Abstract
Cell-based tissue repair of the tooth and – tooth-supporting – periodontal ligament (PDL) is a new attractive approach that complements traditional restorative or surgical techniques for replacement of injured or pathologically damaged tissues. In such therapeutic approaches, stem cells and/or progenitor cells are manipulated in vitro and administered to patients as living and dynamic biological agents. In this review, we discuss the clonogenic potential of human dental and periodontal tissues such as the dental pulp and the PDL and their potential for tooth and periodontal repair and/or regeneration. We propose novel therapeutic approaches using stem cells or progenitor cells, which are targeted to regenerate the lost dental or periodontal tissue.
Keywords: tooth, odontoblast, periodontal ligament, stem cells, dental pulp, implants
Introduction
Our understanding of tooth development and biology of tooth diseases has tremendously advanced in the past two decades. Tooth pathology occurs mainly as a result of periodontal disease or carious lesions. Recent insights on the reparative capability of periodontium and dental pulp in conjunction with progress in stem cell biology, molecular biology and material science will enable us to develop novel therapies using engineered biological compounds and cell based therapeutics. In order to achieve a dental engineering therapy it is necessary to address all the cells and tissues involved in the formation, maintenance and repair of the tooth. Resembling other epithelial appendages, the tooth develops from a series of epithelial/mesenchymal interactions. Once formed the mesenchymal components of the tooth (dentine and periodontal ligament [PDL]) persist and are capable of only limited repair in response to injury. Here we will explore how tissue engineering can take advantage of these repair processes enhancing them and achieve artificial but biological tooth repair.
The need for tooth tissue engineering
One of the unwanted effects of increase longevity in the population is the increase of dentition decay. By the end of last century nearly 25% of the US population aged 65 to 75 years old had lost their natural teeth. This problem has a substantial effect in the population’s quality of life [1]. Preventive dental care reduces the setback of dental decay, but the problem persists. The only solution to tooth perish has been restorative prosthetics in the form of implants. Implant technology is indeed ancient, as the earliest known dental prosthetics dates back to 2500 BC in Egypt and the first known tooth replacement was documented from the Mayan culture in ad 600 [2].
Despite the long history of dental implants there are still limitations in functionality and longevity of the implants mainly due to alveolar bone loss. The tooth organ interacts actively with the alveolar bone through the PDL. Implants lack the plasticity and the biological interactions with the bone that the natural tooth has. PDL biology and bioengineering have advanced tremendously addressing the problems with periodontal diseases and have attempted to alleviate the tooth implants side effects [3].
The hard tissues of the tooth’s crown provide a barrier against bacteria. When a traumatic injury or a carious lesion breaks down this barrier, repair takes place to prevent invasion of the pulp chamber by bacteria. The capacity for pulp cells to resist and repair injuries is fundamental for the maintenance of tooth integrity and homeostasis. In the adult pulp, cell division and the secretory activity of odontoblasts are limited [4], but these processes may be re-activated after injury. In the case of severe tooth lesions the spontaneous regenerative power of the periodontium or the dental pulp is often insufficient resulting in tooth lost. In these cases tissue engineering and regenerative medicine could find indication.
Cellular components and development of tooth
The usage of stem cells systems as a tool for tissue engineering has great potential. Stem cell research has resulted in many clinical applications. Examples of cell based therapies include repair of skin [5], bone [6, 7], articular cartilage [8], cardiac tissues [9, 10] and neuronal tissue in Parkinson’s disease [11, 12]. Through a series of epithelial–mesenchymal interactions, teeth share similar patterns of gene expression and morphological events with the early stages of other epithelial appendages like lung, hair and breast. The epithelial–mesenchymal interactions in tooth are regulated by bone morphogenetic protein (BMP)-2, BMP-4 and Midkine [13–16], whereas fibroblast growth factors (FGFs) are involved in cell proliferation and regulation of specific target genes [17–19]. During tooth initiation and morphogenesis Wnt3, Wnt7b, Wnt10a and Wnt10b in conjunction with sonic hedgehog (SHH) regulate cell proliferation, migration and differentiation [20, 21]. From all these molecules, BMP4 and FGF8 constitute essential early oral epithelial signals that have a crucial role in activating specific homeobox genes in the underlying mesenchyme. It has been proposed that these two molecules could control tooth patterning in rodents: BMP4 directs the shape of incisors and FGF8 the shape of molars [22]. The mesenchyme of the developing incisors expresses a specific complement of genes (Msx1, Msx2) regulated by the influence of BMP4 from the epithelium. In the molars the mesenchyme posses a different complement of genes (Dlx1, Dlx2, Barx1) regulated by FGF8 also from the overlying epithelium. The specific complement of these transcription factors dictates the development of the tooth germs towards an incisorform or molariform shape [23]. Based on the restricted expression domains of signalling molecules and homeobox genes in the cranial neural crest cell-derived mesenchyme of the maxilla and mandible, a ‘co-operative genetic interaction’ model has been proposed [22]. The presence of all these transcription factors appears to be required for a transcriptional program responsible for the characteristic growth and morphology of teeth [23]. The molecular mechanisms governing these events have been extensively reviewed in detail [24–28]. These molecular mechanisms can be used as bases to establish possible mechanisms needed for tooth regeneration [29–31]. In the final stages of tooth development, enamel and dentine form as the outcome of the interactions of the mentioned molecules resulting in the differentiation of the oral ectoderm and cranial ectomesenchyme, respectively. These interactions progressively lead to transformation of the tooth germs into complex mineralized structures. Mesenchymal cells form the dental follicle and dental pulp, and the oral ectoderm form the inner dental epithelium. In terms of mineralized tissue, pulp mesenchymal cells differentiate into odontoblasts and inner dental epithelium into ameloblasts. Odontoblasts are the cells responsible for the formation of mineralized dentine, whilst ameloblasts are responsible for the formation of enamel. Once the mineralization of the crown is completed the tooth starts to erupt in the oral cavity, while the root continues to develop. Hertwing’s epithelial root sheath, a derivative from the outer dental epithelium and the inner dental epithelium will spear head the growth of the root [32–34]. Root development will be accomplished together with the organization of innervation, vascularization and anchoring to the surrounding alveolar bone. This anchoring process will be accomplished mainly by the relationship of three main tissues in the periodontium: cementum, alveolar bone and PDL (Fig. 1). PDL contains a great variety of cells and extracellular matrix. The cellular components include osteoblasts, fibroblasts cementoblasts, osteoclasts, cementoclasts, epithelial rests of Malassez and endothelial cells as well as several connective tissue cells [35].
fig 1.
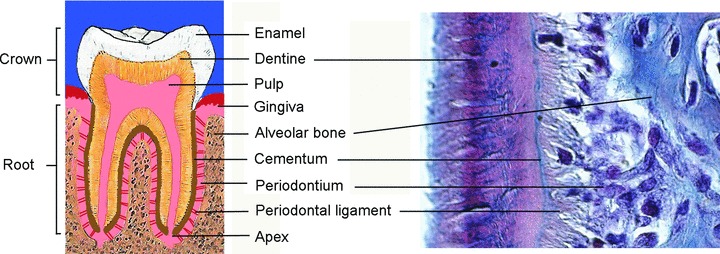
Schematic representation of a human molar and histology of the dental root area showing the various dental tissues.
