Abstract
Circulating tumour cells (CTCs) are independent predictor of prognosis in metastatic breast cancer. Nevertheless, in one third of patients, circulating tumour cells are undetected by conventional methods. Aim of the study was to assess the prognostic value of circulating tumour cells expressing mesenchymal markers in metastatic breast cancer patients. We isolated CTC from blood of 55 metastatic breast cancer patients. CTC were characterized for cytokeratins and markers of epithelial mesenchymal transition. The gain of mesenchymal markers in CTC was correlated to prognosis of patients in a follow-up of 24 months. The presence of mesenchymal markers on CTC more accurately predicted worse prognosis than the expression of cytokeratins alone. Because of the frequent loss of epithelial antigens by CTC, assays targeting epithelial antigens may miss the most invasive cell population. Thus, there is an urgent need to improve detection methods to identify CTC which undergone epithelial mesenchymal transition program.
Keywords: circulating tumour cells, epithelial mesenchymal transition, metastatic breast cancer
Introduction
To date, many studies have reported on the prognostic significance of circulating tumour cells (CTCs) detection in metastatic breast cancer (MBC) [1]. The most currently used approach to detect CTCs, particularly in clinical trials, is the CellSearch(tm) System (Veridex, Warren, NJ, USA), which is the only FDA cleared device for the enumeration of CTC in whole blood. It performs automated immunomagnetic Epithelial Cell Adhesion Molecule (EpCam) based enrichment followed by cytokeratins (CK) staining of cells in blood samples. According to CellSearch, the standard definition of CTC is an EpCam+, CK+ and CD45− nucleated object. Circulating cells with these characteristics are detected in about 60% of MBC patients, where their presence was found an independent predictor of overall survival (OS) and progression free survival (PFS) [2]. In the remaining 40% of patients with metastasis disease, CTC are undetected [3].
Because of CTC genetic and phenotypic heterogeneity, it is theoretically possible that CTC that express EpCam will not express cytokeratins, thus resulting undetected by the CellSearch system. In breast cancer, specifically, loss of CK has been described as an indicator of aggressive disease, and found associated to reduced OS and unfavourable prognostic factors as HR negativity [4]. In this context, it has been suggested that epithelial-to-mesenchymal transition (EMT), which is characterized by loss of CK and gain of mesenchymal markers as vimentin and fibronectin, may be implicated in CTC formation in breast cancer [5].
Recently, it has been observed [6] that a higher proportion of MBC patients with unfavourable prognostic factors (high grade, triple negative disease, brain metastases) had undetectable CTC status, suggesting that an underestimation of CTC by CellSearch may be partly because of CTC undergoing EMT. Aim of this study was to evaluate the prognostic significance of a cell population characterized by lack of CK expression (thus undetected by CellSearch) and gain of mesenchymal markers in blood of MBC patients.
Materials and methods
Patients
This study analysed a population of 55 patients treated between October 2007 and December 2008 for MBC at Sapienza University of Rome. Median follow-up period was 24 months. Before starting any systemic therapy, all patients have been subjected to blood drawing for analysis of CTCs presence. All patients gave their informed consent for the use of their blood samples. The study was approved by local institutional review boards.
Patients population was homogeneous with respect to classical prognostic factors (Table 1).
Table 1.
Characteristics of breast cancer patients
| N | ||
|---|---|---|
| Number of patients | 55 | |
| Median age (yrs) | 52 (range, 34–78) | |
| Staging of the primary tumour | i.v. | 55 (100%) |
| Histology | Ductal | 47 (85%) |
| Lobular | 5 (9%) | |
| Ductal/lobular | (6%) | |
| Grading | 2 | 38 (70%) |
| 3 | 17 (30%) | |
| Estrogen receptor | Pos | 42 (76%) |
| Progesterone receptor | Pos | 36 (65%) |
| HER2 overexpression | Pos (3+) | 18 (32%) |
| Sites of metastasis | ||
| Visceral | 26 (47%) | |
| Non-visceral | 22 (40%) | |
| Visceral + non-visceral | 7 (13%) | |
| Cerebral | 0 |
CTC isolation
Blood samples were maintained at room temperature and processed within a maximum of 12 hrs after blood drawing. CTCs were isolated from 10 cc of peripheral blood by CELLection™ Dynabeads® coated with the monoclonal antibody towards the human EpCam. CELLection™ Epithelial Enrich is designed to optimally enrich bead-free, viable epithelial tumour cells. For each 10 ml blood sample we added 250 μl CELLection™ magnetic beads coated with BerEP4. Epithelial cells bind to the beads in a 30 min incubation. We then lysed the enriched cells with the Lysis Buffer supplied and added 20 μl Dynabeads® Oligo(dT) 25 to capture poly A+ mRNA. From the captured mRNA, a solid cDNA was synthesized. The method has been extensively validated, and is able to detect 1 tumour cell/ml of blood avoiding illegitimate transcription from leukocytes [7].
