Abstract
The peritoneal mesothelium exhibits a high regenerative ability. Peritoneal regeneration is concomitant with the appearance, in the coelomic cavity, of a free-floating population of cells whose origin and functions are still under discussion. We have isolated and characterized this cell population and we have studied the process of mesothelial regeneration through flow cytometry and confocal microscopy in a murine model lethally irradiated and reconstituted with GFP-expressing bone marrow cells. In unoperated control mice, most free cells positive for mesothelin, a mesothelial marker, are green fluorescent protein (GFP). However, 24 hrs after peritoneal damage, free mesothelin+/ GFP+ cells appear in peritoneal lavages. Cultured lavage peritoneal cells show colocalization of GFP with mesothelial (mesothelin, cytokeratin) and fibroblastic markers. Immunohistochemical staining of the peritoneal wall also revealed colocalization of GFP with mesothelial markers and with procollagen-1 and smooth muscle α-actin. This was observed in the injured area as well as in the surrounding not-injured peritoneal surfaces. These cells, which we herein call peritoneal repairing cells (PRC), are very abundant 1 week after surgery covering both the damaged peritoneal wall and the surrounding uninjured area. However, they become very scarce 1 month later, when the mesothelium has completely healed. We suggest that PRC constitute a type of monocyte-derived cells, closely related with the tissue-repairing cells known as ‘fibrocytes’ and specifically involved in peritoneal reparation. Thus, our results constitute a synthesis of the different scenarios hitherto proposed about peritoneal regeneration, particularly recruitment of circulating progenitor cells and adhesion of free-floating coelomic cells.
Keywords: peritoneum, mesothelium, regeneration, progenitor cells, bone marrow, fibrocytes
Introduction
Peritoneal regeneration is a very important issue from the clinical point of view because of the unique physiological properties of the mesothelium, the complications of the abdominal surgery due to serosal adhesions and the disastrous consequences of continued peritoneal dialysis for the mesothelial integrity [1, 2].
The peritoneal mesothelium exhibits a high regenerative ability. The pioneering studies [3] described how peritoneal injuries were able to heal at the same rate independently of their size. Thus, centripetal growth is not enough to account for peritoneal regeneration. The phenomenon was explained by the appearance, immediately after a peritoneal injury, of a population of cells which freely float in the peritoneal cavity and adhere to the damaged surface (reviewed in [4]). However, the origin, nature and precise functions of this population are still uncertain. Some studies have suggested that these cells are peritoneal macrophages that either promote mesothelial proliferation or directly can differentiate into mesothelial cells [5–8]. Other works provide evidence that free-floating cells are just mesothelial cells which have delaminated from other areas of the peritoneum and attach to the injured area to recover their epithelial phenotype [9, 10]. Furthermore, recruitment of sub-mesothelial fibroblast-like cells [11] or mobilization of circulating progenitor cells [12] has also been claimed as a regenerating mechanism.
We aimed to solve the long-lasting dispute about the origin and the nature of the free-floating cells which appear after a peritoneal damage and contribute to mesothelial regeneration by using a murine model lethally irradiated and reconstituted with bone marrow cells from mice constitutively expressing enhanced GFP. We have isolated and characterized the free-floating cell population and we have studied the process of mesothelial regeneration through confocal and scanning electron microscopy. We think that our results constitute a synthesis of the above quoted scenarios proposed about mesothelial regeneration, particularly adhesion of free-floating cells to the damaged surface and recruitment of circulating progenitor cells. We herein provide evidence that bone marrow derived cells migrate into the mesothelial cell layer, acquire a mesothelial-like phenotype and are released to the peritoneal cavity to constitute a free-floating, peritoneal repairing cell population. Interestingly, these cells apparently attach not only to the damaged surface, but also to adjacent areas.
Material and methods
Animals and haematopoietic bone marrow transplants
Eight- to 10-week-old B6.SJL-Ptprca/bPep3b/BoyJxDBA/2 mice (P3D2F1; CD45.1/CD45.2 phenotype) were used as recipients of labelled bone marrow cells. Breeding pairs, originally obtained from Jackson Laboratories (Bar Harbor, ME, USA), were bred at the CIEMAT animal facility (Registration number 28079–21A), allowed food and water ad libitum, and routinely screened for pathogens in accordance with FELASA (Federation of European Laboratory Animal Science Associations) procedures. The F1 mice of C57BL/6J-βactinEGFP (enhanced green fluorescent protein) (kindly provided by Dr. M. Okabe, Osaka, Japan) and DBA/2 mice (BDGF1; CD45.2 EGFP+ phenotype) were used as donors of bone marrow cells. Recipients of bone marrow transplants were irradiated with doses for a myeloablative regimen with a Philips MG324 X-ray equipment (Philips, Hamburg, Germany) set at 300 kV, 10 mA, delivering a total dose 10 Gy, split in two doses, 4 hrs apart, at a dose rate of 1.03 Gy/min.
Bone marrow cells were collected from 6-week-old BDGF1 male mice by flushing tibias and femurs with Iscove’s modified Dulbecco’s media (Gibco Laboratories, Grand Island, NY, USA). Bone marrow cell viability was analysed by the Trypan Blue exclusion method. A total of 107 bone marrow cells were injected into 8- to 10-week-old lethally irradiated female P3D2F1 mice. One and 3 months after transplantation, the haematopoietic engraftment in the peripheral blood was analysed by flow cytometry by analysing the expression of the EGFP and the specific CD45.1 and CD45.2 panleucocyte isoforms using specific monoclonal antibodies (BD Biosciences Pharmingen, San Diego, CA, USA).
