, aortic arch calcification itself is an independent predictor of cardiovascular risk [3]. Recent research showed changes in the mechanical properties of the atherosclerotic lesion and increased inflammation in response to calcification, which may increase the risk of plaque rupture [3, 18, 19]. In contrast to intimal calcification, medial calcification (also known as Mönckeberg’s sclerosis) exclusively involves VSMCs in the absence of inflammation and lipid infiltration. Electron-beam computed tomography (EBCT) and multi-slice computed tomography (MSCT) are reproducible methods to measure coronary calcifications [2, 20]. Among a healthy American population aged 40 to 45 years, prevalence of coronary artery calcifications (CAC) was 19.2% in white patients and 10.3% in Afro-American patients [21]. In another study among healthy persons older than 40 years, CAC prevalence was 29% among men and 19% among women [22]. Strikingly, the prevalence of vascular calcification was higher in men in every decade except between 70 and 80 years. Firstly this difference can be explained by the more favourable risk profile in women in the premenopausal phase causing calcification to develop at an older age. Secondly, men with excess coronary calcification at a younger age might already have died due to their increased cardiovascular risk. The presence of CAC in asymptomatic individuals has been shown to be associated with an increased risk for cardiovascular end-points. Over a variable follow up period from 3 to 4.3 years and depending on the characteristics of the studied population, the relative risk for any coronary event varied from 2.6 in low-risk females to 11.8 in healthy men aged 40 to 50 years [4, 5, 23, 24]. Calcification of the abdominal aorta as seen with CT is more frequently observed in the presence of older age, hypertension, coronary artery disease and peripheral vascular disease [25]. Additionally, the area of calcification on X-ray films of the abdominal aorta is positively correlated with age, systolic blood pressure and aortic stiffness [26]. Limited data exist on the association between femoral artery calcification and cardiovascular mortality. However, in patients with type 2 diabetes, femoral artery calcification is an independent predictor of cardiovascular morbidity and mortality [27, 28].
Table 1.
Risk factors associated with aortic arch calcification as determined in 116,309 patients [3]
| Risk factor | Odds ratio (95% confidence interval)* |
|---|---|
| Age | Men 2.74 (2.58–2.91) |
| Women 3.52 (3.32–3.74) | |
| African descent | Women 1.35 (1.11–1.63) |
| No college education | Men 1.17 (1.00–1.36) |
| Women 1.31 (1.14–1.50) | |
| Total cholesterol > 6.6 mmol/l | Women 1.28 (1.06–1.55) |
| Current smoking | Men 1.30 (1.10–1.53) |
| Women 1.16 (1.01–1.33) | |
| Hypertension† | Men 1.27 (1.11–1.46) |
| Women 1.38 (1.23–1.54) |
Only significant correlations are depicted.
This might also be caused or aggravated by vascular calcification.
At present it is not clear whether measuring vascular calcification with EBCT, MSCT or plain X-ray can be used as a cost-effective strategy for cardiovascular risk stratification. A disadvantage of these techniques is the considerable amount of radiation to which patients or, indeed, asymptomatic individuals are exposed.
Pathobiology of arterial calcification
Experimental animal as well as in vitro studies have revealed several proteins playing a role in the calcification process [29–33]. Unravelling the function and the mechanism of action of these proteins has been a topic for many researchers in the past few years. Some proteins have been identified as inhibitors of calcification, whereas others promote vascular calcification. VSMCs migrated from the media to the intimal layer of the vasculature lose their contractile phenotype, and change into so-called synthetic VSMCs. When these VSMCs become apoptotic in the atherosclerotic lesion they may form the nidus for calcification [34, 35]. Moreover, VSMCs can change their phenotype upon calcification and develop features of osteoblast- or chondrocyte-like cells with respect to gene expression [36]. Table 2 shows proteins involved in calcification subdivided according to their calcification-inhibiting or -promoting properties. Below we discuss these proteins and their biological properties.
Table 2.
