Abstract
Recently the protein myozap, a 54-kD polypeptide which is not a member of any of the known cytoskeletal and junctional protein multigene families, has been identified as a constituent of the plaques of the composite junctions in the intercalated disks connecting the cardiomyocytes of mammalian hearts. Using a set of novel, highly sensitive and specific antibodies we now report that myozap is also a major constituent of the cytoplasmic plaques of the adherens junctions (AJs) connecting the endothelial cells of the mammalian blood and lymph vascular systems, including the desmoplakin-containing complexus adhaerentes of the virgultar cells of lymph node sinus. In light and electron microscopic immunolocalization experiments we show that myozap colocalizes with several proteins of desmosomal plaques as well as with AJ-specific transmembrane molecules, including VE-cadherin. In biochemical analyses, rigorous immunoprecipitation experiments have revealed N-cadherin, desmoplakin, desmoglein-2, plakophilin-2, plakoglobin and plectin as very stably bound complex partners. We conclude that myozap is a general component of cell–cell junctions not only in the myocardium but also in diverse endothelia of the blood and lymph vascular systems of adult mammals, suggesting that this protein not only serves a specific role in the heart but also a broader set of functions in the vessel systems. We also propose to use myozap as an endothelial cell type marker in diagnoses.
Keywords: myozap, adherens junctions, endothelial cells, blood vessels, lymphatic vessels
Introduction
The enormous progress in the elucidation of the ultrastructural organization and molecular composition of the intercellular junctions over the past decades has primarily been based on intense cell fractionation and analytical biochemical work which, together with immunolocalization microscopy, resulted in the classification of four major molecular biologically defined categories: gap junctions, tight junctions, desmosomes and adherens junctions (AJs; [1]). In addition, a number of novel hybrid forms of junctions have been described, including the extensive composite junctions (areae compositae) connecting the cardiomyocytes of the adult mammalian heart, which represent amalgamated forms of both ensembles, the desmosomes and the AJs [1-7]. In particular, in the field of heart development and cardiac function, the definition and molecular analysis of the composite junctions was of great importance as it provided the basis for the molecular explanations of the human mutations which result in arrhythmogenic right ventricular cardiomyopathies, a frequent cause of sudden death in previously healthy individuals [14-16].
Recently, a novel kind of protein called myozap, encoded within the so-called ‘myozap/GRINL1A’ gene complex [17], was discovered as a major constituent of the cardiac composite junctions which fulfills important functions in the heart and perhaps also in other parts of the cardiovascular system [18]. Using in situ hybridization and immunoblots, Seeger et al. [18] could further show that myozap also exists in vascular structures of early embryonal development of the mouse as well as in lung and aorta tissues of different mammalian species. To further explore and extend these findings and to identify and localize myozap out of the heart, we have generated highly sensitive and specific monoclonal and polyclonal antibodies, which have allowed to identify myozap as a general component of the AJ structures of both the myocardium as well as major components of the blood and the lymph vessel systems.
Materials and methods
Cardiac and vascular tissues
Bovine and adult or foetal porcine tissues of freshly killed animals were obtained from a local slaughterhouse (Mannheim, Germany) or from the abattoir of the Institute for Veterinary Genetics (Friedrich-Loeffler-Institut, Mariensee, Germany). Mouse and rat tissues from animals of various ages were obtained as reported [3, 18, 19]. Protocols for rapid freezing and aldehyde fixations of tissue samples were essentially as reported [3, 4, 14–16, 18, 19]. Some cryopreserved or formaldehyde-fixed, paraffin-embedded human tissue samples were kindly provided by Dr. R. Moll (Institute for Pathology, Universities of Marburg and Giessen, Germany) or were obtained from the tissue bank of the National Center for Tumour Diseases (NCT, Heidelberg, Germany). All tissue samples were obtained and processed in compliance with the regulations of the Ethics Committees of the Universities of Heidelberg and Marburg (see also Refs. [3, 7, 15, 16, 18, 19]).
For endothelial monolayer preparations freshly obtained bovine aortae were used directly or kept on ice under rinsing with ice-cold PBS containing CaCl2[20, 21]. The aortae were opened with scissors and prepared so that relatively large areas of endothelium could be harvested by gently scraping off with the edge of a rubber policeman, a glass object slide, or a coverslip. These native endothelial monolayers were then directly spread on another object slide as described [20, 21]. The harvested endothelial cell layers were monitored by microscopy, and well-preserved preparations were chosen for further processing, be it by SDS-PAGE or fixation for 2 min. in methanol, followed by 5 min. in acetone, or by fixation for electron microscopy [15, 16].
Cell cultures
Neonatal rat cardiomyocytes were isolated and cultured for 5–6 days as described [7, 13]. Permanently growing mouse adult cardiomyocyte-derived cells of line HL-1 were kept in culture as described [22]. Human microvascular endothelial cells from dermis (HMVEC-D) or lung (HMVEC-L) were purchased from Lonza (Cologne, Germany) and grown in culture according to Lonza protocols. Human umbilical cord venous endothelial cells (HUVECs), calf pulmonary endothelial cells [23], lung carcinoma A549 cells [24], SV-80 fibroblasts [25] and HaCat keratinocyte cultures [26] all were grown as described [27].
Biochemistry
For preparations of cell lysates, monolayer cell cultures were rinsed twice briefly with PBS and suspended in SDS-PAGE sample buffer containing benzonase (1:1000; Merck, Darmstadt, Germany) using a rubber policeman. Tissue lysates were prepared using the same sample buffer, and hundreds of selected cryostat sections of specific tissue regions were collected. After vigorous homogenization, cell or tissue lysates were heated to and kept at ca. 95°C for 4–5 min., centrifuged at 15,000 g for 10 min. and subjected to SDS-PAGE, followed by transfer to PVDF membranes (ImmobilonP; Millipore, Bedford, USA). 2D-PAGE with isoelectric focusing was performed as described [28]. For immunoblot analyses, horseradish peroxidase-conjugated secondary antibodies were applied in combination with an enhanced chemiluminescence system (ECL; Fisher Scientific, Schwerte, Germany).