BALB/c mice were also used for some experiments not involving labelled bone marrow cells.
Surgical procedure and peritoneal cell collection
Free-floating peritoneal cells were obtained by a modification of the published procedure [10]. Briefly, mice were anaesthetized and opened in the midline. Then, we provoked a slight, superficial abrasion on the right anterior peritoneal wall with a sterile scalpel blade, taking care to avoid haemorrhage. Mice were allowed to recover from anaesthesia and returned to housing. After 24 or 48 hrs the mice were killed by cervical dislocation and the peritoneal cavity was rinsed with 10 ml PBS. Cells were collected by centrifugation at 400 × g for 5 min., and cultured or used immediately for flow cytometry as described below.
Cell culture and flow cytometry
Collected cells from peritoneal lavage were cultured on plastic with DMEM supplemented with 10% foetal bovine serum, penicillin/streptomycin at 37°C and 5% CO2 in a humidified incubator. For positive control we used mouse adult mesothelial cells obtained from explants of omentum on gelatine-coated cover slips.
For flow cytometry, collected cells were incubated on ice for 20 min. with the primary antibody diluted in PBS supplemented with 1% foetal bovine serum and 10 mM HEPES, centrifuged and resuspended in the same buffer. Cells labelled with unlabelled or biotinylated primary antibodies were incubated with the corresponding secondary antibody (usually Cy5-conjugated donkey anti-rat IgG), centrifuged and resuspended again. Negative controls were incubated with isotype IgG and then with the secondary antibody above described. Usually, cells were also incubated with propidium iodide (25 μg/ml) and negative cells were gated to eliminate dead cells from the analysis. The analysis was performed in a DAKO-Cytomation MoFlo Sorter (Dako, Glostrup, Denmark). The primary antibodies used were: rat antimouse CD45, PE conjugated (Pharmigen, Becton Dickinson, Franklin Lakes, NJ, USA, Clone 30-F11) diluted 1:500; rat antimouse mesothelin (MBL D053, clone 295D; MBL, Woburn, MA, USA) diluted 1:50; rat antimouse F4/80, FITC conjugated (eBioscience 11–4801, Clone BM8; eBioscience, San Diego, CA, USA) diluted 1:100.
Histology and immunocytochemistry
Dissected fragments of the right (injured) and the left (intact contralateral) peritoneal walls from mice killed 48 hrs, 1 week or 1 month after surgery were fixed overnight at 4°C in 4% paraformaldehyde (PFA) or at −20°C in Dent’s fixative (Metanol:DMSO 4:1). The tissue was cryoprotected in 15% and 30% sucrose solution, snap frozen in liquid nitrogen-cooled isopentane and embedded in optimal cutting temperature (OCT). Ten micrometre sections were obtained in a cryostat. Fragments of the anterior peritoneal wall of unoperated mice were used as controls.
Cultured cells were fixed for 20 min. at room temperature in 2% PFA or for 20 min. at −20°C in Dent’s fixative, washed in PBS and blocked with 16% sheep serum, 1% bovine serum albumin and 0.5% Triton X-100 in Tris-PBS (SBT). Double immunolabelling was performed incubating with a monoclonal and a polyclonal primary antibody at the same time, using the corresponding secondary biotinylated and/or Cy5-conjugated antibodies (1:100 in SBT) and incubating finally for 45 min. with a complementary fluorochrome–conjugated streptavidin (Sigma-Aldrich, St. Louis, MO, USA), 1:150 in PBS. Nuclei were usually counterstained with propidium iodide or 4′,6-diamidiino-2-phenylindole (DAPI). Colocalization of CD45 with cytokeratin required pre-incubation with a rat anti CD45-PE on live cells, extensive wash and fixation with Cytofix (Becton Dickinson). After washing, the sections were mounted in a 1:1 PBS/glycerol solution and analysed using a Leica TCS SPE laser confocal microscope (Leica Microsystems, Wetzlar, Germany). Primary antibodies used were: polyclonal rabbit anti-cytokeratin (DAKO, Z0622) diluted 1:200; polyclonal rabbit anti-fibroblast specific protein (FSP)1 (A kind gift from Dr. Eric Neilson, Vanderbilt University School of Medicine) diluted 1:100; rat antimouse CD68 (AbD Serotec, Dusseldorf, Germany, MCA1957BT, Clone FA-11) diluted 1:100; polyclonal rabbit anti-mesothelin (A kind gift from Dr. Ira Pastan, NCI-NIH) diluted 1:200; mouse anti-SMC α-actin (Sigma A2547, Clone 1A4) diluted 1:200; goat polyclonal anti-procollagen I (Santa Cruz, Biotechnologies, Heidelberg, Germany; SC-8787) diluted 1:100.
Scanning electron microscopy
Samples of peritoneal walls were fixed in 1% paraformaldehyde, 1% glutaraldehyde in PBS (1 hr) and washed in bidistilled water. Then, the samples were dehydrated in an ethanolic series finishing in 100° ethanol and dried from liquid CO2 by the critical-point method whereas the cell aggregates were air dried. Then all the samples were gold sputted (about 450Å) in a JEOL fine-coat ion sputter (JFC-1100), observed and photographed in a JEOL JSM-840 scanning electron microscope (JEOL, Tokyo, Japan) operated between 10 and 20 kV.