To verify the integrity of extracted RNAs, 5 μl of each cDNA were amplified in PCR buffer containing 25 pmol each of upstream and downstream GAPDH primers as housekeeping gene and 1.25 units of Platinum Taq polymerase (Life Technologies). The absence of leukocytes in the pellet of EpCam positive cells was routinely confirmed by PCR amplifications for CD45 and cytokeratin 8/18/19 (CK8, CK18, CK19), used as markers of epithelial cells. According to standard definition, CTCs were defined as all EpCam positive cells negative for CD45 expression but expressing CK8/18/19. Samples negative for CD45 and negative for CK8/18/19 expression were marked as samples negative for presence of CTCs (as classically defined).
All samples (CK+ and CK−) were then investigated for the presence of vimentin and fibronectin used as markers of EMT.
Sixteen blood samples from healthy volunteers have been analysed for the presence of vimentin and fibronectin.
Statistical analysis
Statistical analysis was performed with BMDP statistical software, version 7 (Statistical Solutions, Saugus, MA, USA) and SPSS (Chicago, version 15.00 for Windows).
PFS was defined as the time elapsed between the date of blood sampling and the date of clinical disease progression or death for any cause. The mean PFS value was 10.9 months.
Kaplan–Meier product-limit method was used to correlate PFS with CK and EMT markers on CTC. Different prognostic groups were compared using the log-rank test. A P value of less than 0.05 was considered statistically significant.
Results
After CTC isolation, 5/55 (9%) samples were found positive for CD45 and discarded. Among the remaining 50 samples, 28 (56%) were found CK+/CD45−, thus CTC positive, according to standard definition. On the contrary, 22/50 (46%) were found CK−/CD45−. These samples, because of the lack of CK expression, would have been considered negative for CTC presence.
The median PFS of the CK-group was 10.2 months versus 11.8 of the CK+. This difference was found not statistically significant (P = 0.299) (Fig. 1A).
fig 1.
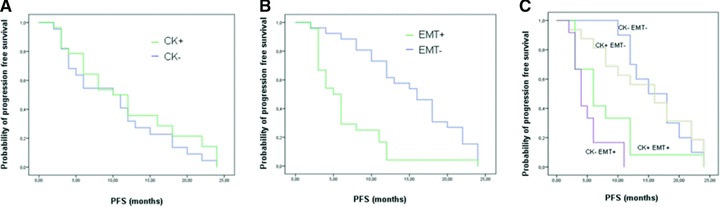
Difference in progression free survival between patients with CK positive versus negative CTCs (A). Difference in progression free survival between patients with mesenchymal markers positive versus negative CTCs (B). Difference in progression free survival of patients according to concomitant expression of CK and mesenchymal markers on CTC (C).
All samples were further analysed for vimentin and fibronectin expression, as EMT markers.
Fibronectin and/or vimentin were found expressed in 24/50 (48%) samples. Independently of the expression of CK, the median PFS of patients with EMT+ markers on CTCs was 6.6 months versus 15.3 months of those with EMT−. This difference was found statistically significant (P = 0.000) (Fig. 1B).
According to the expression of cytokeratins and EMT markers in CTC we had four subgroups of patients: (1) 10/50 (20%) were CK−/EMT−, (2) 12/50 (24%) were CK−/EMT+, (3) 16/50 (32%) were CK+/EMT− ans (4) 12/50 (24%) were CK+/EMT+.
The median PFS of the subgroups were: 16.4, 5.1, 14.6 and 8.1 months, respectively. The difference in PFS between patients with CK−/EMT− and those with CK−/EMT+ CTC was found statistically significant. A similar significant difference in PFS was found between patients with CK+/EMT− and those with CK+/EMT+ CTC (P = 0.000) (Fig. 1C).
The presence of mesenchymal markers in CTC lacking CK expression is shown in Figure 2.
fig 2.
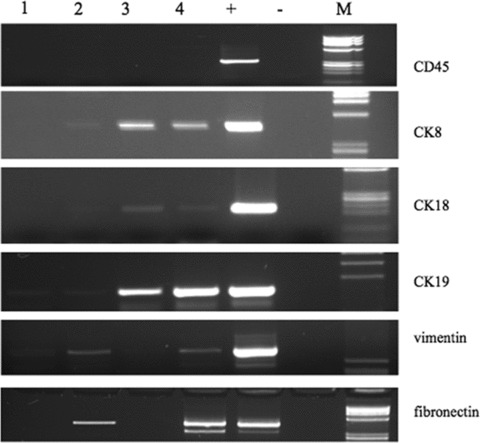
Expression of CD45, CK8/18/19, vimentin and fibronectin in 5 MBC patients (lanes 1–5). Lane 6: positive control (M14 cell line). Lane 7: negative control (sample without RNA). M: molecular size marker.
In all 16 blood samples from healthy donors we failed to find expression of vimentin and fibronectin (Fig. 3).
fig 3.
