Abstract
To study the efficiency of maintaining the reduced tissue environment via pre-treatment with natural antioxidant resveratrol in stem cell therapy, we pre-treated male Sprague-Dawley rats with resveratrol (2.5 mg/kg/day gavaged for 2 weeks). After occlusion of the left anterior descending coronary artery (LAD), adult cardiac stem cells stably expressing EGFP were injected into the border zone of the myocardium. One week after the LAD occlusion, the cardiac reduced environment was confirmed in resveratrol-treated rat hearts by the enhanced expression of nuclear factor-E2-related factor-2 (Nrf2) and redox effector factor-1 (Ref-1). In concert, cardiac functional parameters (left ventricular ejection fraction and fractional shortening) were significantly improved. The improvement of cardiac function was accompanied by the enhanced stem cell survival and proliferation as demonstrated by the expression of cell proliferation marker Ki67 and differentiation of stem cells towards the regeneration of the myocardium as demonstrated by the enhanced expression of EGFP 28 days after LAD occlusion in the resveratrol-treated hearts. Our results demonstrate that resveratrol maintained a reduced tissue environment by overexpressing Nrf2 and Ref-1 in rats resulting in an enhancement of the cardiac regeneration of the adult cardiac stem cells as demonstrated by increased cell survival and differentiation leading to cardiac function.
Keywords: stem cells, myocardial infarction, redox signal, cardiac regeneration, cell proliferation, cell survival
Introduction
Although the results of adult and embryonic stem cell therapy for the infracted myocardium have yielded promising results for the enhanced regeneration of myocardial tissue leading to improved ventricular function [1–3], the effectiveness of stem cell therapy is often blurred from the non-survival of the stem cells due to the oxidative stress in the normal tissue [4]. In recent years, several attempts have been made for the enhancement of the survival and proliferation of stem cells in conjunction with the stem cell therapy. Overexpression of cardiac survival protein Pim-1 in cardiac progenitor cells (CPCs) has yielded promising results as demonstrated by the enhanced proliferation and functional improvement relative to control progenitor cells after myocardial infarction [5]. In another study, intramyocardial injection of hepatocyte growth factor stimulated resident CPCs and potentiated cardiomyocyte regeneration after myocardial infarction by promoting the proliferation and survival of CPCs [6]. Another related study with lipopolysaccharide preconditioning of mesenchymal stem cells before the transplantation surgery, resulted in superior neovascularization resulting in the recovery of cardiac function [7]. Simvastatin improved the efficacy of stem cell in the infracted myocardium. Interestingly, reduced oxidative environment and inflammatory response was noticed in the infracted regions of the animals treated with simvastatin and stem cells [8].
Based on these previously published results, we suggested that a reduction of the oxidative stress in the myocardium could improve the results the stem cell therapy. In this study, we examined the effect of resveratrol on the modification of adult cardiac stem cell therapy in the rats. This study is unique in that we used resveratrol as nutrient to alter the oxidative environment of the myocardium during the stem cell therapy. Our results show that maintaining a reduced tissue environment with resveratrol in rat hearts indeed resulted in the regeneration of infracted heart by adult cardiac stem cells as shown by improved cell survival, differentiation cardiac function.