Proteins involved in arterial calcification
| Inhibitor proteins of arterial calcification | Promoter proteins of arterial calcification |
|---|---|
| MGP | BMP-2 |
| BMP-7 | RANKL |
| OPG | |
| Fetuin-A | |
| OPN |
Matrix Gla protein (MGP)
MGP is a 10 kD vitamin K-dependent protein, first discovered by Price et al.[37]. It is produced by VSMCs and chondrocytes, and accumulates at sites of calcification. Its production is stimulated by an increase in local calcium levels [38]. There is an active and an inactive form depending on whether or not the protein has been carboxylated (i.e. activated) by a vitamin K-driven γ-glutamyl carboxylation. The function of MGP is believed to be a regulator of bone morphogenetic protein type 2 (BMP-2), but it can also bind directly to calcium crystals in the vascular matrix, thereby preventing further calcification growth [39]. Animal studies show that a deficiency or impairment of MGP (blocking vitamin K action by coumarins) lead to rapid and extreme calcification of the vascular matrix [40, 41]. In the human ‘Keutel syndrome’, an autosomal recessive disorder in which patients lack mature MGP, excessive calcification of large arteries is seen [42]. Circulating uncarboxylated MGP levels are inversely proportional to coronary calcification and were significantly lower in patients who underwent PTCA versus a healthy control population [43, 44]. Several experimental studies suggest that MGP, produced in the vascular matrix, is transported to plasma in combination with fetuin-A, forming the fetuin-A-mineral complex [45, 46]. Whether uncarboxylated MGP levels are a reliable reflection of the calcification process in the vascular wall is not fully clear, although from the previously mentioned studies it seems that patients with high cardiovascular risk have lower serum MGP levels [47]. One advantage of MGP is that the protein’s activity completely depends upon vitamin K, and thereby can be modulated by extra vitamin K intake. On the other hand, blocking carboxylation of MGP (i.e. with coumarines) or low vitamin K levels (i.e. deficient intake) results in excessive calcification [48–50].
Bone morphogenetic protein
BMPs are members of the transforming growth factor (TGF)-β superfamily, and play key signalling roles in the maintenance and repair of bone and other tissues in the adult. Their role in vascular calcification is complex. When VSMCs change their phenotype from contractile to synthetic, they enter a state of proliferation in which the expression of smooth muscle markers is diminished. Additionally, they produce large amounts of extracellular matrix proteins and may become osteoblast-like cells. This reduction in smooth muscle marker expression is thought to be crucial in the pathogenesis of atherosclerosis and M○nckeberg’s sclerosis. The loss of smooth muscle markers can be influenced by BMPs. Two BMPs, BMP-2 and BMP-7, have been extensively studied in relation to vascular calcification [51, 52]. Expression of BMP-2 is found in atherosclerotic lesions, in peri-adventitial myofibroblasts and tunica media cells. Induction of BMP-2 in the vasculature is related to oxidative stress, inflammation, oxidized lipids and hyperglycaemia [53–55]. Increased expression of BMP-2 stimulates the osteoregulatory gene MSX-2. Then core binding factor-1 (Cbfa-1 or RUNX2) and osterix, both transcription factors, stimulate differentiation of multipotent vascular mesenchymal cells into ‘osteoblast-like’ cells capable of bone formation and increased intramembranous bone formation in the artery wall [51, 56, 57]. The effect of BMP-2 on bone formation is suggested to be modulated by MGP [31, 58]. Diminished VSMC expression of MGP or inactive MGP may lead to unopposed BMP-2 action and hence vascular calcification. Another important inhibiting mechanism of BMP-2 is mediated by the Smad-6 gene [59]. Smad-6 gene expression is limited to the heart and the vasculature. Interruption of Smad-6 gene function leads to calcification, only in the areas were it is expressed, suggesting an important modulating role of BMP-2 function.
Strikingly, BMP-2 is associated with a decrease in smooth muscle cell markers whereas BMP-7 promotes the VSMC phenotype. The exact mechanism of this difference in action of these very similar proteins is not yet known, although it is mediated by induction of Smad-6 amongst others [60]. BMP-7 promotes increased bone formation and phosphate deposition in bone. High serum phosphate levels and vascular calcification are thus prevented. In chronic kidney disease (CKD) sufficient levels can reverse arterial calcification. However, in patients with CKD, BMP-7 levels (mainly produced by the kidney) are low [61]. A potential protective effect against vascular calcification in these patients might, therefore, be compromised resulting in excessive calcification.
Osteopontin (OPN)
OPN is an acidic extracellular phospho-protein. Phosphoserines within OPN are negatively charged amino acids, and have a strong affinity for hydroxyapatite. It is normally present in mineralized tissues like bones and teeth. Mice lacking OPN are susceptible to vascular calcifications [32]. OPN regulates mineralization in two different ways. On the one hand it inhibits apatite crystal growth, on the other it promotes osteoclast function. In normal arteries OPN is absent, whereas in calcified plaques it is abundantly present. Research suggests that OPN is important in regulating calcification when the artery is injured [62].