Bases for tooth tissue engineering
In the natural course of the life of the tooth crown, the mineralized tissues may be damaged, thus jeopardizing the integrity of the tooth. In the case of dentine this damage can be repaired naturally. Dentine repair can be achieved from surviving odontoblasts (reactionary dentinogenesis) or, in the case of severe damage cells of the dental pulp having stem cells properties are re-activated to form new odontoblasts that will replace the apoptotic odontoblasts and repair the injury. Signalling molecules that are expressed by pulp cells (e.g. BMPs, transforming growth factor [TGF]-β, insulin-like growth factor [IGF]-1) play an important role in the pulp healing during dentine repair. Several studies have shown the importance of these molecules in dentine repair [36–39]. The damage caused to the enamel cannot be repaired naturally because the cells responsible for its formation disappear after the enamel is fully formed. Enamel is the hardest mineralized tissue of the body and acts as a biological barrier that protects the pulp-dentine complex. Enamel interacts with dentine through the dentine/enamel junction. The replacement of enamel with biomaterials, ceramics and precious metals has been well documented and is of common practice in clinic. PDL repair and regeneration has been extensively studied. The complexity of PDL cellular components, extracellular matrix and tissues interactions requires a tight control in the cellular deposition. Cell-occlusive barriers that restrict the repopulation of epithelial and connective tissue in favour of PDL cells and cementoblasts have been used since the mid-1970s [40]. These barriers range from cellulose in earlier procedures to a more convenient usage of synthetic absorbable material. These materials have been used in conjunction with biological factors like BMP2 to enhance the regeneration of bone [41].
The patient as a recipient and as the source of cells
The outcome of all tissue engineering approaches using autologous cell preparations is influenced by the patient selection because the patient is at the same time the source of the cells and the recipient of his/her own treatment.
The identification of a proper indication and the selection of patients are crucial for the evaluation of the efficacy of a treatment. Patient-related factors, such as age, body weight, general health status, size and site of the lesion may influence the outcome of stem cell-based treatments. Tooth damage is the result of different mechanisms of injury combined with the incapacity of intrinsic dental tissue repair. Because the reasons for this can be different in individual patients, it is conceivable that there is no ideal approach to dental tissue repair. However, as our understanding of dental damage and repair mechanisms advance, we might be able to adapt appropriate and more personalized treatment strategies.
In autologous cell-based approaches the patient is also the source of the therapeutic preparation. Consequently, patient-related factors may influence the quality and properties of the therapeutic preparation. Factors include the age of the patient and the healthy or pathological condition of the dental pulp and PDL at the moment of operation. The influence of these factors on the efficacy of cell preparations for cell-based dental treatments has not been investigated exhaustively [42]. Although the entire procedure of stem cell isolation, expansion and preparation is perfectly standardized, cell preparations from every individual patient have to be considered as single/special batches and be quality controlled accordingly. The amount and complexity of quality controls make these procedures expensive, thereby limiting their routine applicability. Nonetheless, autologous cell therapies offer the advantages of minimal risk of disease transmission and of immunological rejection.
Dental stem cell based tissue engineering
The cells represent the active component of cell-based therapies. Hence, besides the patient-related factors discussed above, any cell product will be affected also by the preparation technology. With regard to dental cells, various cell types are needed to form a tooth. In order to tissue engineer a tooth, which is a complex organ, these cells need to come together in a spatially and temporally controlled manner. Because the mature tooth cannot repair or make de novo enamel we will focus on the bioengineering of dentine from pulp and the PDL complex including cementum and alveolar bone. Dental stem cells, originally derived from the ectomesenchyme, are considered a new source of human adult stem cells for regenerative medicine. These can be obtained from either shed primary teeth or extracted permanent teeth (Fig. 2). These stem cells can be used to perform autologous cell replacement. The source of the cells is of most importance and the possibility of harvesting the needed cells from the patient makes this process very attractive. A key question that needs to be addressed is if these dental cells are indeed stem cells. Different studies have provided evidence that dental pulp and PDL cells have mesenchymal stem cell features, based on their ability to differentiate into cartilage, bone, fat, muscle, muscle and neural tissue [43]. Apart from the dental pulp and PDL, mesenchymal stem cells have a diverse distribution in vivo as they can be derived from most, if not all, connective tissues including bone marrow, adipose, periosteum, synovial membrane, skeletal muscle, dermis, pericytes, blood, trabecular bone, human umbilical cord and lung [44].
fig 2.
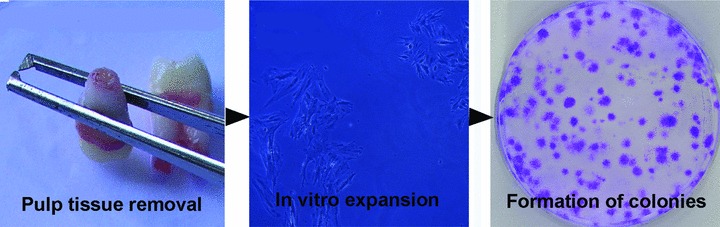
The various steps leading to the formation of single cell colonies derived from human dental pulp.
Dental pulp stem cells (DPSCs)
The pulp has been long recognized as an organ with good reparative and regenerative capacity. Cells present in the dental pulp are capable of terminally differentiate into odontoblast-like cells to form reparative dentine. Gene therapy approaches have been tested and demonstrated the higher odontogenic differentiation ability of pulp cells transfected with growth/differentiation factor 11 [45]. The use of the synthetic glucocorticoid dexamethasone and growth factors like BMP2 to induce differentiation of pulp cells into odontoblast-like cells has been also examined [46, 47]. Adult dental pulp and the pulp of exfoliated deciduous human teeth have also been identified as a potential stem cell source. DPSCs exhibit a multipotent character and the potential to differentiate into chondrocytes, adipocytes [48], osteoblasts/osteocytes [49, 50], myocytes [49], neuronal cells [51] and cardiomyocytes [52]. DPSCs were firstly isolated from the human pulp tissue approximately 10 years ago [53]. DPSCs were isolated from human adult third molars with enzyme treatment of pulp tissues [53, 54]. Pulp tissue from exfoliated deciduous human teeth was also used as a source of DPSCs [55]. These studies demonstrated that the dental pulp contains self-renewing, highly proliferative multipotent stem cells. It has been suggested that these cells reside within perivascular niches [56, 57]. DPSCs are able to form a vascularized pulp-like tissue in vivo, which is surrounded by a layer of odontoblast-like cells [53]. A special feature of DPSCs is their potential for odontoblastic differentiation, as characterized by polarized cell bodies and accumulation of mineralized nodules [58, 59]. Further studies showed that DPSCs do express several stem cell markers including c-kit, CD34, STRO-1 and CD146 [60, 61]. However, the detection of other specific stem cell markers expressed by dental pulp cells is under investigation. DPSCs have the potential to form bone-like tissues when transplanted into immunocompromised animals [62]. Several DPSCs populations can differentiate in vitro into adipocytes, neuronal-like cells and cardiomyocytes as defined by cell morphology and the expression of respective gene markers [57, 63–65]. DPSCs can also undergo osteogenic, chondrogenic and myogenic differentiation in vitro [50, 66–68]. Recently, dental stem cells were shown to be reprogrammed into induced pluripotent stem cells (iPS) with a higher rate compared to other cell types of human origin tried so far [69]. Furthermore, in vivo experimental evidence in animals suggests that DPSCs could provide a novel alternative cell population for repair and/or regeneration of the heart [52], bone [62, 70], muscles [71], brain [51] and tooth [72–74]. Of note, last year the first clinical application for alveolar bone reconstruction using DPSCs was successfully carried out in a patient [75].
The clinical application of DPSCs in regeneration of the pulp/dentin complex
Dental caries is a very common oral disease. It is essentially an infection of the mineral tissues of the tooth, which eventually reaches the dental pulp of the tooth causing inflammation and potentially tooth loss. The dental pulp has important functions to provide nutrients, oxygen and nerve supply to the tooth. In addition, a crucial property of the dental pulp is to foster cells of the immune system that would tackle the infection, and to produce reactionary or reparative dentin formation in response to external stimuli, including bacterial invasion [76–78]. In clinical terms, regeneration of pulp is not yet a routine treatment modality in endodontics. Instead, entire pulp amputation is the choice of treatment, which is followed by the mechanical and chemical disinfection pulp cavity and its filling with an artificial material. Despite that the tooth is saved in its functional position, it is not vital any further and could not fulfil to the full extent its role. The regeneration of the dentine-pulp interface would be the ideal therapy, aiming to re-build a dentinal bridge at the affected site, sealing off the infection and other external stimuli. At present this is not yet achievable. There have been approaches to use growth factors with the appropriate delivery systems, or other regenerative material, as agents for immediate capping of the exposed pulp [79–83]. However, such situations require that the pulp has been exposed to a minimal extent, and inflammation is limited. This is hardly the case in chronic pulpitis that is the most frequent development of deep carious lesions.