RT-PCR
Total RNA was isolated from (1) the CD45+ cell fraction of a peritoneal lavage (48 hrs after surgery) purified with antimouse CD45-coated magnetic immunobeads according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany); (2) mouse mesothelial cells obtained by mild trypsinization of the coelomic cavity and peritoneal lavage (positive control) and (3) peritoneal lavage cells obtained from a mesothelin-null mouse [13] (negative control). RNA was obtained with the RNAeasy MiniKit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. The yield and quantification of RNA obtained was analysed with Nanodrop NP-100 (Thermo Fisher Scientific, Waltham, MA, USA) and normalized to 1 μg to RT-PCR.
Two-step RT-PCR was performed to mRNA amplification with oligo-dT with the First Strand Amplification Kit (Roche Diagnostica, Barcelona, Spain); normalization of RT-PCR product was made with expression of β-actin as reference gene.
PCR amplification was performed with GoTaq Flexi polymerase (Promega, Madison, WI, USA). Following pair of primers were used: 5′TCA GAG TCA TTG TTA TCC ACA GAC; 3′AGT GTG GCC TCC TGG CTT GTC TTT[13], with the following PCR program: 94°C – 5 min.; 94°C – 45 sec., 60°C – 45 sec., 72°C – 45 sec., 35 cycles; 72°C – 5 min.; and 4°C – 5 min.
Results
Flow cytometry of peritoneal lavages after surgery
Peritoneal lavages obtained from mice 24 and 48 hrs after surgery (n = 1 and n = 4, respectively), and from unoperated mice (n = 3), were analysed by flow cytometry. Figure 1 shows the results of a representative experiment. The top rows show the detection of GFP and mesothelin in peritoneal lavage cells. About two thirds of the peritoneal cells were GFP+. Unoperated mice show a population of mesothelin+/GFP– cells accounting for 3.4% of the total peritoneal lavage cells (n = 3, s = 0.74). These cells increased their frequency to 10.4% in the only lavage obtained 24 hrs after surgery, and they represented a 5.0% of the total 48 hrs after surgery (n = 4, s = 1.48). On the other hand, mesothelin+/GFP+ cells were not clearly present in unoperated mice, but a population these cells was found in 24 hrs (16.6% of the total) and 48 hrs peritoneal lavages (6.1%, n = 4, s = 1.48).
fig 1.
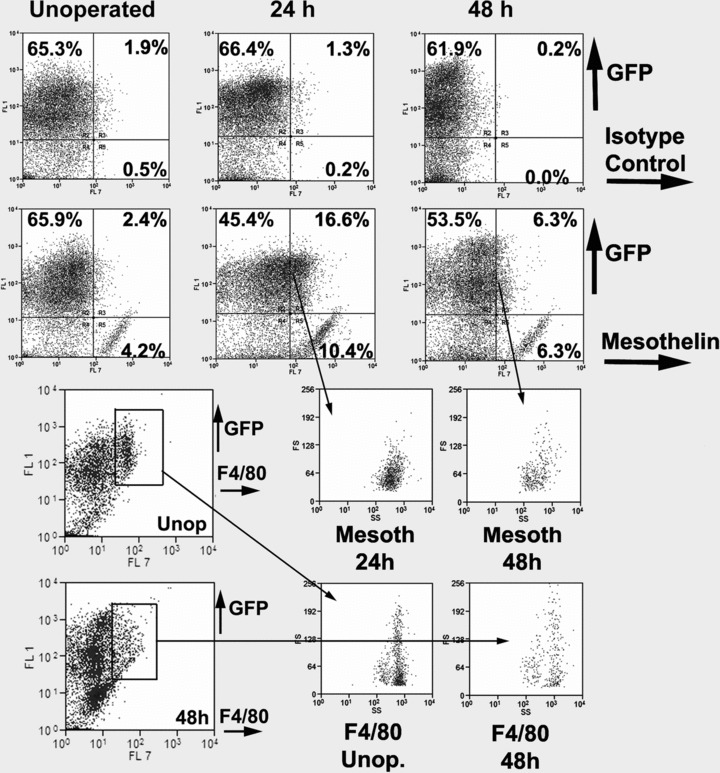
Flow cytometry analysis of the cell population obtained by peritoneal lavage in control unoperated mice, 24 and 48 hrs after injury of the peritoneal wall. Representative result from a series of experiments described in the text. Top row: Isotype control (rat IgG2a) versus GFP. About two thirds of the cells present in the peritoneal lavage are GFP+. Second row: Mesothelin versus GFP. In the unoperated mouse, 4.2% of the cells are mesothelin+/GFP–. They probably are delaminated mesothelial cells. Because these cells can be considered as a positive control of the antibody, we used this population to establish the limit between the mesothelin+ and mesothelin– cells. According to this criterion, 16.6% and 6.3% of the cells were mesothelin+/GFP+ by 24 and 48 hrs after surgery. Note the increase of mesothelin+/GFP– cells by 24 hrs, reaching more than 10% of the total population. Bottom rows: Gating on a F4/80+/GFP+ population obtained from peritoneal lavages originates similar SS/FS profiles from unoperated and operated mice. However, gating on the mesothelin+/GFP+ population originates a different profile, suggesting that both populations are different.
Peritoneal macrophages, detected by F4/80 immunoreactivity, accounted for a 6.8% of the total peritoneal lavage cells 48 hrs after surgery (n = 4, s = 0.88). Virtually all F4/80+ cells were GFP+. Gated mesothelin+/GFP+ and F4/80+ cells showed different profiles in a FS/SS diagram, suggesting that they are different populations (Fig. 1, bottom rows).