RANKL
RANKL is a 316-amino acid transmembrane protein. It is highly expressed by T-cells in lymphoid tissue and by osteoblasts in trabecular bone. RANKL binds to RANK, a 616-amino acid transmembrane receptor which is present amongst others in osteoclasts and their precursors. This binding generates multiple intracellular signals that regulate cell differentiation, function and survival. RANKL action can be blocked by osteoprotegerin (OPG), thereby inhibiting vascular calcification. RANK/RANKL/OPG belong to the tumour necrosis factor (TNF)-α family. RANKL is up-regulated in osteoblasts by 1α 25-dihydroxyvitamin D3, parathyroid hormone (PTH), glucocorticoids, prostaglandin E2, interleukin-1α, TNF-α, interleukin-6, interleukin-11, interleukin-17, calcium or immuno-suppressants like cyclosporin A. Down-regulation of RANKL is mediated through transforming growth factor β. In animal studies RANKL increases osteoclast size and function. Disruption of RANKL results in inhibition of osteoclast formation and function [63–66].
Osteoprotegerin
OPG is a 380 amino acid acting as a soluble (decoy)-receptor for RANKL. It is produced by many tissues in the body, including the cardiovascular system. OPG expression is particularly high in VSMCs and vascular endothelial cells of the aorta and the renal arteries. It prevents the binding of RANKL to RANK [63, 65]. OPG is increased by some of the stimuli that also increase RANKL and by oestrogen, TGF-β and BMP-2. Decreased levels are seen with increased PTH levels, glucocorticoids, prostaglandin E2, insulin-like growth factor-1 or immunosuppressants. A steady balance between RANKL and OPG prevents disorders in bone remodelling and vascular calcification. Low OPG expression leads to unopposed RANKL binding to RANK and thus osteoporosis and vascular calcification, whereas high OPG expression leads to osteopetrosis [67, 68]. Clinically, high serum levels of OPG are associated with atherosclerosis or risk factors for atherosclerotic disease indicating a compensatory increase in OPG levels in response to progressive atherosclerosis and thus OPG may lessen vascular calcification [68–70].
Fetuin-A
Fetuin-A is a serum glycoprotein produced in the liver and present in high serum concentrations (0.4–1.0 g/l) [71]. In end-stage renal disease patients the role of fetuin-A has been extensively studied. With a molecular weight of 56 kD it is non-dialysable. It acts as a negative acute phase protein and is a powerful calcification inhibitor [71–73]. Together with MGP, fetuin-A is able to make up a complex with calcium and phosphate thereby transporting and clearing the insoluble calcium-phosphate salt, and preventing its extra skeletal deposition [74]. In transgenic fetuin-A deficient mice (fetuin-A–/– mice), extra skeletal calcification, including soft tissue and peri-vertebral arterial calcification develop [72]. Large arteries are spared from calcifications, most likely because of up-regulation of other potent calcification inhibitors such as MGP and OPN [75]. In dialysis patients, serum fetuin-A negatively correlates with CAC [76]. It has been shown that low fetuin-A levels were associated with higher all-cause and cardiovascular mortality [77–79]. This effect was partially mediated by inflammation, according to its correlation with higher levels of C-reactive protein.
If there is an imbalance between the calcification protective and calcification inducing factors, progressive vascular calcification can be the result. Figure 1 depicts the different proteins and their role in the calcification process. First the conventional vascular risk factors result in intimal damage and sub-intimal lipid deposition. Subsequently, the inflammatory response induced leads to increased BMP-2. If not balanced by active (carboxylated) MGP, multipotent mesenchymal vascular cells are stimulated to differentiation into ‘osteoblast-like’ cells. At this point the presence of sufficient active MGP is important both for blocking the action of BMP-2 and for binding directly to calcium crystals in the vascular matrix [31, 39, 80, 81]. The use of vitamin K antagonists and/or a low vitamin K diet results in an increase of dysfunctional MGP and thus favouring calcification [41, 82]. In addition, the interactions among RANKL, OPN and OPG influence the rate of calcification. (Fig. 1)
Fig 1.
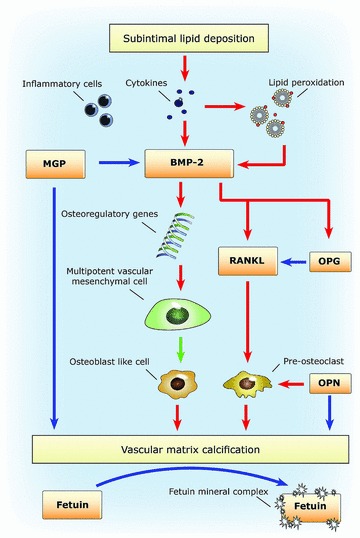
Blue arrows indicate an inhibiting effect and red arrows indicate a stimulating effect.