If regeneration of the dentine-pulp complex can be achieved, this would require the re-vascularization and re-innervation of the pulp and, importantly, the deposition of new dentine by odontoblasts. The use of DPSCs holds a strong promise in this respect, as they can be isolated from the adult dental pulp tissue [53]. When in contact with dentin in vitro, DPSCs can acquire odontoblast-like cell morphology with a polarized cell body and a cell process extending into the existing dentinal tubules [54]. These can be differentiated into odontoblasts that will form dentine [58, 59, 84], endothelial cells that would support the re-vascularization [67, 85] and neurons which could support the re-innervation of the regenerated pulp tissue [63]. Transplantation of DPSCs in mice can regenerate pulp-dentine tissue complexes, with dentinal tubule-like structures [53, 86, 87]. A recent in vivo beagle dog study also demonstrated that a combination of calcium hydroxyapatite and DPSCs was able to regenerate dentin [88]. Although these biological principles prove that pulp regeneration can be achieved via the DPSCs, further studies are required to investigate the potential clinical application in human beings.
Periodontal tissues as a source and niche for stem cells
When considering dental stem cells and their niches, one has also to include the tissues surrounding the teeth. These are termed ‘periodontal tissues’ or periodontium, and consist of the gingiva, the cementum of the tooth root, the surrounding alveolar bone, as well as the interconnecting PDL.
Although the PDL is a physically small tissue, it is unique among the various ligament and tendon systems of the body, in the sense that it is the only soft tissue to span between two distinct hard tissues, namely the cementum of the root and the alveolar bone [89]. This tissue acts as a suspension for the tooth, adapting to the mechanical loads of the oral cavity. Collectively the periodontal tissues are specifically tailored to support the tooth in its functional position. The pathological damage of the periodontal tissues due to a very common inflammatory condition is termed periodontitis. This disease may eventually lead to exfoliation of the adult tooth, due to lack of supporting tissue. One of the major challenges in dentistry has been the regeneration of the disease-affected periodontal tissues, in a manner that recapitulates embryonic tooth development.
Early observations indicated that the periodontal tissues have a regenerative capacity and that multipotent progenitor cells may exist within the periodontium [40]. Extensive studies in experimental animal models have depleted the various periodontal tissues and investigated the regenerative capacities of the remaining tissues each time [90, 91]. It was concluded that the PDL tissue has the regenerative capacity and can form not only the interconnecting collagen tissue, but also alveolar bone and cementum [92–94]. From these early observations, it was concluded that the PDL tissue contains progenitor cells for fibroblasts, osteoblasts and cementoblasts [95]. Several studies demonstrated that PDL from mouse molars contains a slowly dividing population of progenitor cells that is located in perivascular sites [96, 97], as well as in the periosteal and endosteal spaces of the alveolar bone [98]. It was further demonstrated that PDL contains renewable and differentiated populations of cells [99, 100]. Similarly to the other tissues, PDL cells form a heterogeneous cell population composed predominantly of fibroblasts, but also of stem cells that exhibit various developmental potentials. Recent advancement of technologies identifying and characterizing adult stem cells has led to the first substantial evidence that a putative stem-cell population exists within PDL. PDL stem cells (PDLSCs) were positive for the stem cell markers STRO-1 and CD146 and were able to differentiate into cementoblasts, adipocytes and osteoblasts [101]. After culture expansion, these human cells were transplanted into immunocompromised mice and were shown to contribute to periodontal tissue repair forming a PDL/cementum-like structure. Nevertheless, whether this tissue can function to the full extent as a normal PDL tissue is still under examination. Another study has shown that cryopreserved PDL can retain stem cells-like characteristics, suggesting that long term storage is possible [102]. In vitro studies have also found that PDLSCs were capable of differentiating into mesodermal (i.e. adipocytes, osteoblasts, chondrocytes), ectodermal (i.e. neurons), and endodermal (i.e. hepatocytes) lineages [103]. In addition, rat PDLSCs have the capacity to differentiate into vascular cells forming blood vessel-like structures [104]. These findings suggest that the PDL constitutes an important stem cell source not only for the regeneration of periodontal tissues [105], but also for the regeneration of other tissues and organs. Despite the strong promise that PDL holds for regenerative clinical dentistry, there is a however need for further characterization of the PDLSCs.
Gingiva is the oral mucosal tissue that surrounds the teeth and covers the alveolar bone. It functions as a protective mechanical barrier for the tooth supporting tissues and as a biological barrier conferring distinct immunity to oral infection. It is composed of an epithelial layer with an underlying vasculated connective tissue. As the epithelial layer has the capacity for continuous renewal, it is anticipated that this tissue could be a source of stem cells. Cells with mesenchymal stem cell properties have recently been isolated from gingival tissues and characterized [106–108]. These cells have unique immunomodulatory functions, clonogenicity, as well as self-renewal and multi-potent differentiation capacities. Very recently gingival fibroblasts have been evaluated as source of iPS cells. These cells gave rise to high-quality iPS cells suggested that gingival fibroblasts could be promising for cellular reprogramming and pluripotency for future clinical applications [109].
The root cementum is an avascular and unnerved mineralized tissue with ultrastructural similarity to bone that covers the entire root surface. It is the interface between the dentinal tissue and the PDL and contributes to periodontal tissue repair and regeneration after damage. The organic extracellular matrix of cementum contains proteins that selectively enhance the attachment and proliferation of cell populations residing within the PDL space [110–114]. Human cementum-derived cells have been isolated, cloned and characterized in vitro and in vivo [115]. When transplanted subcutaneously in immunodeficient mice, the mineralized matrix produced by these cells exhibited several features identical to cementum, and differed from the mineralized matrix produced by human bone marrow stromal cells [116].
The alveolar bone is a part of the periodontal tissues, functioning as an anchorage of the tooth root to the alveoli and resorbing the forces generated by the function of mastication. Progenitor cells which are responsible for alveolar bone formation lay in the periosteal region, the PDL or around the blood vessels. Alveolar bone marrow is considered as a useful and easily accessible source of progenitor cells, as they have similar osteogenic potential to those derived from the iliac crest [117]. The periosteum is also considered as a suitable cell source for bone regeneration [118–121].
Past, current and future approaches in periodontal regeneration
Periodontal disease is perhaps the most common infectious disease in human beings caused by bacteria present in the oral cavity, which attach on the teeth and cause inflammation of the periodontal tissues. Epidemiological studies show that approximately 7–13% of the global population is at high risk of developing severe forms of destructive periodontitis [122], posing a tremendous burden to health care. This creates a need to develop new therapies directed at the attenuation of the disease and regeneration of the lost tissues, including the PDL and alveolar bone. Periodontal regeneration is defined as reproduction or reconstruction of a functional attachment apparatus consisting of new cementum, alveolar bone and PDL, on a root surface that was previously exposed due to progression of periodontitis.
Early regeneration approaches have focused on providing appropriate conditions for wound healing. These included a range of surgical procedures along with use of bone grafts as tissue substitutes, barrier membranes to prevent the unfavourable tissues from the healing area [92, 123, 124], and more lately, growth factors as means to induce the wound healing capacity of the remaining tissue. One of the very early approaches for regeneration of the lost periodontal tissues was mechanical or surgical removal of the diseased tissues [125, 126], allowing establishment of health via reduction of inflammation. Histological studies in both human beings and animals showed that these approaches do not ensure a predictable outcome of periodontal regeneration and often result in healing with epithelial lining rather than new tissue formation [127, 128]. At a later stage the surgical therapy was combined with the placement of bone grafts in the defect, aiming to stimulating periodontal regeneration. A wide range of bone grafting materials has been applied including autografts (such as intra-oral or iliac crest), and commercially available allografts (i.e. freeze-dried bone), xenografts and alloplasts (i.e. hydroxyapatite, β-tricalcium phosphate) [93, 129, 130]. These materials were expected to fill the space and to either contain the appropriate cell source, or provide the mechanical scaffold for the surrounding cells to repopulate the healing area.
