Abstract
Mechanosensitivity is essential for heart function just as for all other cells and organs in the body, and it is involved in both normal physiology and diseases processes of the cardiovascular system. In this review, we have outlined the relationship between mechanosensitivity and heart physiology, including the Frank–Starling law of the heart and mechanoelectric feedback. We then focused on molecules involved in mechanotransduction, particularly mechanosensitive ion channels. We have also discussed the involvement of mechanosensitivity in heart diseases, such as arrhythmias, hypertrophy and ischaemic heart disease. Finally, mechanobiology in cardiogenesis is described with regard to regenerative medicine.
Keywords: mechanoelectric feedback, mechanotransduction, mechanosensitive ion channels, arrhythmias, ischaemic/reperfusion injury, cardiogenesis, mechanomedicine
Introduction
-
Mechanobiology in normal cardiac physiology
– Enhanced cardiac contraction in response to increased venous return
– Mechanoelectric feedback (MEF)
– Other physiological heart functions involved in mechanosensitivity
-
Mechanotransduction
– Mechanosensitive ion channels
-
Mechanobiology in cardiovascular disease
– Arrhythmias
– Hypertension, hypertrophy and heart failure
– Ischaemic/reperfusion injury and myocardial infarction
Mechanobiology in cardiogenesis
Conclusion
Introduction
The heart is one of the organs whose function is intimately associated with mechanosensitivity. For instance, heart massage is performed as first aid for someone who has fallen down as a result of a cardiac arrest. Actually, this procedure is more than simply pushing out blood from inside the heart because it applies mechanical pressure stimulus to the heart, allowing it to regain normal rhythm. Although its success rate is not very high, the precordial thump, a rapid impact with a clenched fist to a specific place on the sternum, is a measure that may save peoples’ lives by reverting ventricular tachycardia into a normal sinus rhythm [1].
The phenomenon by which a mechanical stimulus to the heart affects its contraction is explained by the concept of mechanoelectric feedback (MEF) [2]. MEF is involved in heart rate regulation. Mechanosensitive ion channels are molecular devices for sensing mechanical stimuli, such as the atrial stretch in MEF. In this review, we discuss the mechanosensitive ion channels that are expressed in the heart.
Cardiovascular disease is the greatest cause of death worldwide, and it is likely to maintain this position until at least 2030 [3]. Mechanosensitivity is inseparably involved in normal cardiac physiology and with cardiovascular diseases that includes arrhythmias, hypertrophy and ischaemia–reperfusion injury. We discuss the current understanding of the mechanosensitivity of the heart and its relation with the pathophysiology of these diseases.
Mechanobiology in normal cardiac physiology
In general, mechanotransduction is involved in cellular functions, such as proliferation, differentiation and apoptosis. Here, we discuss the organ-level response of the heart to mechanical stimuli.
Enhanced cardiac contraction in response to increased venous return
When athletes are at a dead run during a 100 metre sprint or even when we run up the stairs, venous return to the heart increases. Fortunately, the vertebrate heart functions to pump the blood out by increasing cardiac contractile forces when large volumes of blood in the veins return back to the heart and this supports physical exercise. The Frank–Starling law of the heart, which has a history of over 100 years, states that ‘the volume of blood pumped by the heart each minute is determined almost entirely by the rate of venous return’. Although this basic principle has been studied intensely by numerous researchers (reviewed in [4, 5]), it is still being investigated.
This phenomenon is explained by the following three mechanisms: overlapping of actin and myosin filaments in the sarcomere, calcium sensitivity of myofilaments [6, 7] and titin-based passive tension [8]. Although these biophysically well-constructed theories elegantly explain the mechanisms underlying the Frank–Starling law, there is one other phenomenon that cannot be explained by these theories, i.e. the ‘slow force response’, which is a gradual increase in the heart contraction force in minutes, is seen after the immediate increase in contraction force in response to the stretching of cardiomyocytes [9–12]. Although its detailed mechanisms are still under discussion, the involvement of mechanosensitive ion channels expressed in the heart has been suggested [13–15].
Mechanoelectric feedback (MEF)
Electrical excitation of myocytes is converted into mechanical movement of contraction by excitation–contraction coupling. This mechanical movement affects electrical excitation of myocytes. Periodic repeated contraction and relaxation of the heart is constantly modulated by this feedback system called mechanoelectric feedback (MEF). The concept of MEF is important because it is related to the development of arrhythmias and will be discussed later.
The fact that a stretch stimulus alters the membrane potential of cardiomyocytes was determined in the 1960s [16]. A stretch stimulus to the atrium prolongs an action potential's duration [15]. In general, when cardiomyocytes are stretched and/or pressure to the atrium or a ventricle is applied, the membrane potential of the cardiomyocytes depolarizes, the duration of an action potential shortens and the QT interval on an electrocardiogram shortens (see Lab's review in [2]). Stretch-activated channels are suggested to be involved in the MEF of the heart [17].
