Abstract
The term TELOCYTES was very recently introduced, for replacing the name Interstitial Cajal-Like Cells (ICLC). In fact, telocytes are not really Cajal-like cells, they being different from all other interstitial cells by the presence of telopodes, which are cell-body prolongations, very thin (under the resolving power of light microscopy), extremely long (tens up to hundreds of micrometers), with a moniliform aspect (many dilations along), and having caveolae. The presence of telocytes in epicardium and myocardium was previously documented. We present here electron microscope images showing the existence of telocytes, with telopodes, at the level of mouse endocardium. Telocytes are located in the subendothelial layer of endocardium, and their telopodes are interposed in between the endocardial endothelium and the cardiomyocytes bundles. Some telopodes penetrate from the endocardium among the cardiomyocytes and surround them, eventually. Telopodes frequently establish close spatial relationships with myocardial blood capillaries and nerve endings. Because we may consider endocardium as a ‘blood–heart barrier’, or more exactly as a ‘blood–myocardium barrier’, telocytes might have an important role in such a barrier being the dominant cell population in subendothelial layer of endocardium.
Keywords: telocytes, telopodes, endocardium, interstitial Cajal-like cells (ICLCs)
The three-dimensional structure of the heart consists in three important layers-epicardium, myocardium and endocardium—each one being of different thickness, cellular composition and, naturally, having specific role(s). The myocardium is the contractile ‘star’, and its cellular architecture is quite well known [1, 2]. The epicardium is more than a simple cover [3], and recent studies documented the possible key-role of epicardial cells in myocardial development [3–7] and in regeneration/repair [8–12]. Endocardium is usually considered as being only an endothelium, although, from the histological point of view, the endocardium has two layers: the endothelium and the subendothelial loose connective tissue, or subendocardium. Most of the studies concerning endocardium were concentrated on cardiac development [13–18], and the endothelium is the only subject considered. To our knowledge no consistent data about the subendothelial layer is available.
We previously found, by serendipity, within human mammalian myocardium and epicardium the presence of a distinct cell population-telocytes [19]. We coined the term telocytes, which seems internationally accepted [20–22], replacing theformer one: Interstitial Cajal-Like Cells (ICLCs)-that we used during the last 4–5 years [6, 23–26]. The most characteristic features of telocytes are their very long and very thin prolongations, which we named telopodes [19] (free access data is available on http://www.telocytes.com).
We present here several electron microscope images showing clearly the existence of typical telocytes within the subendothelial layer of mouse endocardium. Moreover, telocytes appear as the main interstitial cell type in the loose connective tissue of the subendothelial endocardium.
Small fragments from mouse atrial and ventricular myocardium with endocardium were processed for transmission electron microscopy (EM) according to routine Epon-embedding procedure, as we previously described [11]. Hearts from six 4–12 months old C57BL/6 mice have been examined after the institutional ethical committee approval.
EM showed the presence of telocytes in the subendocardium of mouse heart (Figs 1–4) in atria and ventricles. The cellular component of subendocardium consisted of telocytes (Figs 1–3), fibroblasts (Fig. 4) and nerve endings (Figs 3E and 4). A rough estimation on over 100 low-magnification EM images suggested that about one-third of endothelial cells are underlined by telocytes. The fibrous component of the subendocardium consisted of small amount of collagen and elastic fibres (Figs 1 and 3).
Fig 1.
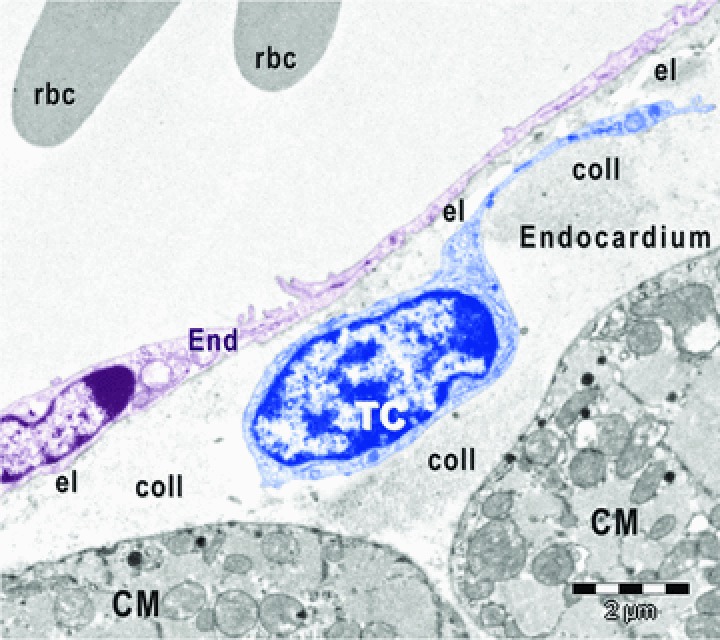
Digitally coloured electron micrograph of mouse atrial endocardium shows a telocyte (TC, blue) beneath endothelial cells (End, burgundy). CM, cardiomyocytes; rbc, red blood cells; el, elastic fibres; coll, collagen fibres.