Detection of mesothelin by RT-PCR in the CD45+ fraction
Mesothelin expression was confirmed by RT-PCR in the CD45+ fraction obtained from a peritoneal lavage 48 hrs after peritoneal injury and purified by magnetic immunobeads (Fig. 2). The amplicon size, 168 bp, was identical to that obtained from a positive control consisting of mesothelial cells obtained from trypsinization of the peritoneal cavity of an adult mouse. However, amplification was not found in the negative control, trypsinized peritoneal cells of a mesothelin-deficient mouse.
fig 2.
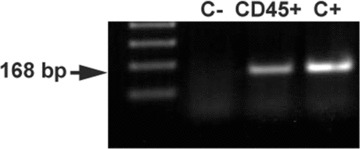
The CD45+ fraction of the peritoneal lavage cells obtained 48 hrs after injury of the peritoneal wall and purified by magnetic immunobeads, shows mesothelin mRNA expression by RT-PCR. Positive control (C+) consisted of mesothelial cells obtained by mild trypsinization of the peritoneal cavity. Negative control (C–) was performed with free peritoneal cells obtained from a mesothelin-null mouse. Normalization of RT-PCR product was made with expression of β-actin as reference gene.
Immunohistochemistry of free peritoneal cells
Colocalization of mesothelial and leucocytic markers was frequent in primary culture of cells obtained from peritoneal lavages 48 hrs after injury, either from mice with GFP-expressing bone marrow or from normal mice (Fig. 3). The colocalization was always found in large, spindle-shaped cells. Cytokeratin showed a characteristic perinuclear dot-like staining pattern (Fig. 3A, colocalization with CD45). This pattern is frequent in primary cultures of mesothelial cells, in which the cytokeratin cytoskeleton seems to collapse, as shown in Figure 3(B), where some mesothelial cells show also the perinuclear dot like pattern whereas others show a still well-developed cytokeratin cytoskeleton (arrowhead in Fig. 3B). CD68 showed an intracytoplasmic pattern and colocalization with mesothelin (Fig. 3C). In peritoneal cells obtained from mice with GFP-expressing bone marrow, GFP colocalized with both mesothelin and cytokeratin in some cells (arrowheads in Fig. 3D) whereas others were GFP–/cytokeratin+/mesothelin+ (white arrows in Fig. 3D). GFP also colocalized with the fibroblastic marker FSP1 in part of the cells (Fig. 3E, E’). Negative control was incubated with isotype IgG and the same secondary antibodies than in the other slides (Fig. 3F).
fig 3.
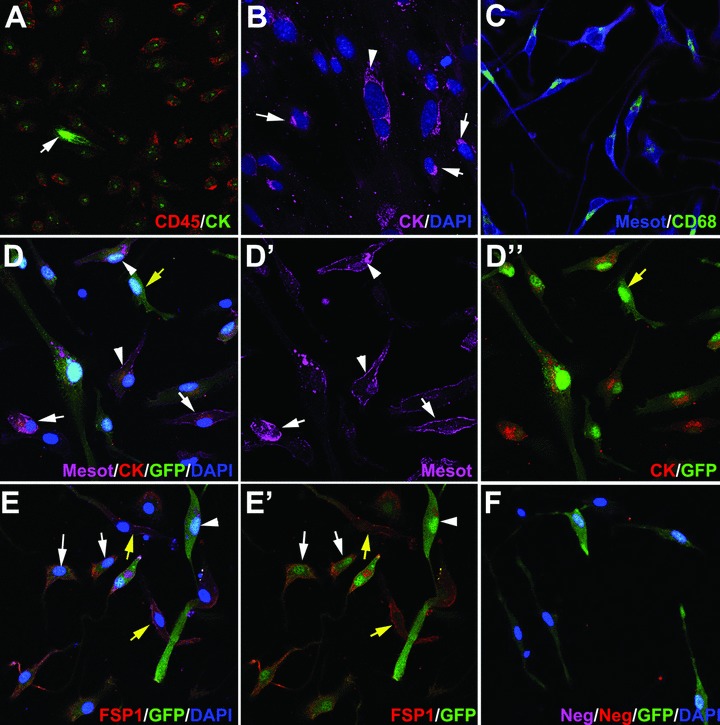
Antigen immunolocalization in adherent cells obtained from peritoneal lavages 48 hrs after injury of the peritoneal wall. (A–C) show cells obtained from normal BALB/c mice whereas (D)–(F) show cells from mice reconstituted with GFP-expressing bone marrow. (A) Virtually all the CD45+ cells show a perinuclear dot-like pattern of cytokeratin immunoreactivity. Note a strongly CD45–/cytokeratin+ immunoreactive cell, probably a delaminated mesothelial cell (arrow). (B) The perinuclear dot-like pattern of cytokeratin immunoreactivity is also present in mesothelial cells migrating from omentum explants, which were used as positive controls (arrows). Other cells still show an extended cytokeratin cytoskeleton (arrowhead). (C) Mesothelin immunoreactivity colocalized with CD68. Note the cytoplasmic and perinuclear CD68 localization. (D) Triple localization of mesothelin, cytokeratin and GFP. This immunostaining revealed different phenotypes, including triple positive cells (arrowheads), mesothelin+/cytokeratin+, GFP– cells which probably are delaminated mesothelial cells (white arrows) and GFP+, mesothelin–/cytokeratin– cells (yellow arrow). (E) The fibroblast marker FSP1 was expressed in many GFP+ cells (white arrows), as well as in GFP– cells (yellow arrows). Other GFP+ cells were negative for FSP1 (arrowhead). (F) Negative control incubated with isotype primary antibodies and with the same secondary antibodies as used in the rest of the figures.