The orientation of cardiomyocytes differs because of the intramyocardial myocyte arrangement, and it is extremely difficult to examine the effects of a mechanical stimulus that is applied to cardiomyocytes during heart contraction. To deal with this problem, models that concurrently simulate the mechanical and electrical aspects of cardiac tissue have been developed using the finite element analysis technique [18]. Interestingly, the mechanism by which a precordial thump can revert arrhythmias into sinus rhythm has been estimated using a simulation model [19].
Other physiological heart functions involved in mechanosensitivity
Atrial natriuretic peptide (ANP) is an important hormone that is involved in blood pressure regulation. ANP secretion is mediated by stretch-activated Cl channels [20]. KATP channels are also suggested to regulate ANP secretion [21]. It is intriguing that healthy women develop ventricular hypertrophy as a result of volume overload and increased stretch in the heart during pregnancy [22]. Stretch-activated c-Src kinase may be involved in this type of mechanically induced hypertrophy.
Mechanotransduction
Each structure that forms the heart seems to be a device that senses mechanical stimuli, including the extracellular matrix, focal adhesion complexes, lipid bilayers, cellular orientation. In fact, each of these structures plays a role in mechanotransduction. In addition, numerous proteins are involved in mechanotransduction, including integrins, Rho kinase, PI3K, integrin-linked kinase, focal adhesion kinase, Src, extracellular signal-regulated kinase, MAP kinase, eNOS and others. These proteins are involved in cellular mechanotransduction pathways that mediate various heart responses, including arrhythmias, hypertrophy and ischaemic heart disease. Here, we have discussed the mechanosensitive ion channels that change their protein conformations in response to mechanical stimuli and induce successive responses of the cardiomyocytes.
Mechanosensitive ion channels
Ion channels that are expressed in the heart and thought to be mechanosensitive are shown in Table 1. The expression levels of some of these channels determined by our group using quantitative RT-PCR are shown in Figure 1. As discussed below, TRPA1, TRPC6, TRPM7, TRPV2 and TREK-1 are involved in the heart's mechanosensitivity. Interestingly, the TREK-1 mRNA expression level is low in the cardiomyocyte cell line H9c2, which suggests that care should be taken when using H9c2 cells for cardiac research.
Table 1.
Ion channels regarded as mechanosensitive in the heart
| Channels | Species | Location |
|---|---|---|
| Sodium channels | ||
| Nav1.5 | ||
| Nav1.6 | ||
| Potassium channels | ||
| TREK-1 | Rat | Cardiomyocyte |
| KATP | Rat | Atrial myocyte |
| SAKCA | Chick | Ventricular myocyte |
| KCNQ | Rat | Cardiomyocyte |
| Calcium channel | ||
| Cav1.2 | Human | Cardiomyocyte |
| Chloride channel | ||
| CFTR | Rabbit | Atrial myocyte, SA node |
| ClC-3? | Rabbit | Atrial myocyte |
| Non-specific cation channels | ||
| TRPA1 | ||
| TRPC1 | Rat | Cardiomyocyte |
| TRPC6 | Rat | Cardiomyocyte |
| TRPM4 | Human | Purkinje fibre, SA node |
| TRPM7 | Human | Atrial fibroblast |
| TRPP2 | ||
| TRPV2 | Human | Cardiac muscle |
| TRPV4 | Human | Atrial myocyte |
Fig. 1.
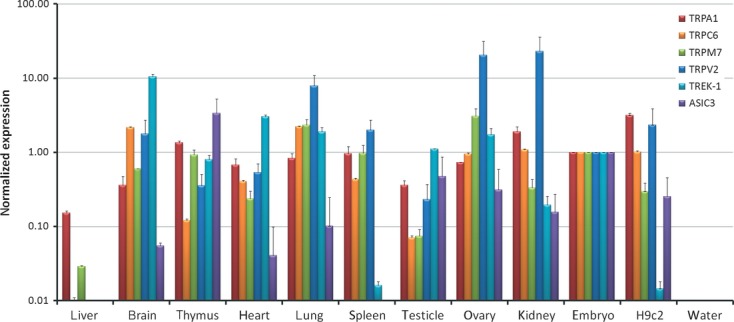
mRNA expressions of mechanosensitive ion channels determined by quantitative RT-PCR. Total RNA samples for liver, brain, thymus, heart, lung, spleen, testicle, ovary and kidney were obtained from 7–8 weeks old Sprague–Dawley rats. Total RNA sample for the embryo was obtained from midterm embryos of Sprague–Dawley rats. These RNA samples were purchased from Ambion. A total RNA sample for the rat ventricular cardiomyocyte cell line H9c2 was also obtained. TaqMan PCR primers were used for TRPA1, TRPC6, TRPM7, TRPV2, ASIC3 and TREK-1 channels' mRNAs. 18S ribosomal RNA was used as an internal control. Relative mRNA levels were calculated using ΔCt values (2∧(40 − ΔCt)) for each PCR run. Finally, the relative mRNA level was normalized to that of the embryo. All data are for three technical replicates.
