Abstract
Due to the extremely limited proliferative capacity of adult cardiomyocytes, human embryonic (pluripotent) stem cell derived cardiomyocytes (hESC-CMs) are currently almost the only reliable source of human heart cells which are suited to large-scale production. These cells have the potential for wide-scale application in drug discovery, heart disease research and cell-based heart repair. Embryonic atrial-, ventricular- and nodal-like cardiomyocytes can be obtained from differentiated human embryonic stem cells (hESCs). In recent years, several highly efficient cardiac differentiation protocols have been developed. Significant progress has also been made on understanding cardiac subtype specification, which is the key to reducing the heterogeneity of hESC-CMs, a major obstacle to the utilization of these cells in medical research and future cell-based replacement therapies. Herein we review recent progress in cardiac differentiation of hESCs and cardiac subtype specification, and discuss potential applications in drug screening and cell-based heart regeneration.
Keywords: pluripotent stem cells, cardiac differentiation, atrial and ventricular specification
Introduction
Human cardiomyocyte are a desirable tool for medical research. Heart disease kills more people than any other disease in industrialized countries, and is becoming the biggest threat to human health in countries with emerging economies. This medical challenge calls for furthermore endeavours to be focussed on heart disease research. In addition, cardiovascular-related toxicity is a major safety concern in pharmacology, and represents the cause of 19% of drug withdrawals from the market [1]. Most cardiac-related research today is conducted using laboratory animals and their heart cells. This raises the question of whether these animals and their cardiomyocytes can fairly reflect human physiology. Toxicity studies comparing the reactions of pharmaceuticals in human and laboratory animals have shown that only 43% of the toxic effects on humans can be predicted by using rodents, and only 63% can be predicted when non-rodents are also included [2]. This suggests that there are clear benefits in using human cardiomyocytes in drug discovery and heart disease research. It is currently impractical to utilize adult human cardiomyocytes in regular medical research due to limited access to human heart tissues and the minimal expansion capacity of cardiomyocytes in vitro. Therefore, it is necessary to develop alternative, reliable and scalable sources of human cardiomyocytes for medical research, and this will require understanding not only the differentiation, but also the physiological maturation of these cells.
For applications such as transplantation therapy for treating myocardial infarction (MI) and cardiotoxicity analyses, it is critical to be able to direct the formation of the correct cell types. To restore the ‘pump’ function of the left ventricle (LV) when treating MI, for example, it is essential to rebuild the damaged LV with ventricular myocytes to optimize electrophysiological integration with recipient tissue and therefore reduce side-effects such as ventricular arrhythmia [3]. In cardiotoxicity testing, the effect of a drug candidate on cardiac electrophysiology, specifically on the interference with ventricular repolarization which raises the risk of ventricular tachycardia arrhythmia, has been one of the major concerns of drug regulatory administrations. Obtaining pure ventricular–like populations from hESCs is critical for the application of human pluripotent stem cell derived cardiomyocytes to cardiotoxicity testing, as guideline ICH S7B emphasizes the importance of evaluating drugs on ventricular repolarization to prevent ventricular tachycardia arrhythmias [1]. Pure atrial- and nodal-like cardiomyocytes are also important for studying atrial and pace-making dysfunctions, and for developing related drugs.
To realize the promise of human cardiomyocytes in both medical research and cell replacement therapy, it is important to establish a reliable source of human cardiomyocytes which can be produced in large quantities. Even though several cell populations, such as cardiac progenitor cells [4,5,6,7,8] and human amniotic membrane-derived mesenchymal stem cells [9] have been shown to have some cardiac differentiation potential, hESCs are much more attractive source for the large-scale production of human cardiomyocytes as they have the capacity to proliferate indefinitely and differentiate into all the cell lineages of the human body [10]. In this review, we will discuss recent progress on the cardiac differentiation of pluripotent stem cells.
Methods for the cardiac differentiation of human embryonic stem cells
Heart development involves a series of highly complex morphogenetic processes that are chronologically regulated by multiple phase-specific signals. Using the chicken as a model, early heart development can be divided subjectively into three phases. In the first phase, before Hamburger & Hamilton stage 3 (HH 3), posterior lateral epiblast cells are stimulated by Activin/TGF-beta and FGF8 from hypoblasts, and by Chordin and Nodal from posterior epiblasts, and move to the distal region of the primitive streak (PS) [11,12]. During the second phase, these cells migrate from the PS to the anterior region of the embryo where they receive inductive signals, including BMP2/4, FGF2/4/8, Crescent, and Wnt11 from the endoderm to form the cardiac mesoderm [13]. In the third phase, cardiac mesoderm cells come to lie under the head folds to form the cardiac crescent, where their fate is determined as cardiac myocytes [14]. Research has shown that BMP4 and bFGF promote hESC differentiation into PS-like populations [15], and WNT/β-catena signalling has biphasic roles during mesoderm formation and the cardiogenesis stages of hESC cardiac differentiation [16].