Stem cell therapy
Sprague-Dawley male rats weighing between 250 and 300 g were fed ad libitum regular rat chow with free access to water until the experimental procedure. All animals used in this study received humane care in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition. The rats were randomly assigned to one of the three groups: (i) left anterior descending coronary artery (LAD) occlusion in control group, (ii) LAD occlusion and stem cell treatment in control group and (iii) LAD occlusion and stem cell treatment in resveratrol- (2.5 mg/kg) treated group. The rats were anesthetized with ketamine (100 mg/kg) / xylazine (10 mg/kg, ip) in combination with buprenorphine (0.5–2.5 mg/kg, s/c, bd), intubated and ventilated at a rate of 70 breaths/min. A left lateral thoracotomy was performed under painless and aseptic conditions. After the chest was opened, the pericardium was cut. A 6–0 polypropylene suture was passed under the LAD at the level of the left atrial appendage. Myocardial ischemia was produced by permanently occluding the LAD. Then stem cells were delivered directly on the myocardium into the bordering regions of ischemia in the second (control) and the third (resveratrol-treated) group. The injection needle was introduced 1 mm into the myocardium at an angle (10–20°) in a cranial direction. After completion of all the surgical procedures, the chest was closed with 4–0 nylon sutures and the ventilation rate was reduced to 50 breaths/min. till the spontaneous respiration starts. The rats were then extubated and kept in a temperature-controlled environment to prevent hypothermia where the rats were continuously monitored. Buprenorphine (0.1 mg/kg s.c.) was administered as an analgesic and gentamycin (1 mg/kg) were used as antibiotic to prevent post-surgical infections. After 28 days of surgery, rats were sedated using isoflurene (3%, inhaled), shaved and placed on a heated pad. Ultrasound gel was spread over the pericardial region, and ultrasound biomicroscope (Vevo 770, Visual-Sonics, Inc., Toronto, ON, Canada). Transthoracic M-mode echocardiography were performed with a Vevo 770 ultrasound system (Agilent Technologies, Andover, MA, USA) equipped with a 25 MHz transducer as described by us earlier to visualize the left ventricle. with a 25 MHz transducer was used to visualize the left ventricle. The left ventricle was analysed in apical, parasternal long axis, and parasternal short axis views for left ventricular inner diameters (LVIDs) both in systole and diastole, and LV functional parameters such as ejection fraction (EF), fractional shortening (FS) and cardiac output (CO). Two-dimensional directed M-mode images of the LV short axis were taken just below the level of the papillary muscles to analyse ventricular wall thickness and chamber diameter. All left ventricular parameters were measured according to the modified American Society of Echocardiography recommended guidelines. All measurements from different animals were averaged and represented as mentioned earlier [9]. After performing M-mode echocardiography, rats were anesthetized with sodium pentobarbital (80 mg/kg, i.p.), (Abbott Laboratories, North Chicago, IL, USA) and with the anticoagulant heparin sodium (500 IU/kg. i.v.) (Elkins-Sinn, Inc., Cherry Hill, NJ, USA). After ensuring sufficient depth of anaesthesia, thoracotomy was performed, hearts were isolated and perfused in the retrograde Langendorff mode at 37°C at a constant perfusion pressure of 100 cm of water (10 kPa) for a 10 min. washout period. The perfusion buffer used in this study consisted of a modified Krebs–Henseleit bicarbonate buffer (in mM: sodium chloride 118, potassium chloride 4.7, calcium chloride 1.7, sodium bicarbonate 25, potassium biphosphate 0.36, magnesium sulphate 1.2 and glucose 10). The hearts were then removed from the apparatus, and frozen with O.C.T. compound, and stored at –20°C. All values were expressed as the mean ± S.E.M. Analysis of variance test followed by Bonferoni’s correction was first carried out to test for any differences between the mean values of all groups. If differences were established, the values of the treated groups were compared with those of the control group by a modified t-test. The results were considered significant if P < 0.05.
Heart tissue samples collected at the end of experiments were frozen with Tissue-Tek embedding medium optimal culture temperature O.C.T. compound and subjected to cryo-sectioning. The obtained specimens (5 μm cuts) were processed for immunofluorescence analysis as described previously [10]. The primary antibodies such as GFP (Cell Signaling Technology, Beverly, MA, USA; 1:100 dilution), c-kit (Cell Signaling Technology; 1:100 dilution), nuclear factor-E2-related factor-2 (Nrf2; Cell Signaling Technology; 1:50 dilution), redox effector factor-1 (Ref-1; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:50 dilution), Ki67 (Santa Cruz Biotechnology; 1:50 dilution) and cardiac myosin (Abcam, Cambridge, MA, USA; 1:100 dilution) were incubated overnight at 4°C. This was followed by incubation with Alexa-Fluor® fluorochrome-conjugated secondary antibodies (Molecular Probes; 1:500 dilution), and the nuclear staining were performed with Topro-3-iodide (Molecular Probes, Carlsbad, CA, USA; 1:500 dilution). The slides were washed and covered with mounting medium, and examined under a fluorescence microscope. Confocal microscopic images were obtained using Zeiss LSM 510 (Thornwood, NY, USA) confocal laser scanning microscope by simultaneous recording in the 488 λ, 530 λ and/or 560 λ channels as appropriate. We monitored the nuclear expression of Nrf2 and Ref-1 7 days and 28 days after LAD occlusion using confocal immunofluorescence microscopy. Our results show that the nuclear expression of Nrf2 and Ref-1 were significantly increased 7 days after LAD occlusion in resveratrol-treated myocardium relative to control (Table 1). However, the nuclear expression of Nrf2 and Ref-1 were not significantly increased 28 days after LAD occlusion (data not shown).