Like other general ion channels, mechanosensitive ion channels change their conformations and become permeable to ions in response to multiple types of stimuli. Conformational changes in ion channels in response to mechanical stimuli have been well studied for the bacterial mechanosensitive channel MscL (mechanosensitive channel of large conductance). An example of mechanosensitive opening of MscS (mechanosensitive channel of small conductance) by coarse-grained molecular dynamics simulation carried out in our laboratory is shown in Figure 2A. As in this simulation, certain types of ion channels change their conformation in response to bilayer tension. Other types of channels sense mechanical stimuli by the interaction with cytoskeletal elements.
Fig. 2.
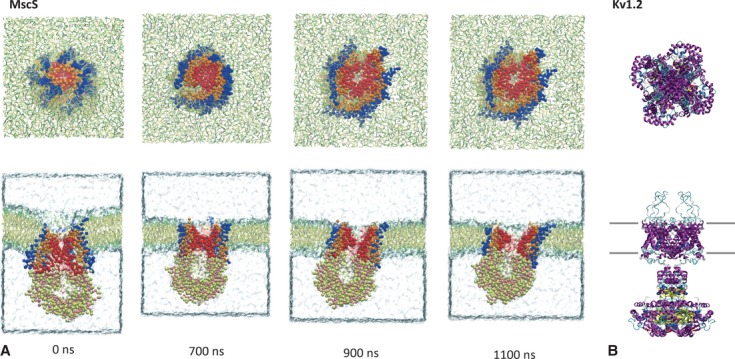
Structure of mechanosensitive ion channels. Upper panels: top views, lower panels: side views. (A) Opening of the bacterial mechanosensitive channel of small conductance (MscS, PDB accession number: 2OAU) in response to lipid bilayer stretch obtained by coarse-grained molecular dynamics simulation. MscS channel is embedded in lipid bilayers consisting of palmitoyloleoylphosphatidylcholine (POPC) and palmitoyloleoylphosphatidylethanolamine (POPE) lipids, which are shown in stick representation. The simulation box is filled with water molecules. Duration after applying a bilayer stretch is indicated in nanoseconds. Note that the channel's pore enlarges over time. (B) Three-dimensional crystal structure of mammalian Kv1.2 channel (PDB accession number: 3LUT) that putatively possesses mechanosensitivity.
In vertebrates, several genes that encode for mechanosensitive ion channels have been identified. For TRPC3 channel, which is genetically close to TRPC1, its three-dimensional structure at 15 Å resolution was obtained by cryo-electron microscopy [23]. However, higher resolution structures, as with bacterial channels, have not yet been obtained, except for the Kv1.2 channel that is thought to have mechanosensitivity (Fig. 2B).
In patch clamp experiments, mechanosensitive ion channels in the heart were found to be stretch-activated channels that became permeable to cations in response to applying negative pressure to the cellular membrane [24]. Later, anion channels that responded to stretch stimulus and swelling [25] were discovered [26]. Cystic fibrosis transmembrane conductance regulator (CFTR), which is a mechanosensitive chloride channel [27], is expressed in cardiac myocytes [28]. Activation of myocardial CFTR channel upon reperfusion after cardiac ischaemia is involved in protection against myocardial injury induced by ischaemic reperfusion [29, 30]. Several potassium ion channels are known to be mechanosensitive. The TREK-1 channel is sensitive to mechanical [31, 32] and thermal [33] stimuli, in addition to arachidonic acid [32] and volatile anaesthetics [34]. Mechanosensitivity of KATP channels was reported in rat atrial myocytes [35]. KATP channels are involved in generating action potentials [36]. We found that one of the big potassium (BK) channels, SAKCA, expressed in chick cardiomyocytes was mechanosensitive [37–39]. The KCNQ channel responds to changes in cellular volume [40].
In addition to these potassium ion channels, TRP channels, which are non-selective cation channels, are also known to be mechanosensitive [41]. The relationships between TRP channels and heart diseases have been vigorously investigated [41–43]. TRP channels that are known to be mechanosensitive and are expressed in the heart, include TRPA1, TRPC1 [44–46], TRPC6 [45, 47], TRPM4 [48], TRPM7, TRPP2, TRPV2 [45, 49] and TRPV4 [50].
TRPA channels (alias, Painless) that are involved in pain sensing in Drosophila are known to be mechanosensitive. Interestingly, these channels are also expressed in the heart and are required for pressure sensing [51]. TRPC1 and TRPC6 channels are expressed in sinoatrial node cells [52]. Impaired touch and hearing sensations observed in TRPC3 and TRPC6 double-knockout mice are caused by abnormal mechanotransduction in sensory nerves and inner ear hair cells [53]. TRPC1 and TRPC6 are stretch-activated channels in the heart. TRPV4 channels are expressed in urothelial cell culture and are permeable to calcium ions in response to stretch stimuli [54]. TRPV4 channels in human corneal endothelial cells are permeable to calcium ions in response to hyposmotic stimulation [55]. TRPV4 channels in capillary endothelial cells have increased cellular calcium levels in response to stretch stimuli, which facilitates the reorientation of these cells [56]. TRPM4 channels are calcium-activated non-selective cation channels that are expressed in the sinoatrial node [57]. These channels are involved in transient inward currents (Iti) in the atrium [58]. TRPM7 channels are major calcium permeable channels in human atrial fibroblasts [59].