Arterial calcification in specific patient populations at high risk for cardiovascular disease
Chronic kidney disease
Elevated circulating levels of phosphate (P), calcium (Ca) and calcium phosphate product (P × Ca) are frequently encountered in dialysis patients and are associated with increased vascular calcifications [83–85]. The calcification process is not exclusively influenced by P and Ca. Many traditional risk factors, such as hypertension and hyperlipidaemia, and non-traditional atherosclerotic risk factors, including lipid oxidation, the presence of advanced glycation end-products, calcitriol and inflammation may affect VSCM-associated calcification, but their precise roles await clarification [53, 86–88]. Although the role of PTH in bone formation is eminent, the importance of PTH for vascular calcification in dialysis patients is less clear. Some studies show an association between PTH levels and vascular calcification, whereas others do not [84, 89, 90]. In addition, associations may differ between groups using calcium-containing or calcium-free phosphate binding therapy [91]. It has been suggested that vascular calcification under uremic conditions may act as a natural ‘stent’ by ‘stabilizing’ plaques [92, 93]. Arterial disease would then lead to less acute ischemic events, but more chronic ischemia and fibrosis through progressive luminal obliteration [92]. Arterial media calcification is mostly localized in muscle-type conduit arteries such as femoral and tibial arteries. These lesions in their most pure form do not obstruct the arterial lumen. This form of calcification has been associated with increased arterial stiffness and mortality [94, 95].
Diabetes mellitus
Type 2 diabetes mellitus (DM2) patients suffer from increased rates of cardiovascular mortality and morbidity, especially in women, due to their extra unfavourable risk profile compared to men with DM2 [96, 97]. Medial artery calcification, i.e. Mönckeberg’s sclerosis, is often seen and is associated with age and the severity of hyperglycaemia [98, 99]. The arteries are stiffened but not occluded [100]. OPG is increased in the tunica media but not in the intima of the vasculature of patients with diabetes [101]. Increased serum levels of OPG are associated with higher HbA1c levels and hypertension in DM2 patients and might be a marker of progressive atherosclerosis with accompanying calcification [102]. The latter might be related to overwhelming risk factors (high glucose levels, high blood pressure or unfavourable lipid profile) for calcification or compromised function of other protective proteins and mechanisms [98, 103]. Clinically, patients with diabetes with medial calcifications have a significant excess risk for total mortality, stroke mortality and cardiovascular mortality than patients without. They also had a significantly higher incidence of coronary heart disease events, stroke events and lower extremity amputations [27, 28].
Treatment and prevention of calcifications
Currently there is no evidence-based treatment regimen that can reduce calcification of large arteries. Experiments using calcium channel blockers or statins have not been convincing [104, 105]. Although the effect of treating cardiovascular risk factors on the progression of large artery calcification has not yet been evaluated, it seems reasonable to treat patients according to current guidelines because of their increased cardiovascular risk. Some current medical treatments, however, may be associated with an increased risk of calcification. For example, treatment of patients for recurrent thrombosis with coumarins results in accelerated calcification [49, 50]. Similarly, in end stage renal disease, treatment of hyperphosphatemia with phosphate binders that contain calcium have been associated with more CAC compared to treatments with non calcium-based phosphate binders [106].
Summary
Arterial calcification is an actively regulated process, which involves different triggers and proteins. Patients with calcification of large arteries have an increased cardiovascular risk when compared to similar patients without calcification. A possible mechanism is that large artery calcification leads to increased arterial stiffness and reflects atherosclerotic burden. Therefore the presence of large artery calcification can be seen as an additional risk factor for cardiovascular events. Although there are no trials that prove risk reduction from aggressive risk management in patients with arterial calcifications, such patients probably benefit when cardiovascular risk factors like hypertension and dyslipidaemia are identified and treated according to current guidelines. Moreover, in patient groups with a strongly elevated risk for arterial calcification, patient-specific measures could possibly prevent arterial calcifications. Whether this will also result in a reduction of cardiovascular risk remains to be proven.
Conflict of interest
The authors confirm that there are no conflicts of interest. There was no commercial funding involved.