To minimize protein degradation, fractionations of cell and tissue lysates were done on ice and with additions of protease inhibitors (Complete Mini Inhibitor Tabs; Roche Diagnostics, Mannheim, Germany). Cells were washed twice in PBS, scraped off the cell culture dish surface with a rubber policeman and disrupted with a Dounce homogenizer (B. Braun Biotech, Melsungen, Germany). Cryopreserved tissue samples were sectioned in a Leica CM3050S research cryostat (Leica, Wetzlar, Germany), and 100–200 sections were usually sampled for homogenization in a Dounce homogenizer. Tissue or cell lysates were centrifuged at 10,000 g for 10 min. and the pellets were treated with 1% Triton X100 in PBS (‘low salt buffer’; ‘PBS+T’), followed by centrifugation and another extraction of the pellets in 1% Triton X100, 0.5 M NaCl in PBS (‘high salt buffer’; ‘PBS+T+S’). After a final centrifugation step, the residual pellets, referred to as ‘cytoskeletal’ or ‘insoluble fraction’ (‘Ins. Fract.’), were suspended by homogenization in sample buffer. The proteins of the supernatants of both types of extractions and of the residual pellets (‘insoluble fraction’) were resuspended in sample buffer and precipitated in methanol (1:4) at –20°C overnight. Methanol was decanted and precipitates were air-dried for few minutes, followed by resuspension in the same volume of sample buffer (50–100 μl), depending of the specific amount of cell or tissue material.
For immunoprecipitation, freshly harvested HL-1 cells were washed in PBS and scraped off the culture dish in the low salt buffer PBS+T mentioned afore or in ‘RIPA buffer’ (150 mM NaCl, 5 mM EDTA, 20 mM HEPES, pH 7.5, 1% Nonidet-P40, 0.2% deoxycholate, 0.1% SDS) as described [29], followed by a brief but intense Dounce homogenizer treatment. Lysates were centrifuged for 10 min. at 15,000 g, and the supernatants were used for immunoprecipitation with antibodies coupled to Dynabeads (Dynal, Hamburg, Germany) according to the supplier's protocol [30].
Identification of the immunoprecipitation bands by electron spray ionization (ESI) was performed in the Protein Analysis Core Facility (Dr. M. Schnölzer) of the German Cancer Research Center.
Generation of antibodies
The full length clone of the protein encoded by the human myozap/GRINL1A complex locus (accession no. ADA68358; cf. [17, 18]) in pCR4-TOPO vector was purchased from ImaGenes GmbH (Berlin, Germany). Polymerase chain reaction was performed to add 5′EcoRI/BglII(BamHI) and 3′SalI/EcoRI restriction sites for integration of the myozap/GRINL1A sequence into the pQE30 protein synthesis vector. Myozap protein was produced in competent M15 pREP4 bacteria and purified using the QIAexpress protein purification system (Qiagen, Hilden, Germany). Recombinant myozap protein was then used to generate monoclonal antibodies (mAb myozap clone 517.67 and mAb myozap clone 101.2) in BALB/c mice, using the Monoclonal Antibody Core Facility (Dr. H. Zentgraf) of the German Cancer Research Center. For the screening of the immunoglobulins secreted by the specific clones, cryostat sections obtained with a Leica CM 3050S cryostat (Leica) from mammalian heart tissue were mounted on 10-well (6.7 mm) diagnostic object slides (Menzel, Braunschweig, Germany). For antibody characterizations SDS-PAGE was performed with lysates containing the recombinant human myozap protein, using lysates of HL-1 cells or of bovine heart muscle tissue. The mAb-containing hybridoma supernatants were examined by immunoblotting using a 28-channel slot blot apparatus (Biometra, Goettingen, Germany).
For direct immunizations, the following polypeptides were synthesized (Peptide Specialty Laboratories, Heidelberg, Germany), representing the aminoterminal (hNT) and the carboxyterminal (hCT) amino acid sequences of the human myozap protein, and conjugated to keyhole limpet cyanin to trigger and enhance the immunoreaction. The polypeptides were mixed with Freud's adjuvants (Sigma, St. Louis, MO, USA) and used for the immunization of guinea pigs to generate polyclonal antibodies to the following two epitope-bearing polypeptides: ‘Myozap GP1A/B (hNT)’: MLRSTSTVTLLSGGAART(+C); ‘Myozap GP2A/B (hCT)’: (C+)YTRVLELTMKKTLT.
Other antibodies
Antibodies used for comparisons have been described [29-31]. In addition, rabbit antisera against VE-cadherin (Cayman Chemical, Ann Arbor, MI, USA) have been used.
Light and electron microscopy
Single- and double-label immunofluorescence microscopy was performed as described elsewhere [3, 7, 15, 19, 29, 30]. For paraffin-embedded tissue sections the antigen retrieval technique was applied for immunofluorescence microscopy, usually for 10 min. at 120°C in a Milestone Microwave Vacuum Histoprocessor (Mikron, Vista, CA, USA) and in 0.1M Tris-buffered saline containing 5% urea (pH 9.5). Images were obtained with a Zeiss confocal laser scanning microscope (LSM 700; Zeiss, Jena, Germany). 4′,6-Diamidino-2-phenylindol (DAPI; Serva, Heidelberg) was applied for staining nuclei [7], and standard transmission electron microscopy as well as immunoelectron microscopy was performed as repeatedly described [3, 7, 18, 19].