Scanning electron microscopy
The injured peritoneal wall, after 48 hrs, showed extensive signs of reparation, with cells adhered to the denudated surface and displaying a variable degree of flattening (arrows in Fig. 4B). Surprisingly the contralateral peritoneal surface (Fig. 4C and D) showed a clear difference as compared with the control (unoperated) mice (Fig. 4A) because of the presence of rounded cells, loosely attached to the peritoneal surface. These features were never observed in control unoperated mice.
fig 4.
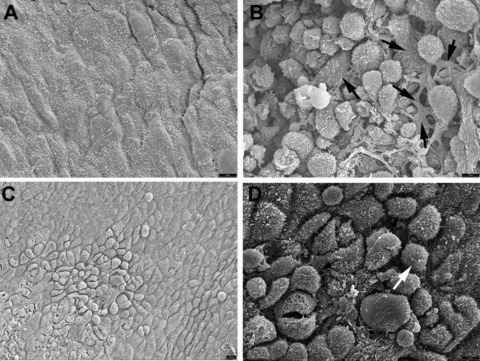
Scanning electron microscopy of the peritoneal surface from a control, unoperated mouse (A), from the injured area 48 hrs after surgery (B) and from the contralateral area of the same mice (C). A higher magnification of the latter is shown in (D). The normal peritoneal surface is squamous and shows abundant microvilli. In the injured surface rounded cells covered by ruffles and microvilli are present. Some cells show different degrees of flattening (arrows). In the contralateral area shown in (C), the surface shows areas of mesothelial activation, with the cells bulging in the lumen and showing signs of detachment. These cells also show microvilli, as shown in (D) (arrow).
Immunohistochemistry of peritoneal walls
After 48 hrs of the peritoneal injury, differences were conspicuous between operated and unoperated mice. Surprisingly, signs of mesothelial activation and remodelation were as intense in the contralateral area as in the injured wall. In sections of peritoneal walls from mice reconstituted with GFP+ bone marrow, GFP+ cells were found in both, the injured and the contralateral peritoneal surfaces (Fig. 5), being slightly more abundant in the latter (32.1% versus 26.1% of the total cell count; n = 8, s = 6.24 and n = 13, s = 13.47, respectively). This difference was not statistically significant. GFP colocalized with mesothelin, especially in the contralateral side and in round cells loosely attached to the peritoneal surface, where mesothelin immunoreactivity was very high (Fig. 5A, C and D). These round cells, partially detached from the peritoneum, were always absent in control, unoperated mice, which only showed a few GFP+ cells inside the peritoneal wall but never in the peritoneal lining (not shown). Intratisular GFP+ cells from operated mice were always mesothelin– (Fig. 5B). Colocalization of cytokeratin with GFP was again more evident in the contralateral areas (Fig. 5F and G). To check the presence of mature macrophages in the tissue we immunocolocalized the F4/80 antigen with GFP. Only a few cells were positive, and they were clearly distinct from the GFP+ cells adhered to the injured surface (Fig. 5H and I). GFP+/FSP1+ cells were present in both, the injured and contralateral areas (Fig. 5K and J). Finally, colocalization of GFP with procollagen was also recorded in both, injured and contralateral peritoneal walls (Fig. 5L and M). In normal mice we also observed, 48 hrs after peritoneal damage, colocalization of mesothelin and cytokeratin with CD45 and CD68 (not shown). This colocalization was never found in control, unoperated mice.
fig 5.
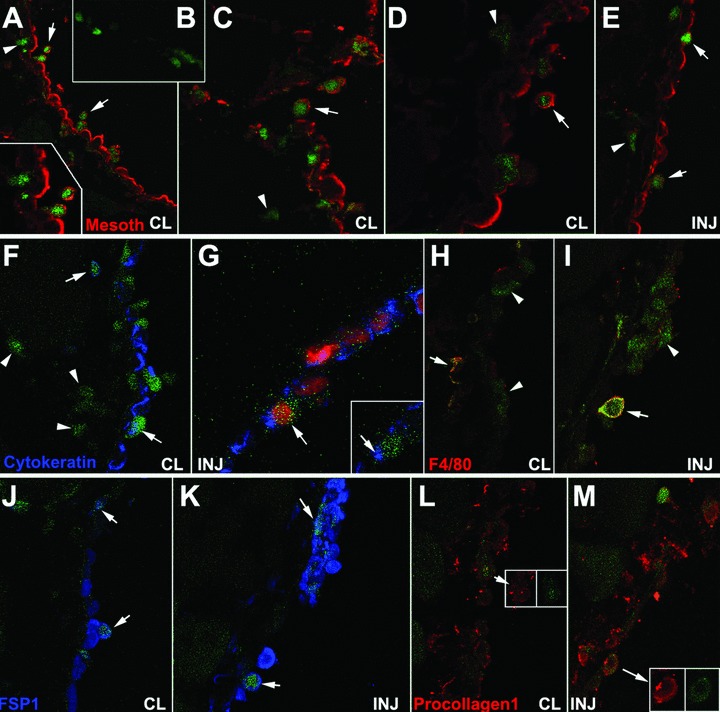
Colocalization of GFP and mesothelin, cytokeratin, F4/80, FSP1 and procollagen-1 in the injured (INJ) or contralateral (CL) peritoneal wall from mice reconstituted with GFP-expressing bone marrow, 48 hrs after surgery. (A–D) Colocalization of GFP and mesothelin in the contralateral areas. Some double-labelled cells are apparently detaching from the mesothelial surface (arrows). GFP+ cells within the tissue are mesothelin– as shown in (B). GFP+/mesothelin– cells are also abundant in sub-mesothelial areas (arrowheads). (E) Double labelled cells can also be seen in the regenerating mesothelium of the injured surface (arrows). GFP+/mesothelin– cells are also present but they are less abundant than in contralateral areas (arrowhead). (F), (G) Colocalization of GFP and cytokeratin can be observed in the contralateral areas (arrows in F) but it is apparently scarcer in the injured ones (G). GFP+/cytokeratin– cells are shown in the contralateral side (arrowheads in F). (H), (I) The macrophage marker F4/80 is present in a few cells from both areas (arrows). However, most of the apparently adhered GFP+ cells in the injured area do not express this macrophage marker (arrowheads). (J), (K) Colocalization of GFP with the fibroblastic marker FSP1 (arrows). (L), (M) Colocalization of GFP with procollagen-1 (arrows).