To date, periodontal regeneration is considered to be biologically possible but clinically unpredictable. As earlier suggested, the cell type that repopulates the root surface after the therapy determines the nature of healing. In this respect, PDLSCs may hold a strong promise for improvement of periodontal regeneration approaches. Recent studies have applied populations of PDLSCs in animal models with improved success outcome for periodontal regeneration [101, 105]. Although similar human studies are not yet available, such cell-based therapy approaches may become visible in the future of periodontal regeneration.
Molecular mechanisms and factors regulating regeneration of periodontal tissues
Predictable periodontal tissue regeneration needs to ideally recapitulate embryonic development, following similar morphogenetic gene expression patterns. Although it is evident that there are many factors that may prove important for controlling periodontal tissue formation, this section focuses on the growth factors and enamel matrix proteins with a proven role in controlling the behaviour of cells within the periodontal tissues.
The principle of therapeutic application of growth factors for the restoration of damaged tissues is based on adult tissue regeneration by mimicking the processes of embryonic and post-natal development [131–134]. The most studied growth factors for periodontal regeneration have been platelet-derived growth factor (PDGF), epidermal growth factor (EGF), FGF, IGF and different BMPs. The PDGF members has been extensively evaluated in both in vitro and in vivo [135–138]. These studies concluded that PDGF alone or in combination with the other growth factors seem to have a positive effect on periodontal healing and regeneration.
Another promising group of growth factors is the BMP family, consisting of members of the TGF-β superfamily. These secretory signal molecules have a variety of functions during morphogenesis and cell differentiation [139–141]. A wide body of evidence from periodontal regeneration studies indicate that BMPs are capable of inducing formation of new alveolar bone and cementum [141–143]. However, because classic BMPs induce the differentiation of cells along an osteogenic pathway, ankylosis can be a frequent side-effect, characterized by the absence of the mediating PDL between the alveolar bone and cementum [144]. Hence, BMPs have not yet been approved for periodontal applications and further experimentation is needed for improving the capacity for periodontal regeneration.
Amelogenins are proteins secreted by ameloblasts and are considered to play a major role in regulating formation of enamel [145]. The rationale for the potential use of enamel proteins in periodontal tissue regeneration therapies is justified by their presence in initial cementum formation, during normal development of the tooth attachment apparatus [146, 147]. The commercially available enamel matrix protein product namely, Emdogain (Straumann AG, Basel, Switzerland), has been in the market for over a decade now [148, 149]. This is a purified acid extract of proteins containing predominantly amelogenin from pig enamel matrix. A large number of studies have investigated the mechanisms of action and clinical efficiency of Emdogain [131, 146, 150–153]. Still, despite the encouraging clinical outcome, the mechanism of action of Emdogain is not clear. It has been suggested that its capacity to initiate the processes of periodontal regeneration relies on the recruitment of cementoblasts onto the root surface, hence stimulating the formation of root cementum. Several in vitro studies suggest that Emdogain could act as a signalling molecule for epithelial–mesenchymal interactions, which can regulate the activity of follicle cells, PDL cells, odontoblasts, gingival fibroblasts and cementoblasts [142, 154–159]. Along with clinical and experimental animal confirming the clinical efficiency of Emdogain in periodontal regeneration procedures [158, 160, 161], recent systematic reviews are also in support of these findings [162–164].
Taken together, these molecular factors are likely to contribute to the regeneration of the periodontal tissues by targeting periodontal progenitors that reside within putative stem cell niches of the periodontium. The potential effects include proliferation and differentiation along pathways parallel to tooth root development. Nevertheless, this should not be over-interpreted. One should consider the multiplicity of the cells and complexity of tissues within the periodontal environment, their responsiveness of cells to the type or dose of the growth factors used, as well as the previously diseased tissue substrate due to inflammation. Therefore, as much as an identical recapitulation of developmental events is desirable, it may be very difficult to achieve due to these reasons.
Further studies will be still needed to identify right cell populations (progenitor or stem cells), signalling molecules that control cell behaviour and scaffold material to act as carriers for the cells and stimulating molecules (i.e. bioreactors). Once these parameters are more clearly defined and the respective wound healing events are clarified, the clinical efficiency of suitable treatment modalities would need to be further assessed. Moreover, as the oral microenvironment is not aseptic, any periodontal regenerative activity involving differentiation of potent stem cells is likely face bacterial challenge. Therefore, it is suggested that any regenerative approach involving stem cells in the oral cavity would need to take this aspect under consideration, in order to achieve predictable and optimal therapeutic outcome.
Scaffolding and material science
The use of scaffolds in tooth repair has been extensively used and studied. Scaffolds are particularly useful when a 3D structure is needed (Fig. 3). The viability and cytotoxicity of 3D scaffolds need to be assessed. Dental pulp cells are viable and show signs of vitality when placed in biodegradable porous calcium polyphosphate scaffolds [165]. Biodegradable scaffolds are very useful because the aim of cell-based tooth tissue engineering should be to utilize cells that will differentiated in correct 3D field and eventually produce a permanent mineralized matrix. Studies using hydroxyapatite–tricalcium phosphate have shown that STRO-1+ dental pulp cells are able to differentiate into odontoblast-like cells producing mineralized matrix [166]. Recently new technologies on material science have made possible the printing of cells on to 3D structures and microenvironments to direct cell differentiation into specific fates [167, 168]. These emerging technologies on material science offer bio-printing as an alternative way of creating 3D structures useful for tissues engineering and should be closely follow.
fig 3.
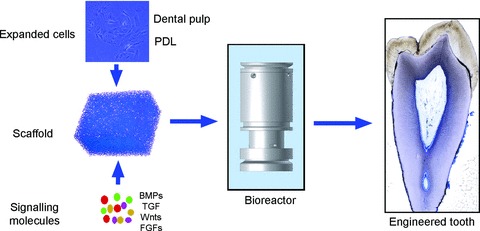
Tissue engineering using dental stem cells, scaffolds and signalling molecules. BMPs: bone morphogenetic proteins; FGFs: fibroblast growth factors; TGF: transforming growth factor.
Conclusions
Although the prospective of tooth tissue engineering is very attractive, we are far from performing routine clinical procedures. Despite the large amount of interest in this field, no clinical trials have been performed for dentine repair and very limited clinical applications are available in periodontal disease treatment. Cell based bioengineering and material sciences have to define conditions for manufacturing consistent and reproducible products, which are quality controlled for safety and efficacy. In addition, cell therapies are in their infancy and many issues need to be taking into account. The use of culture expanded cell populations needs to take into account the possibility of genetic and epigenetic instability, which could possibly result in malignant transformation. The paracrine effects, interactions with the host and immune response following cell transplantation also need to be taking into consideration. For example, the transplantation of bone marrow stem cells to cure diabetes mellitus in an animal model resulted in the reduction of hyperglycaemia by the regeneration of the recipient’s own pancreatic cells. This regeneration was initiated in response to the transplanted cells [169]. In the case of tooth engineering, the possibility of autologous cell replacement and the usage of cells naturally occurring in the site of injury may minimize the risk of side effects. In addition, a better understanding of the biology of tooth repair opens the exciting prospect to develop cell free approaches. The utilization of bioactive factors and biomaterials will support and enhance the intrinsic mechanisms of tooth repair.