Results
Highly sensitive and specific myozap antibodies
The recent discovery of the protein myozap as a novel major constituent of the composite junctions of intercalated disks of the mammalian heart [18] is especially remarkable as this cell molecule represents a protein type in its own right that does not belong to any of the multigene families known. This uniqueness as well as some indications in Northern and Western blots that myozap might also occur in specific cell types of certain other tissues have prompted us to generate highly sensitive and specific monoclonal as well as conventional antibodies that would allow (i) the detection and localization of this protein, even in cells or cell structures containing only very low amounts, (ii) the exploration of myozap for diagnostic problems and (iii) further elucidations of myozap functions. The four types of antibodies obtained all meet these expectations. In the following, however, we present, for the most part, results obtained with murine monoclonal antibodies of clone 517.67.
Immunodetection of myozap and myozap-complexes in biochemical and immunolocalization experiments using myocardiac cells
Using Northern and Western blot tests, protein myozap (Mr 54 kD) had been detected not only in heart but also in placenta and lung tissue [18]. Immunoblot analyses using the novel very sensitive antibodies have now allowed us to identify this protein not only in myocardial tissues of the diverse mammals examined but also in cultures of murine cardiomyocyte-derived, permanently growing HL-1 cells [22] as well as in lung tissue and, in lesser amounts, in certain other tissues such as kidney and liver (Fig. 1, upper panel). Fractionation experiments have further shown that protein myozap is enriched in the Triton-insoluble, cytoskeletal residue fractions containing cell–cell junction structures (Fig. 1, lower panel). Remarkably, in these fractions relatively more protein myozap has been recovered than some of the ‘classic’ cell junction constituents such as N-cadherin and plakoglobin (Fig. 1, lower panel). Two-dimensional gel electrophoresis of the polypeptides present in HL-1 cell lysates, followed by immunoblotting with the new myozap antibodies, further allowed us to distinguish two major variants of this polypeptide with isoelectric points of ca. 6.02 and 6.20. The novel murine monoclonal and guinea pig polyclonal antibodies also specifically and intensely decorated composite junction structures, whether they occurred in frozen myocardial tissue, in primary cultures of neonatal rat cardiomyocytes, or in HL-1 cardiomyocyte cultures (Fig. 2A–C). In all comparative localization experiments using myocardial tissue or HL-1 transformed cardiomyocyte cultures (Fig. 2C–F) protein myozap showed partial colocalization with several other junction plaque proteins, including actin filament-binding ones (for an example, see protein ZO-1; Fig. 2D and E) and members of the armadillo protein family (Fig. 2F presents β-catenin for example).
Fig 1.
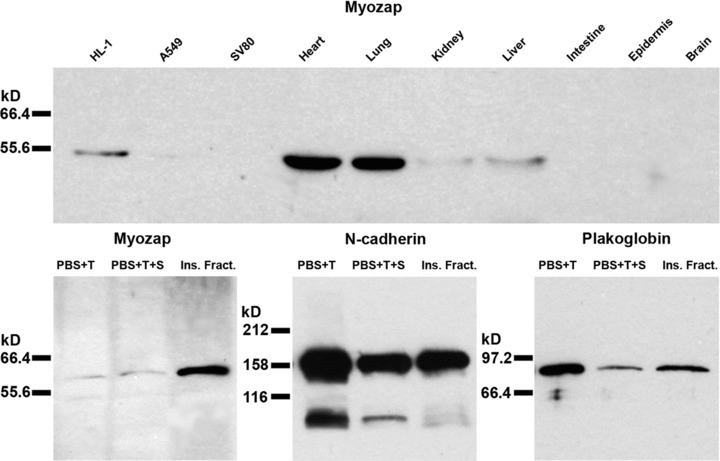
Immunoblot identification of protein myozap after SDS-PAGE of the polypeptides of diverse cell culture lines and tissues as well as cell fractions. Upper panel: Myozap (∼54 kD) detected in cell culture lysates (mouse HL-1 cardiomyocytes, human A549 lung epithelial cells, human SV80 fibroblasts) and in lysates of various mouse tissues (heart, lung, kidney, liver, intestine, epidermis, brain), using a myozap mAb (clone 517.67). Note that HL-1 cells as well as heart and lung tissue contain appreciable amounts of protein myozap, whereas much lesser amounts are detected in kidney and liver lysates and no significant signal has been obtained in intestine, epidermis and brain lysates. Lower panel: Immunoblot demonstrations of myozap in specific cell fractions of mouse HL-1 cardiomyocyte lysates. Note that appreciable amounts of N-cadherin and plakoglobin are also recovered in the ‘low salt’ (PBS + 1% Triton X100; ‘PBS+T’), the ‘high salt’ (PBS + 1% Triton X100 + 0.5 M NaCl; ‘PBS+T+S’) and the residual ‘cytoskeletal’ fractions (insoluble fraction; ‘Ins. Fract.’), while myozap is highly enriched in the cytoskeletal fraction.
Fig 2.