References
- 1.Rennenberg RJ, Kessels AG, Schurgers LJ, et al. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–97. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopp AF, Ohnesorge B, Becker C, et al. Reproducibility and accuracy of coronary calcium measurements with multi-detector row vs electron-beam CT. Radiology. 2002;225:113–9. doi: 10.1148/radiol.2251010173. [DOI] [PubMed] [Google Scholar]
- 3.Iribarren C, Sidney S, Sternfeld B, et al. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–5. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AJ, Bindeman J, Feuerstein I, et al. Coronary calcium independently predicts incident premature coronary heart disease over measured cardiovascular risk factors: mean three-year outcomes in the Prospective Army Coronary Calcium (PACC) project. J Am Coll Cardiol. 2005;46:807–14. doi: 10.1016/j.jacc.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 5.Arad Y, Goodman KJ, Roth M, et al. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–65. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 6.Shaw LJ, Raggi P, Schisterman E, et al. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–33. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. coronary artery calcification in older adults to age 99: prevalence and risk factors. Circulation. 2001;104:2679–84. doi: 10.1161/hc4601.099464. [DOI] [PubMed] [Google Scholar]
- 8.Demer LL, Tintut Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg Award lecture. Arterioscler Thromb Vasc Biol. 2003;23:1739–43. doi: 10.1161/01.ATV.0000093547.63630.0F. [DOI] [PubMed] [Google Scholar]
- 9.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 10.Reslerova M, Moe SM. Vascular calcification in dialysis patients: pathogenesis and consequences. Am J Kidney Dis. 2003(41):S96–9. doi: 10.1053/ajkd.2003.50094. [DOI] [PubMed] [Google Scholar]
- 11.Nakano Y, Forsprecher J, Kaartinen MT. Regulation of ATPase activity of transglutaminase 2 by MT1-MMP: implications for mineralization of MC3T3-E1 osteoblast cultures. J Cell Physiol. 2010;223:260–9. doi: 10.1002/jcp.22034. [DOI] [PubMed] [Google Scholar]
- 12.Alam MU, Kirton JP, Wilkinson FL, et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–8. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 13.Collett GD, Sage AP, Kirton JP, et al. Axl/phosphatidylinositol 3-kinase signaling inhibits mineral deposition by vascular smooth muscle cells. Circ Res. 2007;100:502–9. doi: 10.1161/01.RES.0000258854.03388.02. [DOI] [PubMed] [Google Scholar]
- 14.Kelly RP, Tunin R, Kass DA. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ Res. 1992;71:490–502. doi: 10.1161/01.res.71.3.490. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsuka S, Kakihana M, Watanabe H, et al. Chronically decreased aortic distensibility causes deterioration of coronary perfusion during increased left ventricular contraction. J Am Coll Cardiol. 1994;24:1406–14. doi: 10.1016/0735-1097(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, Ohtsuka S, Kakihana M, et al. Decreased aortic compliance aggravates subendocardial ischaemia in dogs with stenosed coronary artery. Cardiovasc Res. 1992;26:1212–8. doi: 10.1093/cvr/26.12.1212. [DOI] [PubMed] [Google Scholar]
- 17.Jensky NE, Criqui MH, Wright MC, et al. Blood pressure and vascular calcification. Hypertension. 2010;55:990–7. doi: 10.1161/HYPERTENSIONAHA.109.147520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vengrenyuk Y, Carlier S, Xanthos S, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci USA. 2006;103:14678–83. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 20.Ohnesorge B, Flohr T, Fischbach R, et al. Reproducibility of coronary calcium quantification in repeat examinations with retrospectively ECG-gated multisection spiral CT. Eur Radiol. 2002;12:1532–40. doi: 10.1007/s00330-002-1394-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee TC, O’Malley PG, Feuerstein I, et al. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 22.Callaway M, Richards P, Goddard P, et al. The incidence of coronary artery calcification on standard thoracic CT scans. Br J Radiol. 1997;70:572–4. doi: 10.1259/bjr.70.834.9227248. [DOI] [PubMed] [Google Scholar]
- 23.Wong ND, Hsu JC, Detrano RC, et al. Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol. 2000;86:495–8. doi: 10.1016/s0002-9149(00)01000-6. [DOI] [PubMed] [Google Scholar]
- 24.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–6. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita M, Nishikimi N, Sakurai T, et al. Relationship between aortic calcification and atherosclerotic disease in patients with abdominal aortic aneurysm. Int Angiol. 2000;19:276–9. [PubMed] [Google Scholar]
- 26.Nakamura U, Iwase M, Nohara S, et al. Usefulness of brachial-ankle pulse wave velocity measurement: correlation with abdominal aortic calcification. Hypertens Res. 2003;26:163–7. doi: 10.1291/hypres.26.163. [DOI] [PubMed] [Google Scholar]