Gating of voltage-gated channels is also modulated by mechanical stimuli. Although their physiological role in the heart remains to be elucidated, the mechanosensitivity of Cav1.2 [60], Nav1.5 [61, 62] and Nav1.6 [63] has been reported in expression studies.
Mechanobiology in cardiovascular disease
Arrhythmias
Arrhythmias are heart diseases, in which the involvement of mechanosensitivity has been extensively studied. MEF theory indicates that interruption in the normal MEF cycle will lead to arrhythmias. Stretching of the atrium produces changes in action potential shapes and causes arrhythmia [64]. Mechanosensitive ion channels are thought to be directly involved in the process, in which cardiac tissue stretching induces changes in membrane potentials. TRPV4 channels might be involved in the development of arrhythmia via delayed after polarization [50]. TRPM4 channels are highly expressed in the cellular membranes of Purkinje fibres, and their overexpression has been suggested to cause progressive familial heart block type I [65]. A TRPM4 mutation causes conduction block in the heart [66]. It has been reported that an arrhythmia that developed as a result of hypoxia/reperfusion could be suppressed by the TRPM4 channel inhibitor 9-phenanthrol [67]. TRPM7 channels have been suggested to be involved in heart fibrogenesis during atrial fibrillation [59]. As mentioned above, several mechanosensitive ion channels have been suggested to be involved in the pathophysiology of arrhythmias. However, the development of effective cures needs additional research.
Hypertension, hypertrophy and heart failure
It is known that TRPM4 expression is increased in hypertensive rats [58]. TRPM4 channels could be the cause of delayed after depolarization seen in these rats. In addition, TRPM4-deficient mice exhibit hypertension via increased catecholamine secretion [68]. Hypertension and valvular disease cause mechanical stimulation of cardiomyocytes, which induces hypertrophy of these cells via signal transduction pathways.
Hypertrophic responses are mediated by intracellular calcium levels. Store-operated channels (SOC) are regarded as the calcium source. TRPC1 and TRPC6 channels are candidate SOCs. Recently, the relationship between TRPC channels and cardiac hypertrophy has been revealed [42, 69]. TRPC channels are necessary mediators of pathological cardiac hypertrophy [70]. TRPC channels' expression is up-regulated during pressure overload to the heart [71]. In addition, TRPC6 channels are key components of a calcium-dependent regulatory loop involved in cardiac hypertrophy [72]. TRPC6 channels mediate hypertrophic responses in cardiomyocytes; however, they suppress fibrotic responses in cardiac fibroblasts [73]. Progressive pathological hypertrophy develops into heart failure. Stretch-induced apoptosis can lead to heart failure [74]. TRPC6 channel expression is up-regulated in failing hearts [75]. The mechanosensitivity of these channels, which may be involved in the pathophysiology of heart failure, should be the focus of a future study.
Ischaemic/reperfusion injury and myocardial infarction
Ischaemic heart disease is a leading cause of death worldwide [3]. Short duration of ischaemia prior to sustained ischaemia can reduce injury caused by ischaemia–reperfusion injury [76]. This phenomenon is called ‘ischemic preconditioning’. Interestingly, stretch stimuli to the heart were found to have a preconditioning effect on ischaemia–reperfusion injury [77]. This ‘stretch preconditioning’ disappears when KATP channels are blocked [78, 79]. As mentioned earlier, KATP channels are mechanosensitive. On the other hand, CFTR channels, which are involved in cell volume regulation after osmotic swelling, play a role in ischaemic preconditioning [29, 80] and post-conditioning [30]. However, the interplay between KATP and CFTR mechanisms still remains to be elucidated. Further studies on the mechanisms involved in stretch preconditioning may lead to the development of new treatments for ischaemic heart diseases.
Prolonged ischaemia and successive reperfusion induce myocardial infarction, which may accompany arrhythmias. Cardiac mechanosensitivity has been suggested to be the cause of arrhythmogenesis in myocardial infarction. For example, a simulation study demonstrated that premature ventricular beats originated from the ischaemic border where mechanical strain was discontinuous, which may contribute to spontaneous arrhythmias [81]. TRPC6 protein expression is increased in rat myocardial infarction [82]. Future research may reveal whether increased TRPC6 expression is involved in the facilitated mechanosensitivity of cardiomyocytes at the border zone in myocardial infarction.
Mechanobiology in cardiogenesis
During development, cells, tissues and organs assume their characteristic shapes by sensing mechanical stimuli and responding to them. The heart is an organ that first starts functioning in vertebrate embryos. Appropriate elasticity is required for calcium excitation and contraction of the cardiomyocytes [83]. In fact, substrate stiffness influences the heart rate contraction forces, the cytoskeletal structure and intracellular calcium levels in cardiomyocytes [84, 85].