Recently, significant progress has been made on improving the cardiac differentiation efficacy of hESCs (outlined in Fig. 1A). Initially, hESCs were differentiated into cardiomyocytes spontaneously using a procedure called embryoid body (EB) formation, a method derived from mouse cardiac differentiation protocols. Although the differentiation efficacy was lower than 1%, embryonic atrial-like, ventricular-like and node-like myocytes were identified based on action potential (AP) measurements [17]. By partially mimicking the embryonic cardiac development process, several highly efficient cardiac differentiation protocols have been established in recent years. Laflamme et al. sequentially treated hESCs with Activin A and BMP4 for 5 days in a monolayer culture system and reported that this cardiac differentiation procedure gave a cardiac differentiation efficiency of above 30% 2007. By further manipulating WNT signalling with dickkopf-related protein 1 (DKK1) at the later stages of cardiac differentiation, Yang et al. increased cardiac differentiation efficiency to over 50% 2008. They also showed that during the cardiac mesoderm stage, differentiated hESCs could be divided into three distinct populations based on the expression levels of vascular endothelial growth factor receptor-2 (KDR) and C-KIT; a KDRhigh/C-KIT+ population containing haematopoietic and vascular progenitors, a KDRlow/C-KITneg population containing cardiac progenitors, and a KDRneg/C-KIT+ population consisting of undifferentiated ESCs, primitive-streak-like cells and endodermal cells. Our preliminary results indicate that KDR expression is repressed when cells are treated with Noggin (Ngn). Studies with zebrafish have shown that retinoic acid (RA) signalling restricts the cardiac progenitor pool, and exposure of the anterior lateral plate mesoderm of zebrafish embryos to RA antagonist BMS189453 (RAi) causes uncommitted lateral mesodermal cells to become myocardial progenitors [20]. Based on this information, we added Ngn and RAi to our differentiation system, and achieved cardiac differentiation efficiencies of over 70% [21]. Zambidis' group has published a complex method in which a controlled number of hESCs were seeded in V-bottomed 96-well plates for EB formation. The physiological conditions of early embryos were then mimicked by stage-exposing EBs to physiological oxygen (5%), BMP4, bFGF, insulin and human serum albumin (HSA). The final cardiac differentiation efficacy obtained reached 64–89% [22]. Even though each study reported impressive cardiac differentiation efficacies, a commonly known problem with all the protocols mentioned is that it is sometimes difficult for other laboratories to adopt them. Furthermore, the cardiac differentiation efficacy of a given protocol is different when applied to different pluripotent stem cell lines [23]. Sometimes, a given protocol and cell line can even yield significant differences from one experiment to the next in one laboratory. This instability is one of the major hurdles in the broad application of pluripotent stem cell derived cardiomyocytes. Continued endeavours to optimize the cardiac differentiation procedures of pluripotent stem cells are essential for fully realizing their potential in heart regeneration. Compared with the natural process of heart development, the inductive signals provided by these protocols are obviously incomplete. In our opinion, the non-cardiac cells in the cultures may provide some important, but as yet overlooked cardiac inducing signals. Therefore, the details of procedures, such as the EB size used in suspension culture systems and the cell density used for seeding monolayer culture systems, although hard to control practically, are crucial for the success of hESC cardiac differentiation, and should be documented thoroughly.
Fig 1.