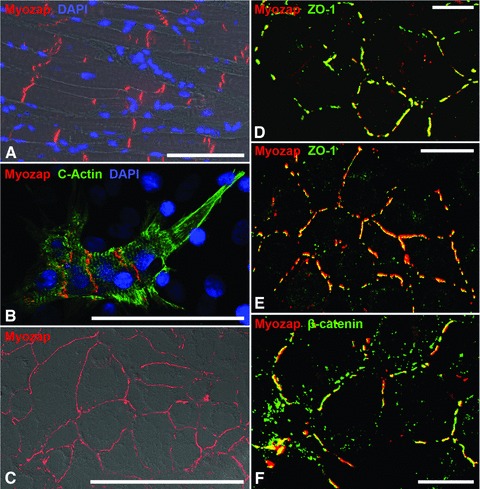
Immunofluorescence microscopy of protein myozap in the composite junctions of mammalian cardiomyocytes. (A) Immunofluorescence micrograph of a cryostat section through bovine myocardium using myozap mAb (clone 517.67, red), in combination with nuclear DAPI staining (blue) and phase contrast microscopy. Note the intense positive reaction of the intercalated disks (IDS). (B) Double-label immunofluorescence microscopy of primary cardiomyocyte cultures of neonatal rat hearts, using murine mAbs to α-cardiac actin (green) and polyclonal guinea pig antibodies to myozap (red), in comparison with DAPI staining (blue). Note the specific reaction of the composite junctions of the IDS. (C–F) Cardiomyocyte-derived HL-1 mouse cells grown in dense culture monolayers and labelled with myozap mAb (red), in a differential interference contrast (DIC) image (C) or in comparison with the actin microfilament-associated junctional protein ZO-1 (D and E; green) and the major adherens junction plaque protein β-catenin (F; green). Note the intense, specific reaction of the composite junctions of the intercalated disk-derived structures (A, B). Note also the colocalization of myozap with parts of the structures positive for further adherens junction-associated plaque proteins (yellow merged colour in D–F). Bars: 100 μm (A–C); 20 μm (D–F).
When immunoprecipitation experiments using these myozap antibodies were performed with extracts from cardiac tissue samples or from HL-1 cell cultures, followed by SDS-PAGE with subsequent silver staining and immunoblotting, enrichments of certain other polypeptides were noted (Fig. 3A). After the very rigorous extraction conditions in detergent-rich RIPA buffer, four proteins consistently co-immunoprecipitated with myozap, which were not found in controls, i.e. pellets from lysates incubated with anti-species immunglobulin-coupled beads alone. When these polypeptide bands were extracted and used for ESI mass spectometry (Fig. 3A and B) they were identified as desmoplakin and plectin (Fig. 3B).
Fig 3.
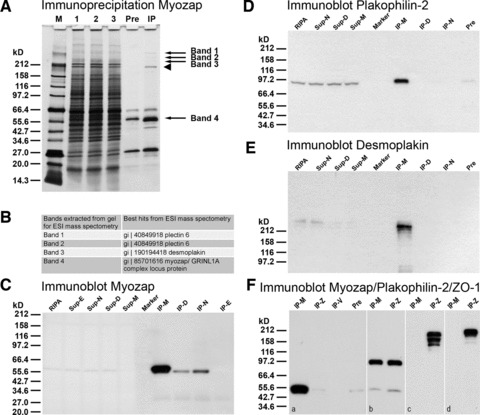
Results of immunoprecipitation experiments using myozap mAb (clone 517.67) and mouse cardiomyocyte HL-1 cell lysates. (A) SDS-PAGE of polypeptides of fractions obtained in immunoprecipitation experiments as seen after silver staining: lane M: broad range Mr reference molecules; lane 1: initial RIPA buffer lysate; lane 2: supernatant of the ‘preclearing step’; lane 3: supernatant after myozap immunoprecipitation; lane ‘Pre’: polypeptides of the ‘preclearing step’ absorbed to dynabeads; lane ‘IP’: lysate of myozap immunoprecipitate. Bands 1–4 denoted by arrows have been used for electron spray ionization (ESI) mass spectometry. Band 4, arrow denotes the band containing both myozap as well as residual desmin and IgG heavy chain. Arrowhead, immunoglobulin (IgM heavy chain residual contamination). (B) Table of results of ESI mass spectometry of the extracted bands 1–4 (see arrows in A). Note that, together with myozap (band 4), three other proteins are detected in the immunoprecipitates: desmoplakin (in band 3), plectin (in bands 1 and 2) and the intermediate filament protein desmin (in band 4). (C) Immunoblot reactions with myozap mAb: lane ‘RIPA’: initial RIPA buffer lysate; lane ‘Sup-E’: supernatant of immunoprecipitation using E-cadherin antibodies; lane ‘Sup-N’: supernatant of N-cadherin immunoprecipitation; lane ‘Sup-D’: supernatant of desmoglein-2 (Dsg-2) immunoprecipitation; lane ‘Sup-M’: supernatant of myozap immunoprecipitation; lane ‘Marker’: broad range protein Mr markers (New England Biolabs, Frankfurt, Germany); Mr values are indicated on the left margin; lane ‘IP-M’: myozap immunoprecipitate; lane ‘IP-D’: desmoglein-2 immunoprecipitate; lane ‘IP-N’: N-cadherin immunoprecipitate; lane ‘IP-E’: E-cadherin immunoprecipitate (negative control). (D, E) Fractions as in B, showing immunoblot reactions with antibodies to plakophilin-2 (D) and desmoplakin (E). Lane designations as in C. Note that under these conditions immunocomplexes of myozap with desmoplakin and plakophilin-2 are identified. (F) Specific parallel immunoprecipitations using antibodies to protein myozap (IP-M; F, a), protein ZO-1 (IP-Z; F, a), VE-cadherin (IP-V; F, a), in comparison with the ‘pre-clearing’ pellet (‘Pre’; see also above) as negative control. For comparison, immunoprecipitates obtained with antibodies to plakophilin-2 (b) and to protein ZO-1 using RIPA buffer (c) or Triton-X100-containing buffer (d) are shown. The specific immunoblot reactions with antibodies to myozap (a), plakophilin-2 (b) and protein ZO-1 (c, d) are shown.