Acknowledgments
N.B. was supported by a grant from the University of Zurich (Switzerland). C.D.B. is a Fellow of the Medical Research Council, UK.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Murray PE, Garcia-Godoy F. The outlook for implants and endodontics: a review of the tissue engineering strategies to create replacement teeth for patients. Dent Clin North Am. 2006;50:299–315. doi: 10.1016/j.cden.2005.11.009. , x. [DOI] [PubMed] [Google Scholar]
- 2.Ring ME. A thousand years of dental implants: a definitive history–part 2. Compend Contin Educ Dent. 1995;16:1132. , 4, 6 passim. [PubMed] [Google Scholar]
- 3.Taba M, Jin Q, Sugai JV, et al. Current concepts in periodontal bioengineering. Orthod Craniofac Res. 2005;8:292–302. doi: 10.1111/j.1601-6343.2005.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitsiadis TA, De Bari C, About I. Apoptosis in developmental and repair-related human tooth remodeling: a view from the inside. Exp Cell Res. 2008;314:869–77. doi: 10.1016/j.yexcr.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Naughton GK. From lab bench to market: critical issues in tissue engineering. Ann NY Acad Sci. 2002;961:372–85. doi: 10.1111/j.1749-6632.2002.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 6.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 7.Vacanti CA, Bonassar LJ, Vacanti MP, et al. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–4. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 8.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 9.Stamm C, Westphal B, Kleine HD, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–6. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 10.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 11.Bjorklund A. Cell therapy for Parkinson’s disease: problems and prospects. Novartis Found Symp. 2005;265:174–86. [PubMed] [Google Scholar]
- 12.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10:S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 13.Bluteau G, Luder HU, De Bari C, et al. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1–9. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- 14.Kratochwil K, Dull M, Farinas I, et al. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–94. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- 15.Nadiri A, Kuchler-Bopp S, Haikel Y, et al. Immunolocalization of BMP-2/-4, FGF-4, and WNT10b in the developing mouse first lower molar. J Histochem Cytochem. 2004;52:103–12. doi: 10.1177/002215540405200110. [DOI] [PubMed] [Google Scholar]
- 16.Vainio S, Karavanova I, Jowett A, et al. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 17.Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–33. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- 18.Kettunen P, Laurikkala J, Itaranta P, et al. Associations of FGF-3 and FGF-10 with signaling networks regulating tooth morphogenesis. Dev Dyn. 2000;219:322–32. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1062>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Kettunen P, Thesleff I. Expression and function of FGFs-4, -8, and -9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev Dyn. 1998;211:256–68. doi: 10.1002/(SICI)1097-0177(199803)211:3<256::AID-AJA7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Dassule HR, McMahon AP. Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol. 1998;202:215–27. doi: 10.1006/dbio.1998.8992. [DOI] [PubMed] [Google Scholar]
- 21.Khan M, Seppala M, Zoupa M, et al. Hedgehog pathway gene expression during early development of the molar tooth root in the mouse. Gene Expr Patterns. 2007;7:239–43. doi: 10.1016/j.modgep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Mitsiadis TA, Smith MM. How do genes make teeth to order through development. J Exp Zool B Mol Dev Evol. 2006;306:177–82. doi: 10.1002/jez.b.21104. [DOI] [PubMed] [Google Scholar]
- 23.Cobourne MT, Mitsiadis T. Neural crest cells and patterning of the mammalian dentition. J Exp Zool B Mol Dev Evol. 2006;306:251–60. doi: 10.1002/jez.b.21084. [DOI] [PubMed] [Google Scholar]
- 24.Caton J, Tucker AS. Current knowledge of tooth development: patterning and mineralization of the murine dentition. J Anat. 2009;214:502–15. doi: 10.1111/j.1469-7580.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thesleff I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647–8. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 26.Thesleff I. The genetic basis of tooth development and dental defects. Am J Med Genet A. 2006;140:2530–5. doi: 10.1002/ajmg.a.31360. [DOI] [PubMed] [Google Scholar]
- 27.Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–23. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 28.Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 29.Mitsiadis TA, Graf D. Cell fate determination during tooth development and regeneration. Birth Defects Res C Embryo Today. 2009;87:199–211. doi: 10.1002/bdrc.20160. [DOI] [PubMed] [Google Scholar]
- 30.Thesleff I, Jarvinen E, Suomalainen M. Affecting tooth morphology and renewal by fine-tuning the signals mediating cell and tissue interactions. Novartis Found Symp. 2007;284:142–53. doi: 10.1002/9780470319390.ch10. ; discussion 53–63. [DOI] [PubMed] [Google Scholar]
- 31.Thesleff I, Wang XP, Suomalainen M. Regulation of epithelial stem cells in tooth regeneration. C R Biol. 2007;330:561–4. doi: 10.1016/j.crvi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- 33.Sonoyama W, Seo BM, Yamaza T, et al. Human Hertwig’s epithelial root sheath cells play crucial roles in cementum formation. J Dent Res. 2007;86:594–9. doi: 10.1177/154405910708600703. [DOI] [PubMed] [Google Scholar]
- 34.Yokohama-Tamaki T, Ohshima H, Fujiwara N, et al. Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133:1359–66. doi: 10.1242/dev.02307. [DOI] [PubMed] [Google Scholar]
- 35.Mariotti A. The extracellular matrix of the periodontium: dynamic and interactive tissues. Periodontol 2000. 1993;3:39–63. doi: 10.1111/j.1600-0757.1993.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 36.Mitsiadis TA, Rahiotis C. Parallels between tooth development and repair: conserved molecular mechanisms following carious and dental injury. J Dent Res. 2004;83:896–902. doi: 10.1177/154405910408301202. [DOI] [PubMed] [Google Scholar]
- 37.Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair. Crit Rev Oral Biol Med. 2001;12:425–37. doi: 10.1177/10454411010120050501. [DOI] [PubMed] [Google Scholar]
- 38.Smith AJ, Matthews JB, Hall RC. Transforming growth factor-beta1 (TGF-beta1) in dentine matrix. Ligand activation and receptor expression. Eur J Oral Sci. 1998;106:179–84. doi: 10.1111/j.1600-0722.1998.tb02173.x. [DOI] [PubMed] [Google Scholar]
- 39.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 40.Melcher AH. On the repair potential of periodontal tissues. J Periodont. 1976;47:256–60. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 41.Lee YM, Nam SH, Seol YJ, et al. Enhanced bone augmentation by controlled release of recombinant human bone morphogenetic protein-2 from bioabsorbable membranes. J Periodont. 2003;74:865–72. doi: 10.1902/jop.2003.74.6.865. [DOI] [PubMed] [Google Scholar]
- 42.De Bari C, Pitzalis C, Dell’Accio F. Reparative medicine: from tissue engineering to joint surface regeneration. Regen Med. 2006;1:59–69. doi: 10.2217/17460751.1.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Karaoz E, Dogan BN, Aksoy A, et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol. 2010;133:95–112. doi: 10.1007/s00418-009-0646-5. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum AJ, Grande DA, Dines JS. The use of mesenchymal stem cells in tissue engineering: a global assessment. Organogenesis. 2008;4:23–7. doi: 10.4161/org.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakashima M, Iohara K, Ishikawa M, et al. Stimulation of reparative dentin formation by ex vivo gene therapy using dental pulp stem cells electrotransfected with growth/differentiation factor 11 (Gdf11) Human Gene Ther. 2004;15:1045–53. doi: 10.1089/hum.2004.15.1045. [DOI] [PubMed] [Google Scholar]