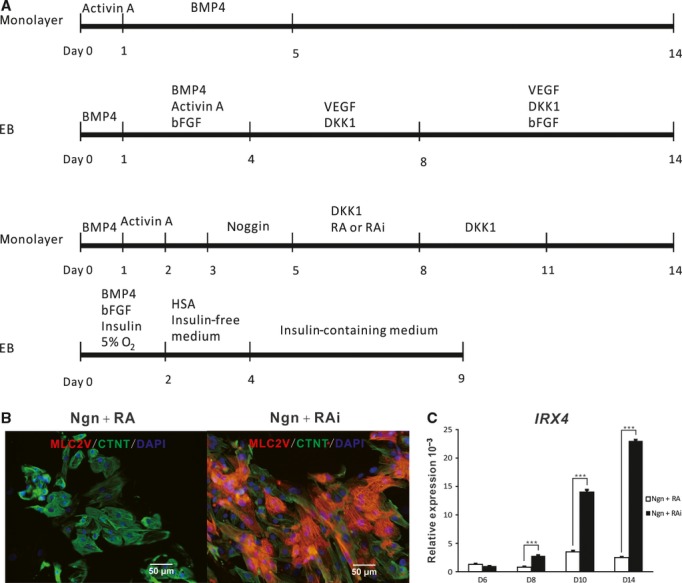
Direct differentiation and characterization of cardiomyocytes from human embryonic stem cells. (A) Representative protocols for the direct differentiation of hESCs into cardiomyocytes. The top timeline shows a protocol developed by Laflamme et al. in which hESCs are sequentially treated with Activin A and BMP4 in a monolayer culture format 2007. The second timeline shows a cardiac differentiation method established by Yang et al. that involves EB formation and treatment with combinations of Activin A, BMP4, bFGF, DKK1 and VEGF for the time intervals indicated 2008. The third timeline stands for a protocol derived by our group in which monolayer hESCs are chronologically induced with Activin A, BMP4, bFGF, Ngn and Dkk1, and either RA or its inhibitor RAi as indicated [21]. The bottom timeline shows the protocol developed by Burridge et. al, in which EBs are formed and stage-exposed to physiological oxygen (5%), BMP4, bFGF, insulin and human serum albumin (HSA) 2011. (B) Immunostaining of 60-day-old Ngn + RA and Ngn + RAi induced cultures for MLC-2V (red) and cTNT (green) using the protocol described by the third timeline in (A). (C) Quantitative RT-PCR analysis of the kinetics of Irx-4 gene expression in Ngn + RA and Ngn+RAi treated cultures using the protocol described by the third timeline in (A). Average Irx-4 expression, normalized to GAPDH, is shown.
In addition to improving cardiac differentiation efficacy, progress has been made on the cardiac subtype specification of hESC-CMs. Laflamme's group have shown that 20% of the hESC-CMs induced with Activin A and BMP4 are embryonic nodal cells, and that inhibition of NRG-1β/ErbB signalling enhances the proportion of hESC-CMs with a nodal phenotype [24]. Of interest, Melkoumian et al., performed with the same protocol as Laflamme's group, except that the Matrigel matrix was substituted with a synthetic surface made of acrylate conjugated with a vitronectin active domain peptide, obtained a relatively homogeneous embryonic ventricular myocyte population (88% ventricular myocytes and 12% atrial myocytes, with no nodal myocytes) 2010. A study using chicken embryos has shown that inhibition of RA signalling within critical periods produces embryos with oversized ventricles and smaller or missing atria, and that exogenous addition of RA results in reverted phenotypes [26]. Based on this information, we examined the roles of RA signalling in the cardiac subtype specification of differentiated hESCs, and found that retinoid signals regulate atrial versus ventricular specification during the cardiac differentiation of hESCs. When the RA inhibitor BMS 189453 is added to cultures, expression of the ventricular-chamber-specific genes Irx-4 and Mlc-2v are significantly elevated and 83% of the hESC-CMs have embryonic ventricular-like APs (Fig. 1B and C); although 94% of the hESC-CMs have embryonic atrial-like APs when RA is applied [21]. Hrt1 and Hrt2, which are expressed in atrial and ventricular precursors respectively [27], are differentially expressed in RA and RAi treated cultures.
Maturation of ESC-differentiated cardiomyocytes
The maturation of hESC-CMs is a long and poorly understood process. During human heart development, embryonic and neonatal cardiomyocytes proliferate until soon after birth, and the continued growth of the heart is largely dependent on the increase in myocyte size over a period of about 10–20 years before the adult size is reached [3]. The gene expression profiles and physiological properties of hESC-CMs also change with time; for example, the number of hESC-CMs that express ventricular-specific sarcomeric protein MLC-2V increases over time, stabilizing after 90 days of differentiation [28]. The density of AP-related currents such as Ica, Ikr, Ito and Ik1, increases from early to later stages of hESC-CM differentiation, reflecting the maturation process [29]. One of the major differences between atrial-like and ventricular-like APs is that atrial-like APs have a shorter AP duration than that of ventricular-like APs. Blocking Ito currents with TTX and Cd2+ in rabbit cardiomycoytes demonstrates that reducing the density of Ito enlarges the duration of atrial-like APs but has minimal impact on the duration of ventricular APs [30]. Therefore, the weak Ito current of newly differentiated cardiomyocytes may be the reason why it is hard to distinguish embryonic atrial- and ventricular-like APs at early stages of hESC-CM differentiation [31,32], but relatively easy at later stages of differentiation [17,21,33,34,35].