Detailed immunoblot analyses of immunoprecipitation experiments using HL-1 cell lysates revealed special molecular binding of myozap to the cadherins, desmoglein Dsg-2 (Fig. 3C, lane IP-D), and N-cadherin (Fig. 3C, lane IP-N), whereas E-cadherin served as a negative control (Fig. 3C, lane IP-E). In addition, protein myozap was also demonstrated in complexes with the plaque proteins plakophilin-2 (Fig. 3D) and desmoplakin (Fig. 3E) and, in lesser amounts and less regularly, β-catenin (not shown). Vice versa, when more diluted HL-1 cell lysates in RIPA or Triton-X100-containing buffer were used for immunoprecipitations with myozap antibodies the pelleted myozap complexes (Fig. 2Fa) were not demonstrably complexed with protein ZO-1 (Fig. 3Fa, lane IP-Z). However, under the same conditions myozap was found to be associated with plakophilin-2 in nearly equimolar amounts with protein ZO-1 (Fig. 3Fb). Finally, under our experimental conditions protein myozap was not detected in the protein ZO-1 immunoprecipitates (Fig. 3Fc, d).
Identification of protein myozap in adhering junctions connecting blood vessel endothelial cells
Using the novel antibodies to myozap in immunofluorescence microscopy we noted extensive reactions specific for endothelial cells of blood vessels of a wide range of calibres and in all kinds of tissues examined, from the zonulae adhaerentes of veins of different calibres to arteries and arterioles and even the smallest capillaries (Fig. 4A and B). The endothelia of small foetal capillaries, including those of human placenta, were also myozap-positive (Fig. 4C and D), thus explaining the Northern blot results of Seeger et al. [18]. In all types of blood vessels examined, the myozap antibodies showed colocalization with β-catenin (e.g.Fig. 4D) and all the other endothelial armadillo proteins. By contrast, the AJs of the one-layered placental epithelium were positive for E-cadherin but negative for myozap whereas β-catenin was also seen in colocalization with myozap (Fig. 4C and D).
Fig 4.
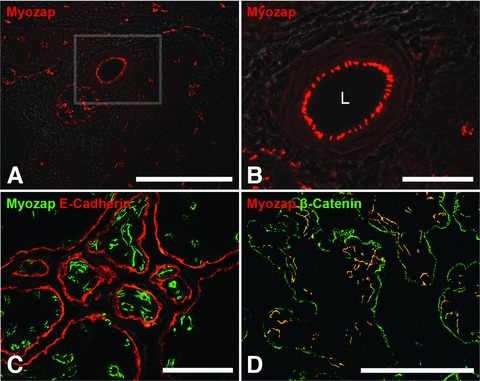
Immunolocalization of protein myozap in adherens junctions connecting endothelial cells of blood vessels in cryostat sections through bovine tongue (A, B) and human placenta (C, D). (A) The endothelial AJs of blood vessels of different calibres are specifically and intensely positive for myozap. (B) Higher magnification of the region in the square marked in (A). Note the specific myozap immunostaining of AJs in capillaries as well as in an arteriole. L: lumen. (C) Cryostat section through human placenta reacted with antibodies to myozap (green) and E-cadherin (red). The AJs of the microvasculature are strongly positive for myozap, whereas the epithelial junctions are negative for myozap but positive for E-cadherin. (D) Merged image showing the colocalization (yellow) of protein myozap (red) and β-catenin (green) in microvascular endothelial AJs whereas only β-catenin is positive in the AJs of the placental epithelium. Bars: 200 μm (A), 50 μm (B), 100 μm (C, D).
Myozap also appeared to be remarkably enriched in the endothelium of the alveolar part of the lung (Fig. 5A–E’’) but was absent from the desmoplakin-positive, single-layered bronchial epithelium (Fig. 5A–C). In contrast, plakoglobin consistently showed co-localization with protein myozap in the endothelial cells but not in the alveolar epithelia (Fig. 5D–D’’). The endothelial localization was also demonstrable by the co-localization of myozap with VE-cadherin (Fig. 5E’’).
Fig 5.
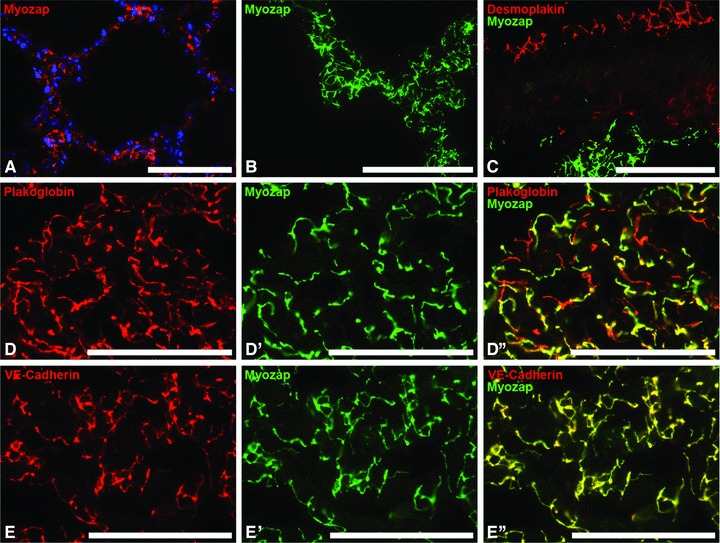
Immunofluorescence microscopy of protein myozap in vascular endothelial AJs of lung alveolar tissue of various species and after different modes of fixation. (A) Formaldehyde-fixed, paraffin-embedded adult porcine lung as seen after antigen retrieval and immunoreaction with myozap mAb 517.67 (red), in combination with nuclear DAPI staining (blue). (B) Myozap immunoreaction (green) on cryostat sections of adult mouse alveolar tissue. (C) Immunofluorescence labelling of the desmosomes in rat lung epithelium (desmoplakin, red), in comparison with myozap immunostaining (green) of the alveolar capillaries. (D, D’, D’’) Double label immunofluorescence microscopy of plakoglobin (red in D) and myozap (green in D’) in alveolar regions, seen on cryostat sections through mouse lung. (D’’) Merged image of (D) and (D’). Note colocalization of myozap with plakoglobin in microvascular endothelial cells (yellow merge-colour in D’’), plakoglobin immunostaining only in the AJs connecting alveolar epithelial cells (red labelling pattern in D’’). (E, E’, E’’) Double-label immunofluorescence microscopy of AJs of blood capillaries positive for VE-cadherin (red in E) as well as for myozap (green in E’). Note the almost complete colocalization of VE-cadherin and myozap (yellow merge colour) in the AJs of the endothelium (E’’). Bars: 100 μm (A), 50 μm (B–E’’).