Another key factor involved in cardiogenesis is cyclic stretching of cardiomyocytes, which is caused by pulsatile changes in cardiac internal pressure. Ott et al. performed interesting experiments, in which cardiac cells were reseeded onto a decellularized heart matrix [86]. When pulsatile perfusion was applied, thick viable cardiac muscles were obtained, whereas thin, weak muscles were obtained in a non-perfusion environment. Cyclic mechanical stretching influences both the expression and localization of connexin 43 [87]. Cyclic stretching also induces orientation of cardiomyocytes that is transverse to the stretch axis [88]. Thus, mechanical forces can affect intercellular communications via gap junction channels in the heart. Changes in blood flow patterns can impair cardiac septation and valve formation (reviewed in [89]).
In recent years, numerous attempts have been made to generate cardiomyocytes from embryonic stem cells, induced pluripotent stem cells and cardiac stem cells to find the means to repair adult hearts after heart attacks or other injuries [90–92]. Considering that the heart is an organ that is constantly exposed to mechanical stimuli, applying mechanical stimuli may be a key for generating robust cardiomyocytes from stem cells.
Conclusion
The normal differentiation of tissues and organs, including the heart, is facilitated by mechanical stimuli during development. Cardiac mechanosensitivity is indispensable for normal heart physiology, as seen in the Frank–Starling law and MEF. Heart diseases have a significant impact on human health. Although the relationship between heart mechanosensitivity and the pathophysiologies of arrhythmias, hypertrophy and ischaemic heart disease is being revealed, further research needs to be conducted to apply this knowledge in finding effective remedies. Applying mechanical stimuli to stem cells is anticipated to contribute to the successful cellular induction of cardiomyocytes.
Conflicts of interest
We declare that there are no conflicts of interest associated with this article.
References
- 1.Pellis T, Kohl P. Extracorporeal cardiac mechanical stimulation: precordial thump and precordial percussion. Br Med Bull. 2009;93:161–77. doi: 10.1093/bmb/ldp045. [DOI] [PubMed] [Google Scholar]
- 2.Lab MJ. Mechanoelectric feedback (transduction) in heart: concepts and implications. Cardiovasc Res. 1996;32:3–14. [PubMed] [Google Scholar]
- 3.The global burden of disease: 2004 update. Geneva: World Health Organization; 2008. [Google Scholar]
- 4.Campbell KS. Impact of myocyte strain on cardiac myofilament activation. Pflugers Arch. 2011;462:3–14. doi: 10.1007/s00424-011-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cazorla O, Lacampagne A. Regional variation in myofilament length-dependent activation. Pflugers Arch. 2011;462:15–28. doi: 10.1007/s00424-011-0933-6. [DOI] [PubMed] [Google Scholar]
- 6.Shiels HA, White E. The Frank-Starling mechanism in vertebrate cardiac myocytes. J Exp Biol. 2008;211:2005–13. doi: 10.1242/jeb.003145. [DOI] [PubMed] [Google Scholar]
- 7.Korte FS, Feest ER, Razumova MV, et al. Enhanced Ca2+ binding of cardiac troponin reduces sarcomere length-dependence of contractile activation independently of strong crossbridges. Am J Physiol Heart Circ Physiol. 2012;303:H863–70. doi: 10.1152/ajpheart.00395.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda N, Terui T, Ohtsuki I, et al. Titin and troponin: central players in the frank-starling mechanism of the heart. Curr Cardiol Rev. 2009;5:119–24. doi: 10.2174/157340309788166714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen DG, Jewell BR, Murray JW. The contribution of activation processes to the length-tension relation of cardiac muscle. Nature. 1974;248:606–7. doi: 10.1038/248606a0. [DOI] [PubMed] [Google Scholar]
- 10.Kentish JC, Wrzosek A. Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J Physiol. 1998;506(Pt 2):431–44. doi: 10.1111/j.1469-7793.1998.431bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward ML, Williams IA, Chu Y, et al. Stretch-activated channels in the heart: contributions to length-dependence and to cardiomyopathy. Prog Biophys Mol Biol. 2008;97:232–49. doi: 10.1016/j.pbiomolbio.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 12.von Lewinski D, Kockskamper J, Zhu D, et al. Reduced stretch-induced force response in failing human myocardium caused by impaired Na(+)-contraction coupling. Circ Heart Fail. 2009;2:47–55. doi: 10.1161/CIRCHEARTFAILURE.108.794065. [DOI] [PubMed] [Google Scholar]
- 13.Lab MJ, Zhou BY, Spencer CI, et al. Effects of gadolinium on length-dependent force in guinea-pig papillary muscle. Exp Physiol. 1994;79:249–55. doi: 10.1113/expphysiol.1994.sp003758. [DOI] [PubMed] [Google Scholar]
- 14.Ruknudin A, Sachs F, Bustamante JO. Stretch-activated ion channels in tissue-cultured chick heart. Am J Physiol. 1993;264:H960–72. doi: 10.1152/ajpheart.1993.264.3.H960. [DOI] [PubMed] [Google Scholar]