Fig 4.
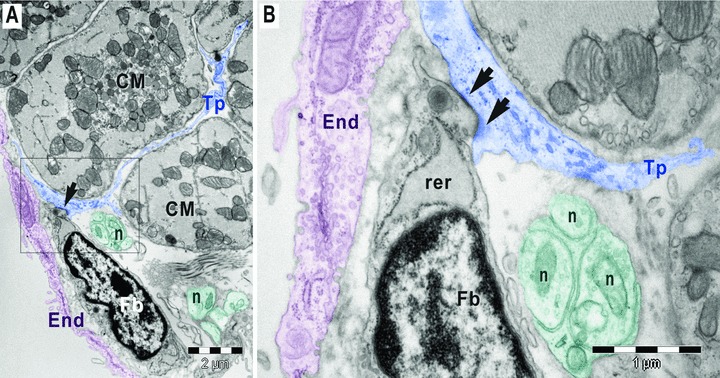
(A,B). Digitally coloured electron micrographs of mouse atrial endocardium (End, burgundy). Square marked area in A is enlarged in B. A gap junction (arrows) connects a subendocardial fibroblast (Fb) with a telopode (Tp) of a myocardial telocyte. The fibroblast has large rough endoplasmic reticulum (rer). The nerve endings (n) could be observed in between telopode and fibroblast; CM, cardiomyocytes.
Fig 3.
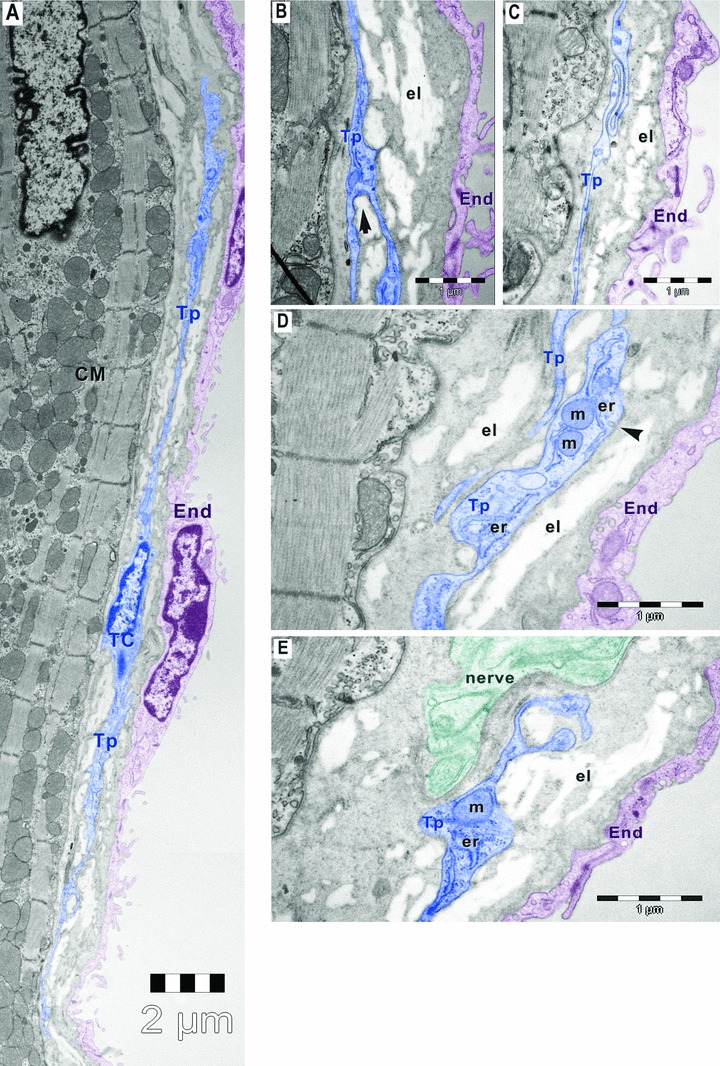
Digitally coloured electron micrographs of mouse atrial endocardium (End, burgundy). Small amount of elastin (el) is visible beneath the endothelial cells (burgundy). (A) Electron micrograph of mouse atrial endocardium shows a 30-μm-long telocyte (TC, blue) with two thin (50–500 nm) telopodes (Tp) beneath endothelial cells of endocardium. (B) Telopode with dichotomous pattern of branching (arrow). (C) Telopode with a sinuous segment having less than 50 nm thickness. (D) Dilatation of telopode hosting mitochondria (m), endoplasmic reticulum (er) and caveolae (arrowhead). (E) Telopode in close apposition with a nerve ending (n); CM, cardiomyocytes.