Finally, in relatively large endothelial detachment (‘scraping off’) preparations from bovine aortae, myozap also showed extensive decoration of cell–cell boundaries, in partial colocalization with VE-cadherin (Fig. 6A, A’) as well as with α-catenin and the armadillo proteins β-catenin, protein p120 and plakoglobin (not shown).
Fig 6.
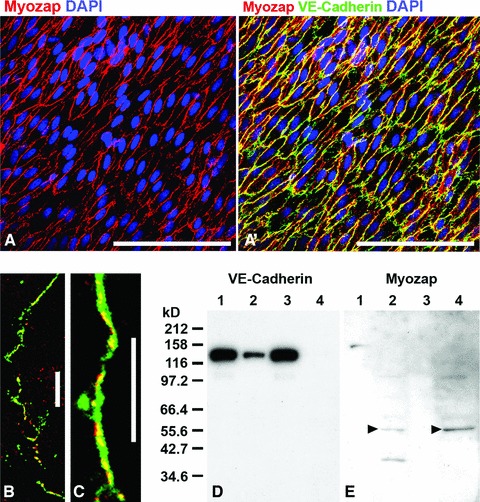
Bovine aortic endothelium in a fresh detached cell layer preparation showing the reaction of protein myozap in the zonulae adhaerentes of a bovine aorta. (A) Immunolocalization of myozap (red) in the detached endothelium. (A’) Same region as in (A) but in an optical channel showing both VE-cadherin (green) and myozap (red) reactions with far-reaching colocalization (yellow merge colour). (B, C) Laser-scanning double-label immunofluorescence microscopy of cultured human endothelial HUVEC cells, showing the partial association of myozap (red) with other adherens junction proteins such as (both in green) β-catenin (B) or VE-cadherin (C). (D, E) Results of fractionation experiments of HUVEC cell lysate proteins after SDS-PAGE of polypeptides and immunoblotting with antibodies to VE-cadherin (D) and myozap (E) are shown. Presented are proteins of the supernatants (lanes 1 and 3) and the remaining pellet fractions (lanes 2 and 4) after lysis using Triton-X100 (lanes 1 and 2) or RIPA (lanes 3 and 4) buffer, showing the relative recoveries of VE-cadherin (D) in comparison with protein myozap (E) which is predominantly detectable in the pellet fractions of the detergent-containing protein lysates (arrowheads). The masses of the molecular size markers (kD) are presented on the left margin of D. Bars: 100 μm (A, A’); 20 μm (B, C).
Thus, not unexpectedly we also found protein myozap in partial association with β-catenin (Fig. 6B) and VE-cadherin (Fig. 6C) of the junctions connecting cultured endothelial cells of the HUVEC type, in immunofluorescence labelling (Fig. 6B and C) as well as in the pellet fractions of cell protein lysates in detergent-containing buffers (Fig. 6D and E). These experiments showed again the markedly low solubility of protein myozap, most of which was recovered in the pelletable residues, i.e. dissociated from the major endothelial junction glycoprotein, VE-cadherin (compare Fig. 6D and E).
As in some tissues the epithelial and the microvascular structures in lung alveoli are very thin and very closely associated with each other we also applied electron microscopy (Fig. 7A presents, for example, a cross-section through such an association of a capillary and an alveolar epithelial structure). Immunoelectron microscopy then clearly showed that in such tissues protein myozap was exclusively located in the plaques of the AJs of the vascular endothelial cells (Fig. 7B and C) where it often could even be resolved as specific for the cytoplasmic plaque structures and absent from the membrane-to-membrane contact and interspace region (Fig. 7D).
Fig 7.
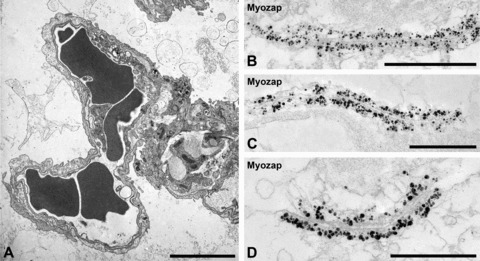
(A) Transmission electron micrographs of sections through mouse lung alveoli. Note the close neighbourhood of capillary endothelial (left part) and respiratory alveolar epithelial structures (right part) structures (erythrocytes appear as dark cells in the lumen of microcapillaries). (B–D) Single-label immunoelectron microscopy showing the specific ultrastructural localization of protein myozap in the zonulae adhaerentes connecting endothelial cells. Note that the cytoplasmic plaques of the endothelial AJ system are entirely and strongly decorated by myozap-antibodies coupled to colloidal gold particles (with silver enhancement), whereas such labelling is absent from the membrane contact region and the intercellular connection structures (D). Bars: 5 μm (A), 1 μm (B), 0.5 μm (C and D).