- 15.Tavi P, Han C, Weckstrom M. Mechanisms of stretch-induced changes in [Ca2+]i in rat atrial myocytes: role of increased troponin C affinity and stretch-activated ion channels. Circ Res. 1998;83:1165–77. doi: 10.1161/01.res.83.11.1165. [DOI] [PubMed] [Google Scholar]
- 16.Penefsky ZJ, Hoffman BF. Effects of stretch on mechanical and electrical properties of cardiac muscle. Am J Physiol. 1963;204:433–8. [Google Scholar]
- 17.Kelly D, Mackenzie L, Hunter P, et al. Gene expression of stretch-activated channels and mechanoelectric feedback in the heart. Clin Exp Pharmacol Physiol. 2006;33:642–8. doi: 10.1111/j.1440-1681.2006.04392.x. [DOI] [PubMed] [Google Scholar]
- 18.Vetter FJ, McCulloch AD. Mechanoelectric feedback in a model of the passively inflated left ventricle. Ann Biomed Eng. 2001;29:414–26. doi: 10.1114/1.1366670. [DOI] [PubMed] [Google Scholar]
- 19.Trayanova NA, Constantino J, Gurev V. Models of stretch-activated ventricular arrhythmias. J Electrocardiol. 2010;43:479–85. doi: 10.1016/j.jelectrocard.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han JH, Bai GY, Park JH, et al. Regulation of stretch-activated ANP secretion by chloride channels. Peptides. 2008;29:613–21. doi: 10.1016/j.peptides.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Saegusa N, Sato T, Saito T, et al. Kir6.2-deficient mice are susceptible to stimulated ANP secretion: K(ATP) channel acts as a negative feedback mechanism? Cardiovasc Res. 2005;67:60–8. doi: 10.1016/j.cardiores.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Eghbali M, Wang Y, Toro L, et al. Heart hypertrophy during pregnancy: a better functioning heart? Trends Cardiovasc Med. 2006;16:285–91. doi: 10.1016/j.tcm.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Mio K, Ogura T, Kiyonaka S, et al. The TRPC3 channel has a large internal chamber surrounded by signal sensing antennas. J Mol Biol. 2007;367:373–83. doi: 10.1016/j.jmb.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 24.Craelius W, Chen V, el-Sherif N. Stretch activated ion channels in ventricular myocytes. Biosci Rep. 1988;8:407–14. doi: 10.1007/BF01121637. [DOI] [PubMed] [Google Scholar]
- 25.Duan DD. The ClC-3 chloride channels in cardiovascular disease. Acta Pharmacol Sin. 2011;32:675–84. doi: 10.1038/aps.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagiwara N, Masuda H, Shoda M, et al. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol. 1992;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WK, Wang D, Duan Y, et al. Mechanosensitive gating of CFTR. Nat Cell Biol. 2010;12:812. doi: 10.1038/ncb2053. [DOI] [PubMed] [Google Scholar]
- 28.Gadsby DC, Nagel G, Hwang TC. The CFTR chloride channel of mammalian heart. Annu Rev Physiol. 1995;57:387–416. doi: 10.1146/annurev.ph.57.030195.002131. [DOI] [PubMed] [Google Scholar]
- 29.Diaz RJ, Armstrong SC, Batthish M, et al. Enhanced cell volume regulation: a key protective mechanism of ischemic preconditioning in rabbit ventricular myocytes. J Mol Cell Cardiol. 2003;35:45–58. doi: 10.1016/s0022-2828(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 30.Uramoto H, Okada T, Okada Y. Protective role of cardiac CFTR activation upon early reperfusion against myocardial infarction. Cell Physiol Biochem. 2012;30:1023–38. doi: 10.1159/000341479. [DOI] [PubMed] [Google Scholar]
- 31.Xian TaoL, Dyachenko V, Zuzarte M, et al. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res. 2006;69:86–97. doi: 10.1016/j.cardiores.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Maingret F, Patel AJ, Lesage F, et al. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–6. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Shepherd N, Creazzo TL. Temperature-sensitive TREK currents contribute to setting the resting membrane potential in embryonic atrial myocytes. J Physiol. 2008;586:3645–56. doi: 10.1113/jphysiol.2008.153395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel AJ, Honore E, Lesage F, et al. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–6. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 35.Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–83. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- 36.Snyders DJ. Structure and function of cardiac potassium channels. Cardiovasc Res. 1999;42:377–90. doi: 10.1016/s0008-6363(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 37.Tang QY, Qi Z, Naruse K, et al. Characterization of a functionally expressed stretch-activated BKca channel cloned from chick ventricular myocytes. J Membr Biol. 2003;196:185–200. doi: 10.1007/s00232-003-0637-8. [DOI] [PubMed] [Google Scholar]
- 38.Qi Z, Chi S, Su X, et al. Activation of a mechanosensitive BK channel by membrane stress created with amphipaths. Mol Membr Biol. 2005;22:519–27. doi: 10.1080/09687860500370703. [DOI] [PubMed] [Google Scholar]
- 39.Naruse K, Tang QY, Sokabe M. Stress-Axis Regulated Exon (STREX) in the C terminus of BK(Ca) channels is responsible for the stretch sensitivity. Biochem Biophys Res Commun. 2009;385:634–9. doi: 10.1016/j.bbrc.2009.05.105. [DOI] [PubMed] [Google Scholar]