Subendocardial telocytes were similar with those founded in myocardium and epicardium and showed typical features with small oval-shaped cellular body (Figs 1–3) and very long, thin cellular process named telopodes (Figs 2 and 3). The subendocardial telocytes often send telopodes inside the myocardium and were connected with myocardial telocytes (Fig. 2).
Fig 2.
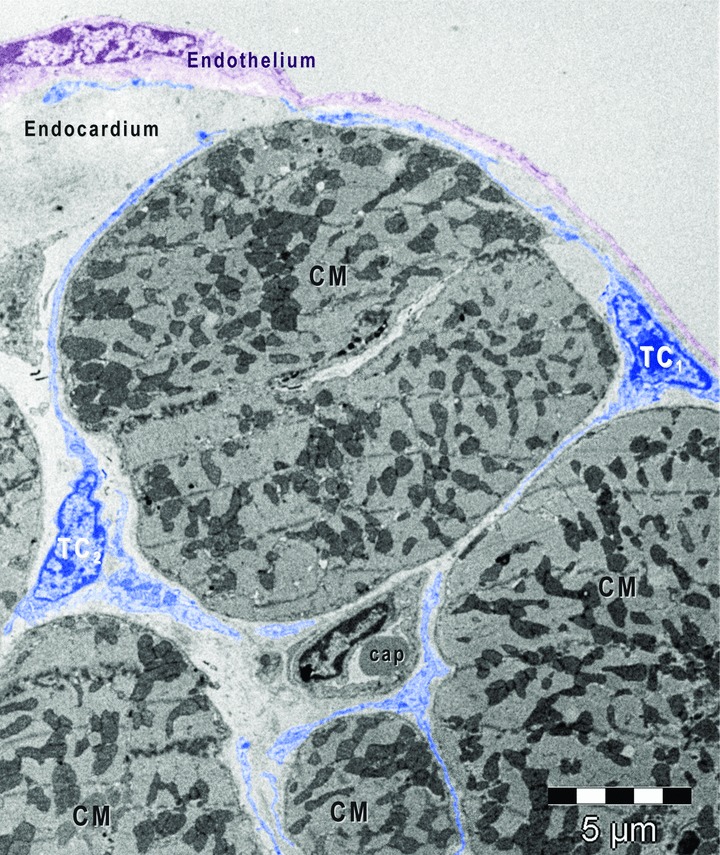
Digitally coloured electron micrograph of mouse ventricular endocardium (burgundy). Telocytes (coloured in blue) make an interstitial network in the heart. Subendocardial telocytes (TC1) send telopodes between cardiomyocytes (CM) and communicate with myocardial telocytes (TC2). Cap, capillary.
The telopodes showed characteristic dichotomous pattern of branching (Fig. 3B), sinuous trajectory (Fig. 3C), thin segments alternating with small dilatations that accommodate mitochondria, endoplasmic reticulum and caveolae (Fig. 3D). Such units, caveolae and the adjacent endoplasmic reticulum (+/− mitochondria) are considered Ca2+ accumulation/releasing units [27, 28]. Also, telocytes were in close vicinity with nerve endings (Figs 3E and 4) and established contacts with other interstitial cells (Fig. 4) in the subendocardial space.
This paper comes to confirm our hypothesis [21, 23–25] that telocytes make a tridimensional cardiac network at the interstitial level and integrate all constitutive layers of mouse heart via their unusual long prolongations, telopodes (Figs 2 and 3A). In fact, the interstitium should be considered a distinct compartment of the heart containing three distinct but highly cooperative networks: vascular network, neural network and telocytes network. The role of telocytes network is still speculative: heterocellular signalling, cardiac renewing, immune surveillance and/or mechanotransduction [11, 12, 19]. We cannot overlook the fact that endocardium is, at least conceptually, something like blood–brain barrier, mainly a ‘blood–heart barrier’ or a ‘blood–myocardium barrier’[29]. Apparently, telocytes, which seem to be the main population in the sub-epithelial layer of endocardium, might have a role in this ‘blood–myocardium barrier’.