Identification of protein myozap in the various kinds of junctions connecting endothelial and special endothelium-derived cells of the lymphatic system
In all four mammalian species examined, we have also identified protein myozap as a major component of the AJs connecting the endothelial cells of the lymphatic system, including the three-dimensionally organized, multiramified cells of the virgultar endothelium system of the lymph node sinus and various other lymph vessel endothelia the cells of which are known to be connected by a special kind of desmoplakin-containing junction, the complexus adhaerens[14–16, 33–37]. The double-label immunolocalizations of Fig. 8A–A’’ and B–B’’ clearly show that the vast majority of the myozap label coincides with desmoplakin in the lymph node sinus cells but is totally absent from the adjacent desmosomes of the lymph node follicles (F in Fig. 8A–A’’). In addition, however, in higher resolution cross-sections through such sinus (Fig. 8B–B’’) short intercepts positive for desmoplakin only could also be identified (see specifically Fig. 8B’’), a minor difference explained by the exceptionally extended desmopakin-positive plaques characteristic of some complexus adhaerens junctions [14–16, 32–35].
Fig 8.
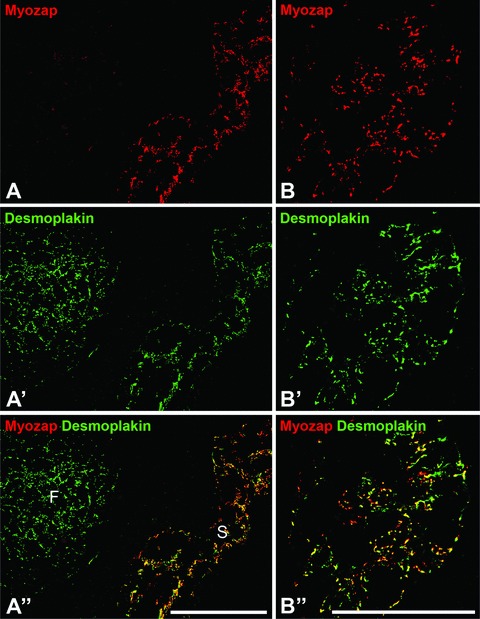
Double label immunofluorescence microscopy of protein myozap in junctions connecting the endothelial cells of the lymphatic vessel system, here on cryostat sections through bovine (A) and human (B) lymph nodes. Note that the complexus adhaerens junctions connecting the endothelial cells of the virgultar arrangements in the lymph node sinus regions are positive for myozap (red in A and B) and desmoplakin (green in A’ and B’) resulting in yellow merge labelling (A’’ and B’’). In contrast, the desmosomes of the follicular dendritic reticulum (F) are strongly positive for desmoplakin only (green in A’ and A’’). Bars: 100 μm.
Discussion
Our results show that the junctional plaque protein myozap, which shares some molecular organizational similarities but no true sequence homology with ERM (ezrin, radixin, moesin) proteins, is not specific to the composite junctions (areae compositae) of the intercalated disks connecting the cardiomyocates but also occurs in the AJs connecting the endothelial cells of most, probably all blood and lymph vessels. This conclusion has been made possible by the high sensitivity of a series of novel mono- and polyclonal antibodies specific for this polypeptide. With these antibodies we have also detected this architectonically important protein in embryogenesis, in which so far only its mRNA had been shown, as well as in parts of the vasculature of both the arterial and the venous system. Using the novel antibodies our results now allow the general conclusion that myozap occurs in almost all tissues, namely as a general and major endothelial junction component of the vascular elements present. This broader expression also explains the previous observations that myozap also occurs as a structural protein in non-cardiac organs such as placenta and adult lung (this study and [18]).
The high stability of myozap and its high resistance to extractions as demonstrated in the present report, together with its specific and tight binding to other adhering junction plaque molecules such as desmoplakin, plakoglobin and plakophilin-2, indicate that this molecule is deeply rooted and stably integrated in the plaque structure. Interestingly, the myozap-binding desmosomal ‘marker’ proteins desmoplakin and plakophilin-2, which are known to be absent in most forms of endothelial junctions of the adult blood and lymph vessel system, have been found in the junctions of certain specific endothelial vessels and cell cultures as well as in embryonal angiogenesis [14–16, 32–39]. On the other hand, myozap is also a major plaque component in zonulae adhaerentes of the great majority of blood and lymph vessels in which these desmosomal type proteins are absent. Clearly, it will be a focus of future experimental work to identify the complex partners of myozap in the majority of endothelia, which are known to be devoid of desmoplakin and plakophilin-2. In yeast-2-hybrid screening experiments Seeger et al. [18] had noted myozap binding to protein ZO-1, and in specific transfection experiments using human embryonal kidney cell cultures (line HEK293T) these authors identified coimmunoprecipitation complexes of tagged constructs of protein ZO-1 with tagged myozap constructs. Therefore, it was to some surprise that we had to realize that in our experiments – in the buffer dilutions and exposure timed chosen – protein ZO-1 – generally known as a major junctional plaque protein of adherens and tight junctions (e.g. Ref. [40]; for a special review on the problems of ‘stickiness’ of proteins of this category see also Ref. [41]) – was dissociated from myozap.
As one of myozap's functions we hypothesize a potential major regulatory role in the assembly and maintenance of diverse types of vascular adhering junctions as well as of cardiac junctions, as is also evident from the cardiomyopathies recently reported in morpholino knock-down experiments in zebrafish [18]. These findings, combined with its demonstrated role in serum response factor (SRF)-dependent transcription, define myozap as a novel molecule critical for both topological stability and organisation of cell and tissue architecture as well as in surface-nucleus signalling. In addition, we suspect that myozap plays a role in the regulations of the plaque anchorage of actin filaments and hence also in cytoskeletal organization and cell contractions as we are impressed by its tight and complex interactions with the plaque protein plectin, present in various kinds of junction plaques (e.g.[42, 43]). Finally, protein myozap may also be useful as a cell type marker in developmental biology and in pathology, in particular as a valuable immunohistological reagent in the diagnosis of certain diseases.