- 46.Alliot-Licht B, Bluteau G, Magne D, et al. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;321:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]
- 47.Iohara K, Nakashima M, Ito M, et al. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004;83:590–5. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- 48.Waddington RJ, Youde SJ, Lee CP, et al. Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs. 2009;189:268–74. doi: 10.1159/000151447. [DOI] [PubMed] [Google Scholar]
- 49.de Mendonca Costa A, Bueno DF, Martins MT, et al. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19:204–10. doi: 10.1097/scs.0b013e31815c8a54. [DOI] [PubMed] [Google Scholar]
- 50.Carinci F, Papaccio G, Laino G, et al. Comparison between genetic portraits of osteoblasts derived from primary cultures and osteoblasts obtained from human pulpar stem cells. J Craniofac Surg. 2008;19:616–25. doi: 10.1097/SCS.0b013e31816aabc8. ; discussion 26–7. [DOI] [PubMed] [Google Scholar]
- 51.Nosrat IV, Widenfalk J, Olson L, et al. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol. 2001;238:120–32. doi: 10.1006/dbio.2001.0400. [DOI] [PubMed] [Google Scholar]
- 52.Gandia C, Arminan A, Garcia-Verdugo JM, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–45. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 53.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang GT, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73. doi: 10.1016/j.joen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 57.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 58.About I, Bottero MJ, de Denato P, et al. Human dentin production in vitro. Exp Cell Res. 2000;258:33–41. doi: 10.1006/excr.2000.4909. [DOI] [PubMed] [Google Scholar]
- 59.Couble ML, Farges JC, Bleicher F, et al. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int. 2000;66:129–38. doi: 10.1007/pl00005833. [DOI] [PubMed] [Google Scholar]
- 60.Laino G, Graziano A, d’Aquino R, et al. An approachable human adult stem cell source for hard-tissue engineering. J Cell Physiol. 2006;206:693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- 61.Menicanin D, Bartold PM, Zannettino AC, et al. Identification of a common gene expression signature associated with immature clonal mesenchymal cell populations derived from bone marrow and dental tissues. Stem Cells Dev. 2010;19:1501–10. doi: 10.1089/scd.2009.0492. [DOI] [PubMed] [Google Scholar]
- 62.Graziano A, d’Aquino R, Laino G, et al. Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif. 2008;41:1–11. doi: 10.1111/j.1365-2184.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arthur A, Rychkov G, Shi S, et al. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787–95. doi: 10.1634/stemcells.2007-0979. [DOI] [PubMed] [Google Scholar]
- 64.Arminan A, Gandia C, Bartual M, et al. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18:907–18. doi: 10.1089/scd.2008.0292. [DOI] [PubMed] [Google Scholar]
- 65.Balic A, Aguila HL, Caimano MJ, et al. Characterization of stem and progenitor cells in dental pulps of the erupted and unerupted murine molars. Bone. 2010;46:1639–51. doi: 10.1016/j.bone.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laino G, d’Aquino R, Graziano A, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394–402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 67.d’Aquino R, Graziano A, Sampaolesi M, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–71. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 68.Zhang W, Walboomers XF, Shi S, et al. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–23. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]
- 69.Yan X, Qin H, Qu C, et al. iPS cells reprogrammed from mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev. 2009;19:469–80. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graziano A, d’Aquino R, Laino G, et al. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4:21–6. doi: 10.1007/s12015-008-9013-5. [DOI] [PubMed] [Google Scholar]
- 71.Kerkis I, Ambrosio CE, Kerkis A, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic. J Transl Med. 2008;6:35. doi: 10.1186/1479-5876-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onyekwelu O, Seppala M, Zoupa M, et al. Tooth development: 2. Regenerating teeth in the laboratory. Dent Update. 2007;34:20–2. doi: 10.12968/denu.2007.34.1.20. [DOI] [PubMed] [Google Scholar]
- 73.Cordeiro MM, Dong Z, Kaneko T, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–9. doi: 10.1016/j.joen.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 74.Nedel F, Andre Dde A, de Oliveira IO, et al. Stem cells: therapeutic potential in dentistry. J Contemp Dent Pract. 2009;10:90–6. [PubMed] [Google Scholar]
- 75.d’Aquino R, De Rosa A, Lanza V, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- 76.Bergenholtz G. Evidence for bacterial causation of adverse pulpal responses in resin-based dental restorations. Crit Rev Oral Biol Med. 2000;11:467–80. doi: 10.1177/10454411000110040501. [DOI] [PubMed] [Google Scholar]
- 77.Bjorndal L. Presence or absence of tertiary dentinogenesis in relation to caries progression. Adv Dent Res. 2001;15:80–3. doi: 10.1177/08959374010150012101. [DOI] [PubMed] [Google Scholar]
- 78.Durand SH, Flacher V, Romeas A, et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J Immunol. 2006;176:2880–7. doi: 10.4049/jimmunol.176.5.2880. [DOI] [PubMed] [Google Scholar]
- 79.Tziafas D, Belibasakis G, Veis A, et al. Dentin regeneration in vital pulp therapy: design principles. Adv Dent Res. 2001;15:96–100. doi: 10.1177/08959374010150012501. [DOI] [PubMed] [Google Scholar]
- 80.Tziafas D, Pantelidou O, Alvanou A, et al. The dentinogenic effect of mineral trioxide aggregate (MTA) in short-term capping experiments. Int Endod J. 2002;35:245–54. doi: 10.1046/j.1365-2591.2002.00471.x. [DOI] [PubMed] [Google Scholar]
- 81.Rutherford RB. BMP-7 gene transfer to inflamed ferret dental pulps. Eur J Oral Sci. 2001;109:422–4. doi: 10.1034/j.1600-0722.2001.00150.x. [DOI] [PubMed] [Google Scholar]
- 82.Sloan AJ, Rutherford RB, Smith AJ. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000;45:173–7. doi: 10.1016/s0003-9969(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 83.Goldberg M, Six N, Decup F, et al. Bioactive molecules and the future of pulp therapy. Am J Dent. 2003;16:66–76. [PubMed] [Google Scholar]
- 84.Yang X, van der Kraan PM, van den Dolder J, et al. STRO-1 selected rat dental pulp stem cells transfected with adenoviral-mediated human bone morphogenetic protein 2 gene show enhanced odontogenic differentiation. Tissue Eng. 2007;13:2803–12. doi: 10.1089/ten.2006.0439. [DOI] [PubMed] [Google Scholar]
- 85.Nakashima M, Iohara K, Sugiyama M. Human dental pulp stem cells with highly angiogenic and neurogenic potential for possible use in pulp regeneration. Cytokine Growth Factor Rev. 2009;20:435–40. doi: 10.1016/j.cytogfr.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Batouli S, Miura M, Brahim J, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976–81. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- 87.Iohara K, Zheng L, Ito M, et al. Regeneration of dental pulp after pulpotomy by transplantation of CD31(-)/ CD146(-) side population cells from a canine tooth. Regen Med. 2009;4:377–85. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- 88.Ji YM, Jeon SH, Park JY, et al. Dental stem cell therapy with calcium hydroxide in dental pulp capping. Tissue Eng Part A. 2010;16:1823–33. doi: 10.1089/ten.TEA.2009.0054. [DOI] [PubMed] [Google Scholar]
- 89.McCulloch CA, Lekic P, McKee MD. Role of physical forces in regulating the form and function of the periodontal ligament. Periodontol 2000. 2000;24:56–72. doi: 10.1034/j.1600-0757.2000.2240104.x. [DOI] [PubMed] [Google Scholar]
- 90.Karring T, Nyman S, Lindhe J. Healing following implantation of periodontitis affected roots into bone tissue. J Clin Periodontol. 1980;7:96–105. doi: 10.1111/j.1600-051x.1980.tb01952.x. [DOI] [PubMed] [Google Scholar]