- 27.Lehto S, Niskanen L, Suhonen M, et al. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 28.Niskanen L, Siitonen O, Suhonen M, et al. Medial artery calcification predicts cardiovascular mortality in patients with NIDDM. Diabetes Care. 1994;17:1252–6. doi: 10.2337/diacare.17.11.1252. [DOI] [PubMed] [Google Scholar]
- 29.Shanahan CM, Proudfoot D, Farzaneh-Far A, et al. The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr. 1998;8:357–75. doi: 10.1615/critreveukargeneexpr.v8.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 30.Proudfoot D, Skepper JN, Shanahan CM, et al. Calcification of human vascular cells in vitro is correlated with high levels of matrix Gla protein and low levels of osteopontin expression. Arterioscler Thromb Vasc Biol. 1998;18:379–88. doi: 10.1161/01.atv.18.3.379. [DOI] [PubMed] [Google Scholar]
- 31.Zebboudj AF, Shin V, Bostrom K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J Cell Biochem. 2003;90:756–65. doi: 10.1002/jcb.10669. [DOI] [PubMed] [Google Scholar]
- 32.Speer MY, McKee MD, Guldberg RE, et al. Inactivation of the osteopontin gene enhances vascular calcification of matrix Gla protein-deficient mice: evidence for osteopontin as an inducible inhibitor of vascular calcification in vivo. J Exp Med. 2002;196:1047–55. doi: 10.1084/jem.20020911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhore CR, Cleutjens JP, Lutgens E, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 34.Clarke MC, Littlewood TD, Figg N, et al. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–38. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 35.Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–57. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 36.Shanahan CM, Cary NR, Metcalfe JC, et al. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J Clin Invest. 1994;93:2393–402. doi: 10.1172/JCI117246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price PA, Urist MR, Otawara Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Commun. 1983;117:765–71. doi: 10.1016/0006-291x(83)91663-7. [DOI] [PubMed] [Google Scholar]
- 38.Farzaneh-Far A, Proudfoot D, Weissberg PL, et al. Matrix gla protein is regulated by a mechanism functionally related to the calcium-sensing receptor. Biochem Biophys Res Commun. 2000;277:736–40. doi: 10.1006/bbrc.2000.3747. [DOI] [PubMed] [Google Scholar]
- 39.Zebboudj AF, Imura M, Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–94. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- 40.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 41.Price PA, Faus SA, Williamson MK. Warfarin causes rapid calcification of the elastic lamellae in rat arteries and heart valves. Arterioscler Thromb Vasc Biol. 1998;18:1400–7. doi: 10.1161/01.atv.18.9.1400. [DOI] [PubMed] [Google Scholar]
- 42.Munroe PB, Olgunturk RO, Fryns JP, et al. Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet. 1999;21:142–4. doi: 10.1038/5102. [DOI] [PubMed] [Google Scholar]
- 43.Schurgers LJ, Teunissen KJ, Knapen MH, et al. Novel conformation-specific antibodies against matrix {gamma}-carboxyglutamic acid (Gla) protein. undercarboxylated matrix gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–33. doi: 10.1161/01.ATV.0000173313.46222.43. [DOI] [PubMed] [Google Scholar]
- 44.Jono S, Ikari Y, Vermeer C, et al. Matrix Gla protein is associated with coronary artery calcification as assessed by electron-beam computed tomography. Thromb Haemost. 2004;91:790–4. doi: 10.1160/TH03-08-0572. [DOI] [PubMed] [Google Scholar]
- 45.Price PA, Lim JE. The inhibition of calcium phosphate precipitation by fetuin is accompanied by the formation of a fetuin-mineral complex. J Biol Chem. 2003;278:22144–52. doi: 10.1074/jbc.M300744200. [DOI] [PubMed] [Google Scholar]
- 46.Price PA, Thomas GR, Pardini AW, et al. Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J Biol Chem. 2002;277:3926–34. doi: 10.1074/jbc.M106366200. [DOI] [PubMed] [Google Scholar]
- 47.Cranenburg EC, Vermeer C, Koos R, et al. The circulating inactive form of matrix Gla protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res. 2008;45:427–36. doi: 10.1159/000124863. [DOI] [PubMed] [Google Scholar]
- 48.Geleijnse JM, Vermeer C, Grobbee DE, et al. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam Study. J Nutr. 2004;134:3100–5. doi: 10.1093/jn/134.11.3100. [DOI] [PubMed] [Google Scholar]
- 49.Koos R, Mahnken AH, Muhlenbruch G, et al. Relation of oral anticoagulation to cardiac valvular and coronary calcium assessed by multislice spiral computed tomography. Am J Cardiol. 2005;96:747–9. doi: 10.1016/j.amjcard.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Schurgers LJ, Aebert H, Vermeer C, et al. Oral anticoagulant treatment: friend or foe in cardiovascular disease. Blood. 2004;104:3231–2. doi: 10.1182/blood-2004-04-1277. [DOI] [PubMed] [Google Scholar]
- 51.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res. 2005;97:105–14. doi: 10.1161/01.RES.00000175571.53833.6c. [DOI] [PubMed] [Google Scholar]