The electrophysiological maturation of hESC-CMs is influenced by the presence of non-cardiac cells in the culture, however, the underlying molecular mechanisms are still largely undiscovered [36]. Mechanical and electrical stimuli are important factors promoting the maturation of hESC-CMs in vitro. Forced contractile stimuli and electrical signals which mimic those of the heart facilitate hESC-CM realignment, strengthen synchronous contractions and increase the density of the gap junction in hESC-CMs in vitro [37,38].
Perspective
The hESC-CMs, particularly myocytes with a ventricular phenotype, are of great interest for cell-based heart regeneration. Arrhythmia, the most severe side-effect of cell replacement treatments for heart infarction [39,40], is primarily caused by the incompatibility of electrophysiological properties between engrafted cells and the recipient heart. Because nodal, atrial and ventricular myocytes have different electrophysiological properties, implantation of relatively homogeneous hESC-CMs with ventricular phenotypes is essential for the electrophysiological integration of engrafted cells with the recipient heart ventricle, and reducing the risk of ventricular arrhythmia [3]. Unlike their adult counterparts, hESC-CMs are immature and beat spontaneously. Engrafting hESC-CMs with spontaneous excitability could thus potentially trigger arrhythmia in cell-based heart repair therapies [41]. During human development, the maturation of the human heart takes at least 9 months, and possibly even up to 20 years, and involves mechanical and electrical stimuli. Although it is extremely difficult to imagine that this long-term process could be shortened or mimicked in vitro, special attention should at least be paid to eliminating the spontaneous excitability of hESC-CMs.
In addition to their potential applications in cell therapy, hESC-CMs are an ideal tool for cardiotoxicity testing. Several cardiac and non-cardiac drugs, which have passed animal toxicity tests, have been withdrawn from the market due to their cardiovascular toxic effects in humans [42]. Very often, due to limited access to human tissues, the first time that a drug candidate is tested on human material is in clinical trials. Using human ESC-CMs for in vitro cardiotoxicity analysis may help to overcome problems of species variation between human and laboratory animals. Cardiotoxic studies performed with hESC-CMs with a ventricular subtype have shown that the drug responses of hESC-CMs were highly predictive of clinically observed cardiotoxic effects, despite the relative immaturity of hESC-CMs [43]. There is increasingly strict regulation on the release of candidate drugs due to their potential interference with ventricular repolarization which may be the cause of ventricular tachycardia arrhythmia. The ready availability of ventricular-like myocytes derived from hESCs is therefore of extensive interest due to the requirements of ICH Safety Guideline S7B [44].
One of the challenges for realizing the promise of hESC-CMs is their capacity for large-scale production. A key difference between currently available hESC cardiac differentiation methods [18,19,21,22] is the involvement of the EB formation process. The size of EBs is critical for efficient cardiac differentiation. Unlike their mouse counterparts, controlling the cardiac differentiation process of human ESCs is challenging, as the formation of EBs of relatively uniform size is difficult. On the other hand, monolayer culture is comparatively easy to handle and suitable for large-scale culture. One big hurdle for large-scale production of human cardiomyocytes using human pluripotent stem cells is the instability of currently available protocols. Clearly, more effort should be invested in identifying cardiac inducing factors, and further optimizing the induction culture system. Because hESC derivatives do not proliferate a lot during cardiac differentiation, obtaining large numbers of cardiomyocytes is also dependent on the presence of large numbers of un-differentiated hESCs as starting materials. Therefore, large-scale production of hESC-CMs also depends on the large-scale production of hESCs.
Although there are still many challenges to overcome before broad application of hESC-CMs is possible in medical research, significant progress has been made on improving cardiac differentiation efficacy and cardiac subtype specification of hESC-CMs. These advances not only open up a new era in heart-related research, but may also make heart cell transplantation therapy one step closer to reality.