- 91.Nyman S, Karring T, Lindhe J, et al. Healing following implantation of periodontitis-affected roots into gingival connective tissue. J Clin Periodontol. 1980;7:394–401. doi: 10.1111/j.1600-051x.1980.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 92.Nyman S, Gottlow J, Karring T, et al. The regenerative potential of the periodontal ligament. An experimental study in the monkey. J Clin Periodontol. 1982;9:257–65. doi: 10.1111/j.1600-051x.1982.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 93.Nielsen IM, Ellegaard B, Karring T. Kielbone in healing interradicular lesions in monkeys. J Periodontal Res. 1980;15:328–37. doi: 10.1111/j.1600-0765.1980.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 94.Parlar A, Bosshardt DD, Unsal B, et al. New formation of periodontal tissues around titanium implants in a novel dentin chamber model. Clin Oral Implants Res. 2005;16:259–67. doi: 10.1111/j.1600-0501.2005.01123.x. [DOI] [PubMed] [Google Scholar]
- 95.Gottlow J, Nyman S, Karring T, et al. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984;11:494–503. doi: 10.1111/j.1600-051x.1984.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 96.Gould TR, Melcher AH, Brunette DM. Migration and division of progenitor cell populations in periodontal ligament after wounding. J Periodontal Res. 1980;15:20–42. doi: 10.1111/j.1600-0765.1980.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 97.McCulloch CA. Progenitor cell populations in the periodontal ligament of mice. Anat Rec. 1985;211:258–62. doi: 10.1002/ar.1092110305. [DOI] [PubMed] [Google Scholar]
- 98.Aukhil I, Nishimura K, Fernyhough W. Experimental regeneration of the periodontium. Crit Rev Oral Biol Med. 1990;1:101–15. doi: 10.1177/10454411900010020101. [DOI] [PubMed] [Google Scholar]
- 99.Davidson D, McCulloch CA. Proliferative behavior of periodontal ligament cell populations. J Periodontal Res. 1986;21:414–28. doi: 10.1111/j.1600-0765.1986.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 100.Nemeth E, Kulkarni GW, McCulloch CA. Disturbances of gingival fibroblast population homeostasis due to experimentally induced inflammation in the cynomolgus monkey (Macaca fascicularis): potential mechanism of disease progression. J Periodontal Res. 1993;28:180–90. doi: 10.1111/j.1600-0765.1993.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 101.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 102.Seo BM, Miura M, Sonoyama W, et al. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84:907–12. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- 103.Kawanabe N, Murata S, Murakami K, et al. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation. 2010;79:74–83. doi: 10.1016/j.diff.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 104.Okubo N, Ishisaki A, Iizuka T, et al. Vascular cell-like potential of undifferentiated ligament fibroblasts to construct vascular cell-specific marker-positive blood vessel structures in a PI3K activation-dependent manner. J Vasc Res. 2010;47:369–83. doi: 10.1159/000277724. [DOI] [PubMed] [Google Scholar]
- 105.Kim SH, Kim KH, Seo BM, et al. Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodont. 2009;80:1815–23. doi: 10.1902/jop.2009.090249. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Q, Shi S, Liu Y, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–98. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–68. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tomar GB, Srivastava RK, Gupta N, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–83. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 109.Egusa H, Okita K, Kayashima H, et al. Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS ONE. 5:e12743. doi: 10.1371/journal.pone.0012743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu D, Ikezawa K, Parker T, et al. Characterization of a collagenous cementum-derived attachment protein. J Bone Miner Res. 1996;11:686–92. doi: 10.1002/jbmr.5650110517. [DOI] [PubMed] [Google Scholar]
- 111.Pitaru S, Savion N, Hekmati H, et al. Molecular and cellular interactions of a cementum attachment protein with periodontal cells and cementum matrix components. J Periodontal Res. 1993;28:560–2. doi: 10.1111/j.1600-0765.1993.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 112.Liu HW, Yacobi R, Savion N, et al. A collagenous cementum-derived attachment protein is a marker for progenitors of the mineralized tissue-forming cell lineage of the periodontal ligament. J Bone Miner Res. 1997;12:1691–9. doi: 10.1359/jbmr.1997.12.10.1691. [DOI] [PubMed] [Google Scholar]
- 113.Yonemura K, Raines EW, Ahn NG, et al. Mitogenic signaling mechanisms of human cementum-derived growth factors. J Biol Chem. 1993;268:26120–6. [PubMed] [Google Scholar]
- 114.MacNeil RL, Somerman MJ. Molecular factors regulating development and regeneration of cementum. J Periodontal Res. 1993;28:550–9. doi: 10.1111/j.1600-0765.1993.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 115.Grzesik WJ, Kuzentsov SA, Uzawa K, et al. Normal human cementum-derived cells: isolation, clonal expansion, and in vitro and in vivo characterization. J Bone Miner Res. 1998;13:1547–54. doi: 10.1359/jbmr.1998.13.10.1547. [DOI] [PubMed] [Google Scholar]
- 116.Grzesik WJ, Cheng H, Oh JS, et al. Cementum-forming cells are phenotypically distinct from bone-forming cells. J Bone Miner Res. 2000;15:52–9. doi: 10.1359/jbmr.2000.15.1.52. [DOI] [PubMed] [Google Scholar]
- 117.Matsubara T, Suardita K, Ishii M, et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20:399–409. doi: 10.1359/JBMR.041117. [DOI] [PubMed] [Google Scholar]
- 118.Yamamiya K, Okuda K, Kawase T, et al. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects. J Periodontol. 2008;79:811–8. doi: 10.1902/jop.2008.070518. [DOI] [PubMed] [Google Scholar]
- 119.Breitbart AS, Grande DA, Kessler R, et al. Tissue engineered bone repair of calvarial defects using cultured periosteal cells. Plast Reconstr Surg. 1998;101:567–74. doi: 10.1097/00006534-199803000-00001. ; discussion 75–6. [DOI] [PubMed] [Google Scholar]
- 120.Mizuno H, Hata K, Kojima K, et al. A novel approach to regenerating periodontal tissue by grafting autologous cultured periosteum. Tissue Eng. 2006;12:1227–335. doi: 10.1089/ten.2006.12.1227. [DOI] [PubMed] [Google Scholar]
- 121.Mase J, Mizuno H, Okada K, et al. Cryopreservation of cultured periosteum: effect of different cryoprotectants and pre-incubation protocols on cell viability and osteogenic potential. Cryobiology. 2006;52:182–92. doi: 10.1016/j.cryobiol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 122.Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol 2000. 2002;29:7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 123.Karring T, Cortellini P. Regenerative therapy: furcation defects. Periodontol 2000. 1999;19:115–37. doi: 10.1111/j.1600-0757.1999.tb00151.x. [DOI] [PubMed] [Google Scholar]
- 124.Laurell L, Bose M, Graziani F, et al. The structure of periodontal tissues formed following guided tissue regeneration therapy of intra-bony defects in the monkey. J Clin Periodontol. 2006;33:596–603. doi: 10.1111/j.1600-051X.2006.00951.x. [DOI] [PubMed] [Google Scholar]
- 125.Waerhaug J. The gingival pocket; anatomy, pathology, deepening and elimination. Odontol Tidskr. 1952;60:1–186. ; 70 figures. [PubMed] [Google Scholar]
- 126.Ramfjord SP. Gold Medal Award of the American Academy of Periodontology. J Periodont. 1973;44:726. [PubMed] [Google Scholar]
- 127.Listgarten MA, Rosenberg MM. Histological study of repair following new attachment procedures in human periodontal lesions. J Periodont. 1979;50:333–44. doi: 10.1902/jop.1979.50.7.333. [DOI] [PubMed] [Google Scholar]
- 128.Caton J, Zander HA. Osseous repair of an infrabony pocket without new attachment of connective tissue. J Clin Periodontol. 1976;3:54–8. doi: 10.1111/j.1600-051x.1976.tb01850.x. [DOI] [PubMed] [Google Scholar]
- 129.Schallhorn RG, Hiatt WH. Human allografts of iliac cancellous bone and marrow in periodontal osseous defects. II. Clinical observations. J Periodont. 1972;43:67–81. doi: 10.1902/jop.1972.43.2.67. [DOI] [PubMed] [Google Scholar]
- 130.Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987:7–16. [PubMed] [Google Scholar]