- 52.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–30. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 53.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 54.Fukui N, Zhu Y, Maloney WJ, et al. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am. 2003;85-A:59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- 55.Rifas L, Arackal S, Weitzmann MN. Inflammatory T cells rapidly induce differentiation of human bone marrow stromal cells into mature osteoblasts. J Cell Biochem. 2003;88:650–9. doi: 10.1002/jcb.10436. [DOI] [PubMed] [Google Scholar]
- 56.Ahrens M, Ankenbauer T, Schroder D, et al. Expression of human bone morphogenetic proteins-2 or -4 in murine mesenchymal progenitor C3H10T1/2 cells induces differentiation into distinct mesenchymal cell lineages. DNA Cell Biol. 1993;12:871–80. doi: 10.1089/dna.1993.12.871. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh-Choudhury N, Choudhury GG, Harris MA, et al. Autoregulation of mouse BMP-2 gene transcription is directed by the proximal promoter element. Biochem Biophys Res Commun. 2001;286:101–8. doi: 10.1006/bbrc.2001.5351. [DOI] [PubMed] [Google Scholar]
- 58.Bostrom K, Tsao D, Shen S, et al. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–52. doi: 10.1074/jbc.M008103200. [DOI] [PubMed] [Google Scholar]
- 59.Galvin KM, Donovan MJ, Lynch CA, et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24:171–4. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 60.Dorai H, Sampath TK. Bone morphogenetic protein-7 modulates genes that maintain the vascular smooth muscle cell phenotype in culture. J Bone Joint Surg Am. 2001;83-A:S70–8. [PubMed] [Google Scholar]
- 61.Mathew S, Davies M, Lund R, et al. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur J Clin Invest. 2006;36:43–50. doi: 10.1111/j.1365-2362.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 62.Giachelli CM, Speer MY, Li X, et al. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–22. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 63.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–53. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 64.Sattler AM, Schoppet M, Schaefer JR, et al. Novel aspects on RANK ligand and osteoprotegerin in osteoporosis and vascular disease. Calcif Tissue Int. 2004;74:103–6. doi: 10.1007/s00223-003-0011-y. [DOI] [PubMed] [Google Scholar]
- 65.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–5. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 66.Schoppet M, Preissner KT, Hofbauer LC. RANK ligand and osteoprotegerin: paracrine regulators of bone metabolism and vascular function. Arterioscler Thromb Vasc Biol. 2002;22:549–53. doi: 10.1161/01.atv.0000012303.37971.da. [DOI] [PubMed] [Google Scholar]
- 67.Bucay N, Sarosi I, Dunstan CR, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–8. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004;95:1046–57. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 69.Knudsen ST, Foss CH, Poulsen PL, et al. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur J Endocrinol. 2003;149:39–42. doi: 10.1530/eje.0.1490039. [DOI] [PubMed] [Google Scholar]
- 70.Kazama JJ, Shigematsu T, Yano K, et al. Increased circulating levels of osteoclastogenesis inhibitory factor (osteoprotegerin) in patients with chronic renal failure. Am J Kidney Dis. 2002;39:525–32. doi: 10.1053/ajkd.2002.31402. [DOI] [PubMed] [Google Scholar]
- 71.Ketteler M, Wanner C, Metzger T, et al. Deficiencies of calcium-regulatory proteins in dialysis patients: a novel concept of cardiovascular calcification in uremia. Kidney Int Suppl. 2003;84:S84–7. doi: 10.1046/j.1523-1755.63.s84.21.x. [DOI] [PubMed] [Google Scholar]
- 72.Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–66. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lebreton JP, Joisel F, Raoult JP, et al. Serum concentration of human alpha 2 HS glycoprotein during the inflammatory process: evidence that alpha 2 HS glycoprotein is a negative acute-phase reactant. J Clin Invest. 1979;64:1118–29. doi: 10.1172/JCI109551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coen G, Ballanti P, Balducci A, et al. Renal osteodystrophy: alpha-Heremans Schmid glycoprotein/fetuin-A, matrix GLA protein serum levels, and bone histomorphometry. Am J Kidney Dis. 2006;48:106–13. doi: 10.1053/j.ajkd.2006.03.083. [DOI] [PubMed] [Google Scholar]
- 75.Ketteler M, Westenfeld R, Schlieper G, et al. Pathogenesis of vascular calcification in dialysis patients. Clin Exp Nephrol. 2005;9:265–70. doi: 10.1007/s10157-005-0385-4. [DOI] [PubMed] [Google Scholar]
- 76.Moe SM, Reslerova M, Ketteler M, et al. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD) Kidney Int. 2005;67:2295–304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 77.Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–33. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 78.Stenvinkel P, Wang K, Qureshi AR, et al. Low fetuin-A levels are associated with cardiovascular death: impact of variations in the gene encoding fetuin. Kidney Int. 2005;67:2383–92. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang AY, Woo J, Lam CW, et al. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant. 2005;20:1676–85. doi: 10.1093/ndt/gfh891. [DOI] [PubMed] [Google Scholar]