One week after peritoneal damage, GFP+ cells were observed in the peritoneal surface of both, the injured and the contralateral areas. They were even more abundant than by 48 hrs, and they formed several cell layers (Fig. 6). The proportion of GFP+ cells was similar in both areas (55.7% in contralateral versus 57.7% in injured areas; n = 5, s = 13.16 and n = 6, s = 9.92, respectively). GFP colocalized with cytokeratin (Fig. 6A, contralateral area) and smooth muscle α-actin (Fig. 6B and C, injured area). Cytokeratin staining showed both the extended and the condensed dot-like pattern (Fig. 6A). One month after surgery, the mesothelial lining was again normal, and the number of GFP+ cells had decreased considerably, although it was still possible to find some GFP+ cells occasionally positive for cytokeratin and mesothelin, either at a sub-mesothelial level or integrated in the mesothelial cell layer (Fig. 6D–F).
fig 6.
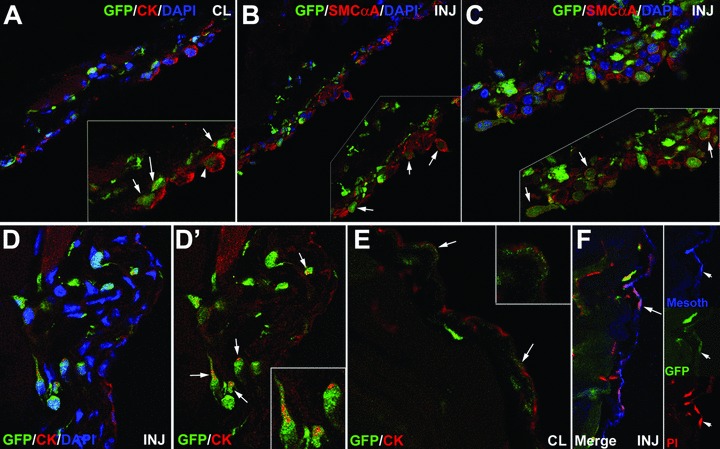
Colocalization of GFP, mesothelial and fibroblastic markers in the contralateral (CL) and injured (INJ) peritoneal wall from mice reconstituted with GFP-expressing bone marrow, 1 week (A–C) and 1 month (D–F) after surgery. (A) Colocalization of cytokeratin and GFP in the injured area 1 week after surgery. Some GFP+ cells show an extended cytokeratin cytoskeleton (arrowhead) whereas others show the perinuclear dot-like cytokeratin pattern (arrows). (B), (C) Colocalization of smooth muscle cell α-actin with GFP in the injured area 1 week after surgery. GFP+ cells are very abundant and form several cell layers covering all the damaged area. Most of these cells express smooth muscle cell α-actin. (D–F) Colocalization of mesothelial markers and GFP in the injured and contralateral areas 1 month after surgery. The mesothelium is completely regenerated. GFP+ cells are present in the sub-mesothelial area, and some of them show a perinuclear cytokeratin dot (arrows in D’). It is still possible to find a few GFP+ cells integrated in the mesothelial lining of both, injured and contralateral areas, expressing cytokeratin (arrows in E) and mesothelin (arrows in F).
Discussion
The nature and origin of the peritoneal repairing cells (PRC) has been subject of a long-lasting dispute. Delaminated mesothelial cells, macrophages, resident mesenchymal or circulating progenitor cells have been alternatively claimed as main agents of the peritoneal regeneration (reviewed in [4]). We think that our results could clarify this point and provide an intermediate model in which most of these points of view can be partially reconciled. According to our findings, the process of peritoneal regeneration apparently involves the recruitment, in the peritoneal wall and within the peritoneal cavity, of bone marrow derived cells which display expression of mesothelial markers (particularly mesothelin), and adhere to the damaged surfaces. We will call this population PRC. These cells have already been quoted by earlier works with different names such as peritoneal exudative cells [14], or tissue repair cells (TRC, [15, 16]), but a characterization of the origin or nature of these cells was hitherto not performed. Most probably PRC are also the same bone marrow derived cells, called ‘myofibroblasts’ and expressing SMCα-actin, that cover fragments of silastic tubing implants in the peritoneal cavity of mice [17].