Table 1.
Regeneration of infracted myocardium with resveratrol-modified cardiac stem cells
| 7 days after infarction | 28 days after infarction | |||
|---|---|---|---|---|
| Expression and nuclear co-localization | (Pixel/nuclear co-localization) | |||
| –Resveratrol | Resveratrol | –Resveratrol | +Resveratrol | |
| Nrf2 | 500 ± 25 | 1125 ± 27* | 27 ± 5 | 322 ± 14* |
| Ref-1 | 710 ± 21 | 2350 ± 35* | 32 ± 5 | 375 ± 20* |
| Ki67 | 105 ± 20 | 650 ± 31* | 85 ± 5 | 603 ± 19* |
P < 0.05 versus–Resveratrol.
Regeneration of myocardium 28 days after myocardial infarction
Cellular proliferation was studied by the expression of Ki67 28 days after LAD occlusion, and our results show that Ki67 staining was remarkably increased in resveratrol-treated myocardium compared to control hearts (Table 1). Cardiac regeneration was studied by staining the cardiac tissue section with GFP. Quantification of GFP staining 28 days after LAD occlusion indicate that GFP staining was remarkably increased in resveratrol-treated myocardium compared to control hearts. Confocal microscopic images showed the significant enhancement of c-kit+ cells 28 days after myocardial infarction in resveratrol-treated samples (Fig. 1A), and the co-expression of SDF-1 and GFP in resveratrol-treated hearts (Fig. 1B) leading to the regeneration of the myocardium in the resveratrol-treated heart (Fig. 1C). M-mode echocardiogram of rats recorded at 28 days after survival surgery reveal that resveratrol treatment enhanced the cardiac stem cell-mediated improvement in cardiac functional parameters such as LVID in systole, EF, LVID in diastole, FS and CO (Table 2).
Fig 1.
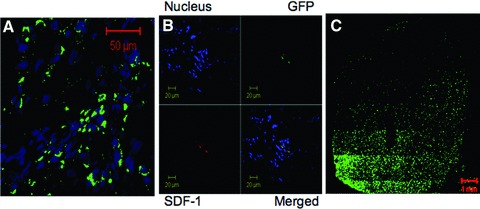
Co-expression of SDF-1 and GFP in the resveratrol-treated hearts. (A) Expression of c-kit (green channel) 28 days after myocardial infarction in resveratrol-treated samples, where the blue channel shows the nucleus. (B) Confocal microscopic images of left ventricular tissue section showing the expression of SDF-1 and GFP 28 days after myocardial infarction in resveratrol-treated samples. (C) Expression of cardiac stem cell mediated GFP (green) in the resveratrol-treated heart.
Table 2.
Improvement of cardiac function after resveratrol-modified stem cell therapy
| Functional parameters | 7 days after infarction | 28 days after infarction | ||
|---|---|---|---|---|
| –Resveratrol | +Resveratrol | –Resveratrol | +Resveratrol | |
| LVID-S | 5.0 ± 0.1 | 5.9 ± 0.2 | 5.5 ± 0.2 | 6.2 ± 0.3* |
| LVID-D | 6.1 ± 0.2 | 6.8 ± 0.3 | 6.0 ± 0.3 | 7.8 ± 0.2* |
| EF | 38 ± 0.4 | 42 ± 0.3 | 40 ± 1.0 | 60 ± 2.2* |
| FS | 38 ± 2.5 | 43 ± 2.6 | 41 ± 3.2 | 58 ± 4.0* |
| CO | 41 ± 1.8 | 50 ± 3.4 | 43 ± 2.3 | 75 ± 4.1* |
LVID-S: left ventricular internal diameter in systole; LVID-D: left ventricular internal diameter in diastole; EF: ejection fraction; FS: fractional shortening; CO: cardiac output.