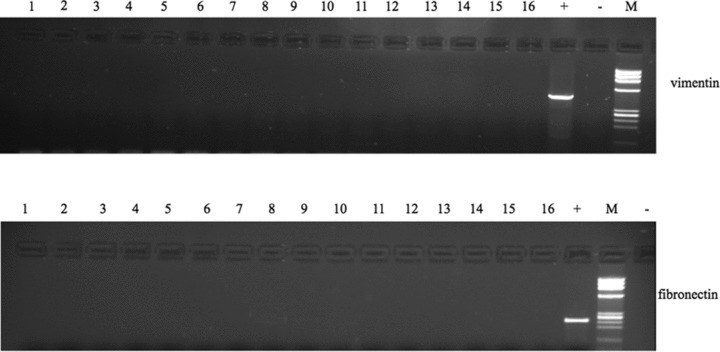
Expression of vimentin and fibronectin in blood from healthy volunteers (lanes 1–16). Lane 17: positive control. Lane 18: negative control. M: molecular size marker.
Discussion
In the era of the ‘microscopic revolution’ cancer has been rethought at a single-cell level. Data are indeed increasing which support the prognostic value of CTC, in terms of PFS and OS, in metastatic breast cancer patients. The intriguing idea of a ‘liquid biopsy’ offered an alternative strategy to monitor cancer evolution and response to therapies [8]. Despite this, a grey area is emerging regarding currently available methods for the detection of CTC. Based on current definition of these cells (EpCam+/CK+/CD45−) approximately one third of metastatic breast cancer patients have no CTC in peripheral blood [3].
In a very recent work, questions have been raised as to whether negative CTCs and ‘undetectable’ CTCs should be differently interpreted [6].
Indeed, in a higher proportion of patients with poor prognosis disease, CTCs were not detected using the CellSearch™ system. This could be partially because of an underestimation of CTC displaying mesenchymal traits, after activation of EMT program.
In this study we explored the hypothesis that circulating cells lacking CK expression and gaining mesenchymal markers, thus undetected through standard CTC isolation methods, may have prognostic significance in metastatic breast cancer patients. Our analysis demonstrates that, independently of the expression of CK on CTC, the groups of patients with worse prognosis are those with CTC expressing mesenchymal markers. Indeed, among the four subgroups of patients examined, a substantial difference in PFS has been observed consistently with the gain of mensenchymal markers. Thus, we may speculate that the expression of CK alone on CTC may not adequately predict prognosis in MBC patients. In fact cells lacking cytokeratins and expressing mesenchymal markers would not have been counted through CellSearch analysis, because classical definition criteria for CTC were not met. Although the potential loss of CTC during the enrichment steps has been widely suggested [9], whether this cell loss has a negative impact in the detection of prognostic CTC was still unanswered. To our knowledge, this is the first study assessing the prognostic relevance of a different CTC population, selected for the expression of mesenchymal markers (vimentin, fibronectin) even in absence of CK expression.
Our results suggest that, because of the loss of epithelial antigens by CTC during EMT, assays targeting epithelial antigens may miss the most invasive pool of CTC. Thus, there is an urgent need to improve detection methods to identify, count and characterize all CTC after EMT.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Nagaiah G, Abraham J. Circulating tumor cells in the management of breast cancer. Clin Breast Cancer. 2010;10:209–16. doi: 10.3816/CBC.2010.n.028. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 3.Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–8. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 4.Vora HH, Patel NA, Rajvik KN, et al. Cytokeratin and vimentin expression in breast cancer. Int J Biol Markers. 2009;24:38–46. doi: 10.1177/172460080902400106. [DOI] [PubMed] [Google Scholar]
- 5.Blick T, Hugo H, Widodo E, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:235–52. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 6.Mego M, De Giorgi U, Dawood S, et al. Characterization of metastatic breast cancer patients with non-detectable circulating tumor cells. Int J Cancer. 2010 doi: 10.1002/ijc.25690. 10.1002/ijc.25690. [DOI] [PubMed] [Google Scholar]
- 7.Hardingham JE, Kotasek D, Farmer B, et al. Immunobead-PCR: a technique for the detection of circulating tumor cells using immunomagnetic beads and the polymerase chain reaction. Cancer Res. 1993;53:3455–8. [PubMed] [Google Scholar]
- 8.Criscitiello C, Sotiriou C, Ignatiadis M. Circulating tumor cells and emerging blood biomarkers in breast cancer. Curr Opin Oncol. 2010;22:552–8. doi: 10.1097/CCO.0b013e32833de186. [DOI] [PubMed] [Google Scholar]
- 9.Jacob K, Sollier C, Jabado N. Circulating tumor cells: detection, molecular profiling and future prospects. Exp Rev Proteom. 2007;4:741–56. doi: 10.1586/14789450.4.6.741. [DOI] [PubMed] [Google Scholar]