Acknowledgments
This work was supported by CNCSIS–UEFISCSU, project number PNII–IDEI 957/2008 (M. Gherghiceanu, L.M. Popescu) and by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/89/1.5/S/64109 (M. Gherghiceanu).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Covell JW. Tissue structure and ventricular wall mechanics. Circulation. 2008;118:699–701. doi: 10.1161/CIRCULATIONAHA.108.797399. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RH, Smerup M, Sanchez-Quintana D, et al. The three-dimensional arrangement of the myocytes in the ventricular walls. Clin Anat. 2009;22:64–76. doi: 10.1002/ca.20645. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B, Pu WT. More than a cover: epicardium as a novel source of cardiac progenitor cells. Regen Med. 2008;3:633–5. doi: 10.2217/17460751.3.5.633. [DOI] [PubMed] [Google Scholar]
- 4.Zhou B, Ma Q, Rajagopal S, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habib M, Caspi O, Gepstein L. Human embryonic stem cells for cardiomyogenesis. J Mol Cell Cardiol. 2008;45:462–74. doi: 10.1016/j.yjmcc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Suciu L, Popescu LM, Regalia T, et al. Epicardium: interstitial Cajal-like cells (ICLC) highlighted by immunofluorescence. J Cell Mol Med. 2009;13:771–7. doi: 10.1111/j.1582-4934.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gittenberger-de Groot AC, Winter EM, Poelmann RE. Epicardium derived cells (EPDCs) in development, cardiac disease and repair of ischemia. J Cell Mol Med. 2010;14:1056–60. doi: 10.1111/j.1582-4934.2010.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gherghiceanu M, Popescu LM. Human epicardium: ultrastructural ancestry of mesothelium and mesenchymal cells. J Cell Mol Med. 2009;13:2949–51. doi: 10.1111/j.1582-4934.2009.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riley PR. The adult epicardium: realizing the potential for neovascular therapy. Arterioscler, Thromb Vasc Biol. 2008;28:803–4. doi: 10.1161/ATVBAHA.108.165191. [DOI] [PubMed] [Google Scholar]
- 10.Kajstura J, Urbanek K, Rota M, et al. Cardiac stem cells and myocardial disease. J Cell Mol Med. 2008;45:505–13. doi: 10.1016/j.yjmcc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris IS, Black BL. Development of the endocardium. Pediatr Cardio. 2010;31:391–9. doi: 10.1007/s00246-010-9642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith TK, Bader DM. Signals from both sides: control of cardiac development by the endocardium and epicardium. Semin Cell Dev Biol. 2007;18:84–9. doi: 10.1016/j.semcdb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meadows KN, Iyer S, Stevens MV, et al. Akt promotes endocardial-mesenchyme transition. J Angiogenes Res. 2009;1:2. doi: 10.1186/2040-2384-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtzman NG, Schoenebeck JJ, Tsai HJ, et al. Endocardium is necessary for cardiomyocyte movement during heart tube assembly. Development. 2007;134:2379–86. doi: 10.1242/dev.02857. [DOI] [PubMed] [Google Scholar]
- 17.Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237:2804–19. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- 18.Lee RK, Stainier DY, Weinstein BM, et al. Cardiovascular development in the zebrafish. II. Endocardial progenitors are sequestered within the heart field. Development. 1994;120:3361–6. doi: 10.1242/dev.120.12.3361. [DOI] [PubMed] [Google Scholar]
- 19.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faussone-Pellegrini MS, Bani D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J Cell Mol Med. 2010;14:1061–3. doi: 10.1111/j.1582-4934.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010 doi: 10.1159/000319467. in press: doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- 23.Hinescu ME, Popescu LM. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J Cell Mol Med. 2005;9:972–5. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popescu LM, Gherghiceanu M, Hinescu ME, et al. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popescu LM, Gherghiceanu M, Mandache E, et al. Caveolae in smooth muscles: nanocontacts. J Cell Mol Med. 2006;10:960–90. doi: 10.1111/j.1582-4934.2006.tb00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gherghiceanu M, Popescu LM. Electron microscope tomography: further demonstration of nanocontacts between caveolae and smooth muscle sarcoplasmic reticulum. J Cell Mol Med. 2007;11:1416–8. doi: 10.1111/j.1582-4934.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adler A, Huang H, Wang Z, et al. Endocardial endothelium in the avascular frog heart: role for diffusion of NO in control of cardiac O2 consumption. Am J Physiol – Heart Circ Physiol. 2004;287:H14–H21. doi: 10.1152/ajpheart.01235.2003. [DOI] [PubMed] [Google Scholar]