PRC do not show the typical antigenic profile of mature peritoneal macrophages because we could not find colocalization of F4/80 with mesothelial or fibroblastic markers although a significant fraction of them expressed the antigen CD68 in the cytoplasm. The expression of another macrophage marker, CD11b, in mesothelial cells during the early stages of development of pleural adhesions had previously been reported [8]. These antigenic similarities with macrophages reveal a common lineage between PRC and these cells, as monocyte-derived cells. Some reports have emphasized the heterogeneity of the monocyte/macrophage population not only in its antigenic profile but also in its differentiation potential which seems to be much wider than supposed [18–20]. However, we think that PRC are closely related to fibrocytes, a monocyte-derived cell type involved in processes of cell repairing and fibrosis [21, 22]. Accordingly to the typical fibrocyte description, PRC are large, spindle-shaped cells that express procollagen-1 and SMCα-actin, and they are rapidly recruited to sites of tissue injury. As far as we know, the FSP1 protein, which is distinctly expressed by PRC, had not been hitherto reported in fibrocytes. However, PRC are different from the original description of fibrocytes by the expression of mesothelial markers such as cytokeratin and mesothelin. On the other hand, fibrocytes express CD34, a marker which was not found in PRC by flow cytometry (data not shown). However, we must be cautious when comparing antigenic analysis of free-floating peritoneal cells with cells obtained from tissues, because the antigenic profile can be modulated by their different localization. Thus, despite minor antigenic differences, PRC could be regarded as a specialized type of fibrocyte specifically involved in peritoneal regeneration. Table 1 shows a comparison of the antigenic profile of PRC, fibrocytes, fibroblasts and macrophages based on our data and those published elsewhere [23].
Table 1.
Expression of markers on macrophages, PRC, fibrocytes and fibroblasts according our own results and data from [23]
| Antigen | Macrophages | PRC | Fibrocytes | Fibroblasts |
|---|---|---|---|---|
| CD45 | + | + | + | – |
| CD68 | + | + | + | ± |
| F4/80 | VAR | – | – | – |
| CD34 | VAR | – | VAR | – |
| Mesothelin | – | + | – | – |
| Cytokeratin | – | + | – | – |
| SMCα-actin | – | + | VAR | VAR |
| FSP1 | NT | + | NT | + |
| Procollagen-1 | ± | ± | ± | + |
NT: Not tested; –: No expression; ±, +,VAR: Low, high and variable expression, respectively.
Besides the active reparation process of the injured peritoneal surface, we have observed striking changes in the contralateral peritoneal wall 48 hrs after injury. The activation of uninjured peritoneal areas surrounding the damaged mesothelium had been previously described [24]. It is possible that, as described elsewhere [10], during the earliest stages of peritoneal regeneration mesothelial cells can be released from uninjured areas to adhere to the damaged ones. This is consistent with our observation of an increase of free GFP–/mesothelin+ cells in the peritoneal cavity 24 hrs after surgery. The subsequent denudation would be repaired by recruitment of bone marrow derived PRC. An alternative explanation is that the supposedly uninjured areas spontaneously lose their mesothelial lining due to surgery. Anyhow, the repairing mechanism mediated by PRC would account for the similar number of these cells found in both, the injured and contralateral areas even 1 week after surgery. PRC would replace the lost mesothelium at both sides, and then they probably are released into the peritoneal cavity. In fact, the characteristic dot-like pattern of cytokeratin immunoreactivity of PRC in culture is typical of epithelial cells which have transformed to a mesenchymal state, a cellular process what involves the collapse of the cytokeratin cytoskeleton. This can be observed in cultured mesothelial cells (Fig. 3B) and it has also been described in some epithelial-derived tumour lines [25]. The PRC-derived mesothelial-like lining seems to be provisional, being probably replaced by the proliferating mesothelium, as suggested by the little number of GFP+ cells found in the mesothelium 1 month after surgery.
Mesothelin is a cell surface glycoprotein widely expressed by mesothelial cells. The pro-mesothelin peptide is proteolitically cleaved by furin giving rise to the secreted MPF (megakaryocyte potentiating factor) and the mature mesothelin, whose physiological function is little known [26]. In fact, mice lacking mesothelin expression are viable and fertile [13]. Giving the sharp increase in mesothelin expression observed during peritoneal regeneration and its proposed role as an adhesion molecule [27, 28], it is conceivable that the physiological function of this protein could be related with the control of the release and adhesion of the free-floating cells, critical processes for the mesothelial regeneration.
In summary, we think that most current and confronted points of view about peritoneal regeneration can be reconciled by the PRC concept, a new cell type, probably a monocyte-derived specialized subtype of fibrocyte, which express mesothelial, fibroblastic, leucocytic and macrophage markers. We also think that the potential of the PRC in tissue regeneration will deserve further attention, given the plasticity reported for the adult mesothelial cells [29, 30]. In this way PRC could be involved not only in the regeneration of the peritoneal mesothelium but also in other concomitant processes of visceral tissue repair.