Acknowledgments
We thank Dr. Joy Fleming for critical discussion and revision of the manuscript. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01020201), the National Basic Research Program of China (2010CB945024 and 2011CB965002) and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-50).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- Mandenius CF, Steel D, Noor F, et al. Cardiotoxicity testing using pluripotent stem cell-derived human cardiomyocytes and state-of-the-art bioanalytics: a review. J Appl Toxicol. 2011;31:191–205. doi: 10.1002/jat.1663. [DOI] [PubMed] [Google Scholar]
- Olson H, Betton G, Robinson D, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- Chen HS, Kim C, Mercola M. Electrophysiological challenges of cell-based myocardial repair. Circulation. 2009;120:2496–508. doi: 10.1161/CIRCULATIONAHA.107.751412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–8. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Mishra R, Vijayan K, Colletti EJ, et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation. 2011;123:364–73. doi: 10.1161/CIRCULATIONAHA.110.971622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Nagai T, Wada H, et al. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol. 2007;176:329–41. doi: 10.1083/jcb.200603014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Miyoshi S, Ikegami Y, et al. Xenografted human amniotic membrane-derived mesenchymal stem cells are immunologically tolerated and transdifferentiated into cardiomyocytes. Circ Res. 2010;106:1613–23. doi: 10.1161/CIRCRESAHA.109.205260. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Curr Top Dev Biol. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- Ladd AN, Yatskievych TA, Antin PB. Regulation of avian cardiac myogenesis by activin/TGFbeta and bone morphogenetic proteins. Dev Biol. 1998;204:407–19. doi: 10.1006/dbio.1998.9094. [DOI] [PubMed] [Google Scholar]
- Wagner M, Siddiqui MA. Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation. Exp Biol Med (Maywood) 2007;232:866–80. [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–35. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Yu P, Pan G, Yu J, et al. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell. 2011;8:326–34. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, et al. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci USA. 2006;103:19812–7. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JQ, Ma Y, Lee Y, et al. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Begemann G, et al. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005;307:247–9. doi: 10.1126/science.1101573. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Jiang J, Han P, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–87. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–40. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Xie Y, Moyes KW, et al. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–86. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkoumian Z, Weber JL, Weber DM, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 2010;28:606–10. doi: 10.1038/nbt.1629. [DOI] [PubMed] [Google Scholar]
- Yutzey K, Gannon M, Bader D. Diversification of cardiomyogenic cell lineages in vitro. Dev Biol. 1995;170:531–41. doi: 10.1006/dbio.1995.1234. [DOI] [PubMed] [Google Scholar]
- Nakagawa O, Nakagawa M, Richardson JA, et al. HRT1, HRT2, and HRT3: a new subclass of bHLH transcription factors marking specific cardiac, somitic, and pharyngeal arch segments. Dev Biol. 1999;216:72–84. doi: 10.1006/dbio.1999.9454. [DOI] [PubMed] [Google Scholar]
- Fu JD, Jiang P, Rushing S, et al. Na+/Ca2+ exchanger is a determinant of excitation-contraction coupling in human embryonic stem cell-derived ventricular cardiomyocytes. Stem Cells Dev. 2009;19:773–82. doi: 10.1089/scd.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartiani L, Bettiol E, Stillitano F, et al. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–44. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol. 1988;405:123–45. doi: 10.1113/jphysiol.1988.sp017325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satin J, Kehat I, Caspi O, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–96. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WZ, Xie Y, Moyes KW, et al. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–86. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Fleischmann BK, Selbert S, et al. Selection of ventricular-like cardiomyocytes from ES cells in vitro. FASEB J. 2000;14:2540–8. doi: 10.1096/fj.00-0002com. [DOI] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–9. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wilson GF, Soerens AG, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Majdi M, Xia P, et al. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev. 2010;19:783–95. doi: 10.1089/scd.2009.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004;101:18129–34. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XM, Zhao YS, Chang HX, et al. Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation. 2006;113:2229–37. doi: 10.1161/CIRCULATIONAHA.105.583039. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- Menasche P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- Kehat I, Khimovich L, Caspi O, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- Lasser KE, Allen PD, Woolhandler SJ, et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:2215–20. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- Braam SR, Tertoolen L, van de Stolpe A, et al. Prediction of drug-induced cardiotoxicity using human embryonic stem cell-derived cardiomyocytes. Stem Cell Res. 2010;4:107–16. doi: 10.1016/j.scr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration International Conference on Harmonisation; guidance on S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; availability. Notice. Fed Regist. 2005;70:61133–4. [PubMed] [Google Scholar]