P < 0.05 versus–Resveratrol.
Concluding remarks
Despite the cell-based therapy for cardiac diseases has yielded promising results for the improvement of cardiac function and regeneration, the stems cells fail to survive for a prolonged time for several factors including the persistence of oxidative environment in the target tissue. In clinical trials for the treatment of acute and chronic myocardial ischemia, a variety of progenitor cells including bone marrow-derived progenitor cells, bone marrow-derived mononuclear circulating progenitor cells, as well as skeletal myoblasts have been used [13]. Even though the results of cell-based therapies are promising, the improvement in cardiac function and myocardial regeneration has not been satisfactory. Several modifications including the reduction of oxidative stress have been made in an attempt to improve the performance of a cell-based therapy by modifying the cells prior to treatment. We suggested that the stem cell homing and proliferation could also be manipulated by modifying the physiological redox environment by proper nutrition. Thus, we examined the effects of adult cardiac stem cell therapy in animals treated with a definite dosage of [11] resveratrol, a polyphenolic compound present in red wine and grapes, which possesses the ability to enhance myocardial survival by changing the intracellular oxidative environment into a reduced environment [12]. We also selected this compound because of its well-known ability to enhance the antioxidant defence mechanism [12]. Our results revealed for the first time that it is possible to maintain a safer niche for the stem cells with proper nutrition, which could result in enhanced proliferation, myocardial regeneration and differentiation leading to an improved cardiac function. To achieve this, we pre-treated the rats with resveratrol, in a pre-determined dosage of 2.5 mg/kg/day [11]. Resveratrol has the potential to donate hydrogen or react with superoxide anion, hydroxyl radicals and lipid peroxyl radicals [14], and has been shown to modulate antioxidant enzymes involved in the phase II response such as haem oxygenase 1 [15]. Furthermore, resveratrol has been shown to increase plasma antioxidant capacity and decrease lipid peroxidation in vivo[16]. In vitro studies show that resveratrol prevents myocardial infarction by chelating metal ions as well as by directly scavenging free radicals [17]. Resveratrol induces the activities of catalase and quinone reductase 1 and reduces the amount of reactive oxygen species (ROS) generated by menadione in the myocardium of guinea pigs [18]. Altogether, these results indicate that resveratrol can suppress pathological oxidative stress in vivo. In this study, we examined the redox status of the myocardium 7 days after the survival surgery by monitoring Nrf2, a master gene of the endogenous antioxidant defence system, in the heart. A recent study showed that redox status in the extracellular compartment regulates intracellular ROS, which play an important role in the activation of Nrf2 and up-regulation of antioxidant and detoxification systems in mouse embryonic fibroblasts [19]. Nrf2 plays a critical role in the protection of the murine heart against pathological cardiac hypertrophy and heart failure via suppressing oxidative stress [20] Nrf2 mediated adaptive response has been shown to protect against superoxide anion-induced oxidative damage [21] and cadmium-induced oxidative stress in mouse embryonic fibroblasts [22]. Because dietary polyphenols including resveratrol are known to elicit anti-oxidant mechanism via activation of Nrf2 [23], we studied the activation of Nrf2 as a redox activation marker in our samples. The nuclear activation of Nrf2 was significantly elevated in resveratrol-treated rats, and a close association between Nrf2 activation and GEFP+ stem cells’ localization was noticed. Further, it is to be noted that endogenous ROS are shown to function as signalling molecules for myogenic differentiation via regulation of Nrf2 and glutathione redox [24] Ape1/Ref-1 is a novel redox component of signal transduction processes that regulate eukaryotic gene expression. Ape1/Ref-1 activates several transcription factors and facilitates their DNA binding via the reduction of a cysteine residue [25]. Three-dimensional molecular structure modelling and virtual screening show that resveratrol docks into a druggable pocket of Ref-1 protein [26]. Previously, we have demonstrated that resveratrol possesses the ability to protect the cells at lower doses as observed during pharmacological preconditioning of the heart, whereas at higher doses it causes cell death as found for cancer cells [11]. In the present study, we have found that Ref-1 activation was significantly increased in resveratrol-treated hearts.