Acknowledgments
This work was supported by grants BFU2008–02384 and SAF2008–1883, (Ministerio de Ciencia e Innovación), RD06/0010/0015 (TerCel network, ISCIII), P06-CTS-01614, P08-CTS-03618 (Junta de AndalucÌa) and LSHM-CT-2005–018630 (VI framework, UE). We thank Dr. Ira Pastan (National Cancer Institute, NIH, Bethesda) for the sending of the anti-mesothelin antibody and their useful advices. We also thank Dr. Eric G. Neilson (Vanderbilt University School of Medicine, Nashville) for the gift of the anti-FSP1 antibody. D. Navas kindly helped us with flow cytometry and confocal microscopy. The authors also thank, S. GarcÌa for irradiating the animals and I. Orman for his expert help with the flow cytometry procedures at the CIEMAT/CIBER-ER.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Yáñez-Mó M, Lara-Pezzi E, Selgas R, et al. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N Engl J Med. 2003;348:403–13. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- 2.Yung S, Chan TM. Intrinsic cells: mesothelial cells – central players in regulating inflammation and resolution. Perit Dial Int. 2009;29:S21–7. [PubMed] [Google Scholar]
- 3.Hertzler AE. The peritoneum. Vol. 1. St. Louis: Mosby; 1919. [Google Scholar]
- 4.Mutsaers SE, Prêle CM, Lansley SM, et al. The origin of regenerating mesothelium: a historical perspective. Int J Artif Organs. 2007;30:484–94. doi: 10.1177/039139880703000606. [DOI] [PubMed] [Google Scholar]
- 5.Eskeland G. Regeneration of parietal peritoneum in rats. 1. A light microscopical study. Acta Pathol Microbiol Scand. 1966;68:355–78. doi: 10.1111/apm.1966.68.3.355. [DOI] [PubMed] [Google Scholar]
- 6.Eskeland G, Kjaerheim A. Regeneration of parietal peritoneum in rats. 2. An electron microscopical study. Acta Pathol Microbiol Scand. 1966;68:379–95. doi: 10.1111/apm.1966.68.3.379. [DOI] [PubMed] [Google Scholar]
- 7.Watters WB, Buck RC. Scanning electron microscopy of mesothelial regeneration in the rat. Lab Invest. 1972;26:604–9. [PubMed] [Google Scholar]
- 8.Amari M, Taguchi K, Iwahara M, et al. Interaction between mesothelial cells and macrophages in the initial process of pleural adhesion: ultrastructural studies using adhesion molecules. Med Mol Morphol. 2006;39:187–92. doi: 10.1007/s00795-006-0340-9. [DOI] [PubMed] [Google Scholar]
- 9.Whitaker D, Papadimitriou J. Mesothelial healing: morphological and kinetic investigations. J Pathol. 1985;145:159–75. doi: 10.1002/path.1711450204. [DOI] [PubMed] [Google Scholar]
- 10.Foley-Comer AJ, Herrick SE, Al-Mishlab T, et al. Evidence for incorporation of free-floating mesothelial cells as a mechanism of serosal healing. J Cell Sci. 2002;115:1383–9. doi: 10.1242/jcs.115.7.1383. [DOI] [PubMed] [Google Scholar]
- 11.Bolen JW, Hammar SP, McNutt MA. Reactive and neoplastic serosal tissue. A light-microscopic, ultrastructural, and immunocytochemical study. Am J Surg Pathol. 1986;10:34–47. doi: 10.1097/00000478-198601000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Wagner JC, Johnson NF, Brown DG, et al. Histology and ultrastructure of serially transplanted rat mesotheliomas. Br J Cancer. 1982;46:294–9. doi: 10.1038/bjc.1982.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–6. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukasawa M, Campeau JD, Yanagihara DL, et al. Mitogenic and protein synthetic activity of tissue repair cells: control by the postsurgical macrophage. J Invest Surg. 1989;2:169–80. doi: 10.3109/08941938909015348. [DOI] [PubMed] [Google Scholar]
- 15.Fukasawa M, Campeau JD, Yanagihara DL, et al. Regulation of proliferation of peritoneal tissue repair cells by peritoneal macrophages. J Surg Res. 1990;49:81–7. doi: 10.1016/0022-4804(90)90114-h. [DOI] [PubMed] [Google Scholar]
- 16.Rodgers KE, diZerega GS. Function of peritoneal exudate cells after abdominal surgery. J Invest Surg. 1993;6:9–23. doi: 10.3109/08941939309141188. [DOI] [PubMed] [Google Scholar]
- 17.Campbell JH, Efendy JL, Han C, et al. Haemopoietic origin of myofibroblasts formed in the peritoneal cavity in response to a foreign body. J Vasc Res. 2000;37:364–71. doi: 10.1159/000025752. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci USA. 2003;100:2426–31. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwana M, Okazaki Y, Kodama H, et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–45. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- 20.Sharifi BG, Zeng Z, Wang L, et al. Pleiotrophin induces transdifferentiation of monocytes into functional endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1273–80. doi: 10.1161/01.ATV.0000222017.05085.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 22.Bucala R. Fibrocytes. New insights into tissue repair and systemic fibroses. Singapore: World Scientific; 2007. [Google Scholar]
- 23.Pilling D, Fan T, Huang D, et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mutsaers SE, Whitaker D, Papadimitriou JM. Mesothelial regeneration is not dependent on subserosal cells. J Pathol. 2000;190:86–92. doi: 10.1002/(SICI)1096-9896(200001)190:1<86::AID-PATH493>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Langner C, Wegscheider BJ, Ratschek M, et al. Keratin immunohistochemistry in renal cell carcinoma subtypes and renal oncocytomas: a systematic analysis of 233 tumors. Virchows Arch. 2004;444:127–34. doi: 10.1007/s00428-003-0948-2. [DOI] [PubMed] [Google Scholar]
- 26.Sathyanarayana BK, Hahn Y, Patankar MS, et al. Mesothelin, stereocilin, and otoancorin are predicted to have superhelical structures with ARM-type repeats. BMC Struct Biol. 2009;9:1. doi: 10.1186/1472-6807-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gubbels JA, Belisle J, Onda M, et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol Cancer. 2006;5:50. doi: 10.1186/1476-4598-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmadbouh I, Chen Y, Louedec L, et al. Mesothelial cell transplantation in the infarct scar induces neovascularization and improves heart function. Cardiovasc Res. 2005;68:307–17. doi: 10.1016/j.cardiores.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Gotloib L, Gotloib LC, Khrizman V. The use of peritoneal mesothelium as a potential source of adult stem cells. Int J Artif Organs. 2007;30:501–12. doi: 10.1177/039139880703000608. [DOI] [PubMed] [Google Scholar]