- 40.Hammami S, Willumsen NJ, Olsen HL, et al. Cell volume and membrane stretch independently control K+ channel activity. J Physiol. 2009;587:2225–31. doi: 10.1113/jphysiol.2008.163550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue R, Jian Z, Kawarabayashi Y. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther. 2009;123:371–85. doi: 10.1016/j.pharmthera.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H, Murakami M, Ohba T, et al. The pathological role of transient receptor potential channels in heart disease. Circ J. 2009;73:419–27. doi: 10.1253/circj.cj-08-1153. [DOI] [PubMed] [Google Scholar]
- 43.Inoue R, Jensen LJ, Shi J, et al. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–31. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 44.Huang H, Wang W, Liu P, et al. TRPC1 expression and distribution in rat hearts. Eur J Histochem. 2009;53:e26. doi: 10.4081/ejh.2009.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunert-Keil C, Bisping F, Kruger J, et al. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. Bmc Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maroto R, Raso A, Wood TG, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–85. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 47.Spassova MA, Hewavitharana T, Xu W, et al. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA. 2006;103:16586–91. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, El Zein L, Kruse M, et al. Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet. 2012;3:374–85. doi: 10.1161/CIRCGENETICS.109.930867. [DOI] [PubMed] [Google Scholar]
- 49.Iwata Y, Katanosaka Y, Arai Y, et al. A novel mechanism of myocyte degeneration involving the Ca2+-permeable growth factor-regulated channel. J Cell Biol. 2003;161:957–67. doi: 10.1083/jcb.200301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guinamard R, Chatelier A, Demion M, et al. Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol. 2004;558:75–83. doi: 10.1113/jphysiol.2004.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senatore S, Rami ReddyV, Semeriva M, et al. Response to mechanical stress is mediated by the TRPA channel painless in the drosophila heart. PLoS Genet. 2010;6:e1001088. doi: 10.1371/journal.pgen.1001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ju YK, Chu Y, Chaulet H, et al. Store-operated Ca2+ influx and expression of TRPC genes in mouse sinoatrial node. Circ Res. 2007;100:1605–14. doi: 10.1161/CIRCRESAHA.107.152181. [DOI] [PubMed] [Google Scholar]
- 53.Quick K, Zhao J, Eijkelkamp N, et al. TRPC3 and TRPC6 are essential for normal mechanotransduction in subsets of sensory neurons and cochlear hair cells. Open Biol. 2012;2:120068. doi: 10.1098/rsob.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mochizuki T, Sokabe T, Araki I, et al. The TRPV4 cation channel mediates stretch-evoked Ca2+ influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21257–64. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mergler S, Valtink M, Taetz K, et al. Characterization of transient receptor potential vanilloid channel 4 (TRPV4) in human corneal endothelial cells. Exp Eye Res. 2011;93:710–9. doi: 10.1016/j.exer.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 56.Thodeti CK, Matthews B, Ravi A, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123–30. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demion M, Bois P, Launay P, et al. TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res. 2007;73:531–8. doi: 10.1016/j.cardiores.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 58.Guinamard R, Demion M, Magaud C, et al. Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension. 2006;48:587–94. doi: 10.1161/01.HYP.0000237864.65019.a5. [DOI] [PubMed] [Google Scholar]
- 59.Du J, Xie J, Zhang Z, et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lyford GL, Strege PR, Shepard A, et al. alpha(1C) (Ca(v)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol. 2002;283:C1001–C8. doi: 10.1152/ajpcell.00140.2002. [DOI] [PubMed] [Google Scholar]
- 61.Beyder A, Rae JL, Bernard C, et al. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J Physiol. 2010;588:4969–85. doi: 10.1113/jphysiol.2010.199034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris CE, Juranka PF. Nav channel mechanosensitivity: activation and inactivation accelerate reversibly with stretch. Biophys J. 2007;93:822–33. doi: 10.1529/biophysj.106.101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang JA, Lin W, Morris T, et al. Membrane trauma and Na+ leak from Nav1.6 channels. Am J Physiol Cell Physiol. 2009;297:C823–34. doi: 10.1152/ajpcell.00505.2008. [DOI] [PubMed] [Google Scholar]
- 64.Nazir SA, Lab MJ. Mechanoelectric feedback in the atrium of the isolated guinea-pig heart. Cardiovasc Res. 1996;32:112–9. [PubMed] [Google Scholar]
- 65.Kruse M, Schulze-Bahr E, Corfield V, et al. Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest. 2009;119:2737–44. doi: 10.1172/JCI38292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stallmeyer B, Zumhagen S, Denjoy I, et al. Mutational spectrum in the Ca(2+)–activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat. 2012;33:109–17. doi: 10.1002/humu.21599. [DOI] [PubMed] [Google Scholar]