In conclusion, our results suggest that cell-based therapy in the ischemic myocardium can be enhanced by nutritional modification of intracellular redox environment with resveratrol via enhanced stem cell survival, proliferation and cardiac regeneration and function compared to only stem cell treated rat hearts. The above effects were shown to be mediated through the ability of resveratrol to enhance the redox potential in the target organ.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann JL, Abarbanell AM, Weil BR, et al. Cell-based therapy for ischemic heart disease: a clinical update. Ann Thorac Surg. 2009;88:1714–22. doi: 10.1016/j.athoracsur.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 3.Domian IJ, Chiravuri M, Van Der Meer P, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–9. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toma C, Mark F. Pittenger MF, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 5.Fischer KM, Cottage CT, Wu W, et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–87. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rota M, Padin-Iruegas ME, Misao Y, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–16. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Y, Zhang F, Wang L, et al. Lipopolysaccharide preconditioning enhances the efficacy of mesenchymal stem cells transplantation in a rat model of acute myocardial infarction. J Biomed Sci. 2009;16:74. doi: 10.1186/1423-0127-16-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YJ, Qian HY, Huang J, et al. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscler Thromb Vasc Biol. 2009;29:2076–82. doi: 10.1161/ATVBAHA.109.189662. [DOI] [PubMed] [Google Scholar]
- 9.Lekli I, Mukherjee S, Ray D, et al. Functional recovery of diabetic mice hearts by glutaredoxin-1 gene therapy: role of Akt-FoxO signaling network. Gene Therapy. 2010;17:478–85. doi: 10.1038/gt.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurusamy N, Lekli I, Gorbunov NV, et al. Cardioprotection by adaptation to ischaemia augments autophagy in association with BAG-1 protein. J Cell Mol Med. 2009;13:373–87. doi: 10.1111/j.1582-4934.2008.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley J, Das S, Mukherjee S, et al. Resveratrol, a unique phytoalexin present in red wine, delivers either survival signal or death signal to the ischemic myocardium depending on dose. J Nutr Biochem. 2009;20:443–52. doi: 10.1016/j.jnutbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Bertelli AA, Das DK. Grapes, Wines, Resveratrol and Heart Health. J Cardiovasc Pharmacol. 2009;54:468–76. doi: 10.1097/FJC.0b013e3181bfaff3. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann JL, Abarbanell AM, Weil BR, et al. Cell-based therapy for ischemic heart disease: a clinical update. Ann Thorac Surg. 2009;88:1714–22. doi: 10.1016/j.athoracsur.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 14.Hung LM, Su MJ, Chu WK, et al. The protective effect of resveratrols on ischaemia-reperfusion injuries of rat hearts is correlated with antioxidant efficacy. Br J Pharmacol. 2002;135:1627–33. doi: 10.1038/sj.bjp.0704637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaga S, Zhan L, Matsumoto M, et al. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;39:813–22. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–6. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 17.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–4. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 18.Floreani M, Napoli E, Quintieri L, et al. Oral administration of trans-resveratrol to guinea pigs increases cardiac DT-diaphorase and catalase activities, and protects isolated atria from menadione toxicity. Life Sci. 2003;72:2741–50. doi: 10.1016/s0024-3205(03)00179-6. [DOI] [PubMed] [Google Scholar]
- 19.Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Ichikawa T, Villacorta L, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol. 2009;29:1843–50. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 21.Osburn WO, Wakabayashi N, Misra V, et al. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454:7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–83. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- 23.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Ding Y, Choi KJ, Kim JH, et al. Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation. Am J Pathol. 2008;172:1529–41. doi: 10.2353/ajpath.2008.070429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishel ML, Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol Aspects Med. 2007;28:375–95. doi: 10.1016/j.mam.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Yang S, Irani K, Heffron SE, et al. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/ redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol Cancer Ther. 2005;4:1923–35. doi: 10.1158/1535-7163.MCT-05-0229. [DOI] [PubMed] [Google Scholar]