- 80.Canfield AE, Doherty MJ, Kelly V, et al. Matrix Gla protein is differentially expressed during the deposition of a calcified matrix by vascular pericytes. FEBS Lett. 2000;487:267–71. doi: 10.1016/s0014-5793(00)02363-2. [DOI] [PubMed] [Google Scholar]
- 81.Murshed M, Schinke T, McKee MD, et al. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–30. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schurgers LJ, Dissel PE, Spronk HM, et al. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z Kardiol. 2001;90:57–63. doi: 10.1007/s003920170043. [DOI] [PubMed] [Google Scholar]
- 83.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 84.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–83. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 85.Oh J, Wunsch R, Turzer M, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–5. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 86.Yamagishi S, Fujimori H, Yonekura H, et al. Advanced glycation endproducts accelerate calcification in microvascular pericytes. Biochem Biophys Res Commun. 1999;258:353–7. doi: 10.1006/bbrc.1999.0625. [DOI] [PubMed] [Google Scholar]
- 87.Jono S, Nishizawa Y, Shioi A, et al. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation. 1998;98:1302–6. doi: 10.1161/01.cir.98.13.1302. [DOI] [PubMed] [Google Scholar]
- 88.Moe SM, Chen NX. Inflammation and vascular calcification. Blood Purif. 2005;23:64–71. doi: 10.1159/000082013. [DOI] [PubMed] [Google Scholar]
- 89.Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267–76. doi: 10.1053/ajkd.2001.24533. [DOI] [PubMed] [Google Scholar]
- 90.Wang AY, Wang M, Woo J, et al. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J Am Soc Nephrol. 2003;14:159–68. doi: 10.1097/01.asn.0000038685.95946.83. [DOI] [PubMed] [Google Scholar]
- 91.Chertow GM, Raggi P, Chasan-Taber S, et al. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1489–96. doi: 10.1093/ndt/gfh125. [DOI] [PubMed] [Google Scholar]
- 92.Goldsmith D, Ritz E, Covic A. Vascular calcification: a stiff challenge for the nephrologist: does preventing bone disease cause arterial disease. Kidney Int. 2004;66:1315–33. doi: 10.1111/j.1523-1755.2004.00895.x. [DOI] [PubMed] [Google Scholar]
- 93.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 94.Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–42. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 95.Guerin AP, London GM, Marchais SJ, et al. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–21. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 96.Qasim AN, Martin SS, Mehta NN, et al. Lipoprotein(a) is strongly associated with coronary artery calcification in type-2 diabetic women. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.02.021. doi: 10.1016/j.ijcard.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–8. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishimura E, Okuno S, Kitatani K, et al. Different risk factors for peripheral vascular calcification between diabetic and non-diabetic haemodialysis patients–importance of glycaemic control. Diabetologia. 2002;45:1446–8. doi: 10.1007/s00125-002-0920-8. [DOI] [PubMed] [Google Scholar]
- 99.Reaven PD, Sacks J. Coronary artery and abdominal aortic calcification are associated with cardiovascular disease in type 2 diabetes. Diabetologia. 2005;48:379–85. doi: 10.1007/s00125-004-1640-z. [DOI] [PubMed] [Google Scholar]
- 100.Lachman AS, Spray TL, Kerwin DM, et al. Medial calcinosis of Monckeberg. A review of the problem and a description of a patient with involvement of peripheral, visceral and coronary arteries. Am J Med. 1977;63:615–22. doi: 10.1016/0002-9343(77)90207-8. [DOI] [PubMed] [Google Scholar]
- 101.Olesen P, Ledet T, Rasmussen LM. Arterial osteoprotegerin: increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF-alpha. Diabetologia. 2005;48:561–8. doi: 10.1007/s00125-004-1652-8. [DOI] [PubMed] [Google Scholar]
- 102.Rasmussen LM, Tarnow L, Hansen TK, et al. Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol. 2006;154:75–81. doi: 10.1530/eje.1.02049. [DOI] [PubMed] [Google Scholar]
- 103.Tanikawa T, Okada Y, Tanikawa R, et al. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 2009;46:572–80. doi: 10.1159/000226225. [DOI] [PubMed] [Google Scholar]
- 104.Motro M, Shemesh J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension. 2001;37:1410–3. doi: 10.1161/01.hyp.37.6.1410. [DOI] [PubMed] [Google Scholar]
- 105.Wong ND, Kawakubo M, LaBree L, et al. Relation of coronary calcium progression and control of lipids according to National Cholesterol Education Program guidelines. Am J Cardiol. 2004;94:431–6. doi: 10.1016/j.amjcard.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–52. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]