- 67.Simard C, Salle L, Rouet R, et al. Transient receptor potential melastatin 4 inhibitor 9-phenanthrol abolishes arrhythmias induced by hypoxia and re-oxygenation in mouse ventricle. Br J Pharmacol. 2012;165:2354–64. doi: 10.1111/j.1476-5381.2011.01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathar I, Vennekens R, Meissner M, et al. Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest. 2010;120:3267–79. doi: 10.1172/JCI41348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guinamard R, Bois P. Involvement of transient receptor potential proteins in cardiac hypertrophy. Biochim Biophys Acta. 2007;1772:885–94. doi: 10.1016/j.bbadis.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 70.Wu X, Eder P, Chang B, et al. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA. 2010;107:7000–5. doi: 10.1073/pnas.1001825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seth M, Zhang ZS, Mao L, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–30. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuwahara K, Nakao K. New molecular mechanisms for cardiovascular disease: transcriptional pathways and novel therapeutic targets in heart failure. J Pharmacol Sci. 2011;116:337–42. doi: 10.1254/jphs.10r28fm. [DOI] [PubMed] [Google Scholar]
- 73.Nishida M, Onohara N, Sato Y, et al. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282:23117–28. doi: 10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- 74.Choudhary R, Baker KM, Pan J. All-trans retinoic acid prevents angiotensin II- and mechanical stretch-induced reactive oxygen species generation and cardiomyocyte apoptosis. J Cell Physiol. 2008;215:172–81. doi: 10.1002/jcp.21297. [DOI] [PubMed] [Google Scholar]
- 75.Kuwahara K, Wang Y, McAnally J, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–26. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Minamino T. Cardioprotection from ischemia/reperfusion injury: basic and translational research. Circ J. 2012;76:1074–82. doi: 10.1253/circj.cj-12-0132. [DOI] [PubMed] [Google Scholar]
- 77.Ovize M, Kloner RA, Przyklenk K. Stretch preconditions canine myocardium. Am J Physiol. 1994;266:H137–46. doi: 10.1152/ajpheart.1994.266.1.H137. [DOI] [PubMed] [Google Scholar]
- 78.Gysembergh A, Margonari H, Loufoua J, et al. Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Physiol. 1998;274:H955–64. doi: 10.1152/ajpheart.1998.274.3.H955. [DOI] [PubMed] [Google Scholar]
- 79.Mosca SM. Cardioprotective effects of stretch are mediated by activation of sarcolemmal, not mitochondrial, ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol. 2007;293:H1007–12. doi: 10.1152/ajpheart.00051.2007. [DOI] [PubMed] [Google Scholar]
- 80.Diaz RJ, Hinek A, Wilson GJ. Direct evidence of chloride ion efflux in ischaemic and pharmacological preconditioning of cultured cardiomyocytes. Cardiovasc Res. 2010;87:545–51. doi: 10.1093/cvr/cvq084. [DOI] [PubMed] [Google Scholar]
- 81.Jie X, Gurev V, Trayanova N. Mechanisms of mechanically induced spontaneous arrhythmias in acute regional ischemia. Circ Res. 2010;106:185–U381. doi: 10.1161/CIRCRESAHA.109.210864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhou R, Hang P, Zhu W, et al. Whole genome network analysis of ion channels and connexins in myocardial infarction. Cell Physiol Biochem. 2011;27:299–304. doi: 10.1159/000327956. [DOI] [PubMed] [Google Scholar]
- 83.Majkut SF, Discher DE. Cardiomyocytes from late embryos and neonates do optimal work and striate best on substrates with tissue-level elasticity: metrics and mathematics. Biomech Model Mechanobiol. 2012;11:1219–25. doi: 10.1007/s10237-012-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bajaj P, Tang X, Saif TA, et al. Stiffness of the substrate influences the phenotype of embryonic chicken cardiac myocytes. J Biomed Mater Res A. 2010;95:1261–9. doi: 10.1002/jbm.a.32951. [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez AG, Han SJ, Regnier M, et al. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys J. 2012;101:2455–64. doi: 10.1016/j.bpj.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 87.Salameh A, Dhein S. Effects of mechanical forces and stretch on intercellular gap junction coupling. Biochim Biophys Acta. 2013;1818:147–56. doi: 10.1016/j.bbamem.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 88.Salameh A, Wustmann A, Karl S, et al. Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res. 2010;106:1592–602. doi: 10.1161/CIRCRESAHA.109.214429. [DOI] [PubMed] [Google Scholar]
- 89.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation. 2010;17:164–78. doi: 10.1111/j.1549-8719.2010.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sachinidis A, Fleischmann BK, Kolossov E, et al. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc Res. 2003;58:278–91. doi: 10.1016/s0008-6363(03)00248-7. [DOI] [PubMed] [Google Scholar]
- 91.Zwi L, Caspi O, Arbel G, et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–23. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 92.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]