- 131.Giannobile WV, Somerman MJ. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol. 2003;8:193–204. doi: 10.1902/annals.2003.8.1.193. [DOI] [PubMed] [Google Scholar]
- 132.Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–96. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 133.Ripamonti U. Recapitulating development: a template for periodontal tissue engineering. Tissue Eng. 2007;13:51–71. doi: 10.1089/ten.2006.0167. [DOI] [PubMed] [Google Scholar]
- 134.Jin Q, Anusaksathien O, Webb SA, et al. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–26. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giannobile WV, Hernandez RA, Finkelman RD, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31:301–12. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 136.Lynch SE, Williams RC, Polson AM, et al. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16:545–8. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 137.Howell TH, Fiorellini JP, Paquette DW, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68:1186–93. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 138.Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2205–15. doi: 10.1902/jop.2005.76.12.2205. [DOI] [PubMed] [Google Scholar]
- 139.Wozney JM, Rosen V, Celeste AJ, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–34. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 140.Ripamonti U, Petit JC. Bone morphogenetic proteins, cementogenesis, myoblastic stem cells and the induction of periodontal tissue regeneration. Cytokine Growth Factor Rev. 2009;20:489–99. doi: 10.1016/j.cytogfr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 141.Wikesjo UM, Qahash M, Thomson RC, et al. rhBMP-2 significantly enhances guided bone regeneration. Clin Oral Implants Res. 2004;15:194–204. doi: 10.1111/j.1600-0501.2004.00971.x. [DOI] [PubMed] [Google Scholar]
- 142.Kemoun P, Laurencin-Dalicieux S, Rue J, et al. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283–94. doi: 10.1007/s00441-007-0397-3. [DOI] [PubMed] [Google Scholar]
- 143.Zhao M, Xiao G, Berry JE, et al. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res. 2002;17:1441–51. doi: 10.1359/jbmr.2002.17.8.1441. [DOI] [PubMed] [Google Scholar]
- 144.Selvig KA, Sorensen RG, Wozney JM, et al. Bone repair following recombinant human bone morphogenetic protein-2 stimulated periodontal regeneration. J Periodontol. 2002;73:1020–9. doi: 10.1902/jop.2002.73.9.1020. [DOI] [PubMed] [Google Scholar]
- 145.Overall CM, Limeback H. Identification and characterization of enamel proteinases isolated from developing enamel. Amelogeninolytic serine proteinases are associated with enamel maturation in pig. Biochem J. 1988;256:965–72. doi: 10.1042/bj2560965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hammarstrom L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–68. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 147.Gestrelius S, Lyngstadaas SP, Hammarstrom L. Emdogain–periodontal regeneration based on biomimicry. Clin Oral Investig. 2000;4:120–5. doi: 10.1007/s007840050127. [DOI] [PubMed] [Google Scholar]
- 148.Heijl L. Periodontal regeneration with enamel matrix derivative in one human experimental defect. A case report. J Clin Periodontol. 1997;24:693–6. doi: 10.1034/j.1600-051x.1997.00693.x. [DOI] [PubMed] [Google Scholar]
- 149.Heijl L, Heden G, Svardstrom G, et al. Enamel matrix derivative (EMDOGAIN) in the treatment of intrabony periodontal defects. J Clin Periodontol. 1997;24:705–14. doi: 10.1111/j.1600-051x.1997.tb00253.x. [DOI] [PubMed] [Google Scholar]
- 150.Bosshardt DD. Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol. 2008;35:87–105. doi: 10.1111/j.1600-051X.2008.01264.x. [DOI] [PubMed] [Google Scholar]
- 151.Maycock J, Wood SR, Brookes SJ, et al. Characterization of a porcine amelogenin preparation, EMDOGAIN, a biological treatment for periodontal disease. Connect Tissue Res. 2002;43:472–6. doi: 10.1080/03008200290000880. [DOI] [PubMed] [Google Scholar]
- 152.Hatakeyama J, Philp D, Hatakeyama Y, et al. Amelogenin-mediated regulation of osteoclastogenesis, and periodontal cell proliferation and migration. J Dent Res. 2006;85:144–9. doi: 10.1177/154405910608500206. [DOI] [PubMed] [Google Scholar]
- 153.Zeichner-David M, Chen LS, Hsu Z, et al. Amelogenin and ameloblastin show growth-factor like activity in periodontal ligament cells. Eur J Oral Sci. 2006;114:244–53. doi: 10.1111/j.1600-0722.2006.00322.x. ; discussion 54–6, 381–2. [DOI] [PubMed] [Google Scholar]
- 154.Nagano T, Iwata T, Ogata Y, et al. Effect of heat treatment on bioactivities of enamel matrix derivatives in human periodontal ligament (HPDL) cells. J Periodontal Res. 2004;39:249–56. doi: 10.1111/j.1600-0765.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 155.Lyngstadaas SP, Lundberg E, Ekdahl H, et al. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001;28:181–8. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 156.Carinci F, Piattelli A, Guida L, et al. Effects of Emdogain on osteoblast gene expression. Oral Dis. 2006;12:329–42. doi: 10.1111/j.1601-0825.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 157.Zeldich E, Koren R, Dard M, et al. Enamel matrix derivative protects human gingival fibroblasts from TNF-induced apoptosis by inhibiting caspase activation. J Cell Physiol. 2007;213:750–8. doi: 10.1002/jcp.21142. [DOI] [PubMed] [Google Scholar]
- 158.Veis A, Tompkins K, Alvares K, et al. Specific amelogenin gene splice products have signaling effects on cells in culture and in implants in vivo. J Biol Chem. 2000;275:41263–72. doi: 10.1074/jbc.M002308200. [DOI] [PubMed] [Google Scholar]
- 159.Messenger MP, Raif el M, Seedhom BB, et al. The potential use of enamel matrix derivative for in situ anterior cruciate ligament tissue engineering: a translational in vitro investigation. Tissue Eng. 2007;13:2041–51. doi: 10.1089/ten.2006.0059. [DOI] [PubMed] [Google Scholar]
- 160.Heden G, Wennstrom JL. Five-year follow-up of regenerative periodontal therapy with enamel matrix derivative at sites with angular bone defects. J Periodontol. 2006;77:295–301. doi: 10.1902/jop.2006.050071. [DOI] [PubMed] [Google Scholar]
- 161.Boyan BD, Weesner TC, Lohmann CH, et al. Porcine fetal enamel matrix derivative enhances bone formation induced by demineralized freeze dried bone allograft in vivo. J Periodontol. 2000;71:1278–86. doi: 10.1902/jop.2000.71.8.1278. [DOI] [PubMed] [Google Scholar]
- 162.Esposito M, Coulthard P, Thomsen P, et al. Enamel matrix derivative for periodontal tissue regeneration in treatment of intrabony defects: a Cochrane systematic review. J Dent Educ. 2004;68:834–44. [PubMed] [Google Scholar]
- 163.Rathe F, Junker R, Chesnutt BM, et al. The effect of enamel matrix derivative (Emdogain) on bone formation: a systematic review. Tissue Eng B Rev. 2009;15:215–24. doi: 10.1089/ten.teb.2008.0065. [DOI] [PubMed] [Google Scholar]
- 164.Venezia E, Goldstein M, Boyan BD, et al. The use of enamel matrix derivative in the treatment of periodontal defects: a literature review and meta-analysis. Crit Rev Oral Biol Med. 2004;15:382–402. doi: 10.1177/154411130401500605. [DOI] [PubMed] [Google Scholar]
- 165.Wang FM, Qiu K, Hu T, et al. Biodegradable porous calcium polyphosphate scaffolds for the three-dimensional culture of dental pulp cells. Int Endod J. 2006;39:477–83. doi: 10.1111/j.1365-2591.2006.01114.x. [DOI] [PubMed] [Google Scholar]
- 166.Zhang W, Walboomers XF, Wolke JG, et al. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng. 2005;11:357–68. doi: 10.1089/ten.2005.11.357. [DOI] [PubMed] [Google Scholar]
- 167.Phillippi JA, Miller E, Weiss L, et al. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells. 2008;26:127–34. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 168.Calvert P. Materials science. Printing cells. Science. 2007;318:208–9. doi: 10.1126/science.1144212. [DOI] [PubMed] [Google Scholar]
- 169.Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotech. 2003;21:763–70. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]