Acknowledgments
We gratefully acknowledge support by a grant-in-aid of the German Ministry for Education and Research (BMBF) in a cooperative research program entitled ‘Standardization of Mesenchymal Stem Cells for Regenerative Medicine (START-MSC2)’. In particular, S.P. thanks the German Science Foundation for funding of a Postdoctoral Research Fellowship (DFG-GZ: Pi 869/1–1) at the University of British Columbia (Vancouver, Canada), and the British Heart Foundation (BHF Centres of Research Excellence Funding) for funding of a subsequent Postdoctoral Training Fellowship at the Queens Medical Research Institute – Centre for Cardiovascular Science (University of Edinburgh, Scotland). Moreover, the authors thank Heiner Niemann (Friedrich-Loeffler-Institute, Mariensee, Germany) for providing fresh foetal and adult porcine tissues and Roland Moll (Institute for Pathology, Universities of Marburg and Giessen, Germany) for providing frozen and formaldehyde-fixed human tissues.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Franke WW. Discovering the molecular components of intercellular junctions – a historical view. Cold Spring Harb Perspect Biol. 2009;1:003061. doi: 10.1101/cshperspect.a003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass-Zubek AE, Godsel LM, Delmar M, et al. Plakophilins: multifunctional scaffolds for adhesion and signaling. Curr Opin Cell Biol. 2009;21:708–16. doi: 10.1016/j.ceb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franke WW, Borrmann CM, Grund C, et al. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol. 2006;85:69–82. doi: 10.1016/j.ejcb.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Franke WW, Rickelt S, Barth M, et al. The junctions that don't fit the scheme: special symmetrical cell-cell junctions of their own kind. Cell Tiss Res. 2009;338:1–17. doi: 10.1007/s00441-009-0849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godsel LM, Getsios S, Huen AC. The molecular composition and function of desmosomes. In: Behrens J, Nelson JW, et al., editors. Cell adhesion. Heidelberg: Springer; 2004. pp. 137–93. [DOI] [PubMed] [Google Scholar]
- 6.Pieperhoff S, Barth M, Rickelt S, et al. Desmosomal molecules in and out of adhering junctions: normal and diseased states of epidermal, cardiac and mesenchymally derived cells. Dermatol Res Pract. 2010:139167. doi: 10.1155/2010/139167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieperhoff S, Schumacher H, Franke WW. The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol. 2008;87:399–411. doi: 10.1016/j.ejcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Corrado D, Basso C, Thiene G. Arrhythmogenic right ventricular cardiomyopathy: an update. Heart. 2009;5:766–73. doi: 10.1136/hrt.2008.149823. [DOI] [PubMed] [Google Scholar]
- 9.Delmar M, McKenna WJ. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ Res. 2010;107:700–14. doi: 10.1161/CIRCRESAHA.110.223412. [DOI] [PubMed] [Google Scholar]
- 10.Gerull B, Heuser A, Wichter T, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet. 2004;36:1162–4. doi: 10.1038/ng1461. [DOI] [PubMed] [Google Scholar]
- 11.Marcus FI, Nava A, Thiene G. Arrhythmogenic RV cardiomyopathy/dysplasia. Heidelberg: Springer; 2007. [Google Scholar]
- 12.Oxford EM, Musa H, Maass K, et al. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–11. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 13.Franke WW, Schumacher H, Borrmann CM, et al. The area composita of adhering junctions connecting heart muscle cells of vertebrates. III. Assembly and disintegration of intercalated disks in rat cardiomyocytes growing in culture. Eur J Cell Biol. 2007;86:127–42. doi: 10.1016/j.ejcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Schmelz M, Franke WW. Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: the syndesmos connecting retothelial cells of lymph nodes. Eur J Cell Biol. 1993;61:274–89. [PubMed] [Google Scholar]
- 15.Hämmerling B, Grund C, Boda-Heggemann J, et al. The complexus adhaerens of mammalian lymphatic endothelia revisited: a junction even more complex than hitherto thought. Cell Tissue Res. 2006;324:55–67. doi: 10.1007/s00441-005-0090-3. [DOI] [PubMed] [Google Scholar]
- 16.Moll R, Sievers E, Hämmerling B, et al. Endothelial and virgultar cell formations in the mammalian lymph node sinus: endothelial differentiation morphotypes characterized by a special kind of junction (complexus adhaerens) Cell Tissue Res. 2009;335:109–41. doi: 10.1007/s00441-008-0700-y. [DOI] [PubMed] [Google Scholar]
- 17.Roginski RS, Mohan Raj BK, Birditt B, et al. The human GRINL1A gene defines a complex transcription unit, an unusual form of gene organization in eukaryotes. Genomics. 2004;84:265–76. doi: 10.1016/j.ygeno.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Seeger TS, Frank D, Rohr C, et al. Myozap, a novel intercalated disc protein, activates serum response factor-dependent signaling and is required to maintain cardiac function in vivo. Circ Res. 2010;106:880–90. doi: 10.1161/CIRCRESAHA.109.213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langbein L, Pape UF, Grund C, et al. Tight junction-related structures in the absence of a lumen: occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol. 2003;82:385–400. doi: 10.1078/0171-9335-00330. [DOI] [PubMed] [Google Scholar]
- 20.Cowin P, Kapprell HP, Franke WW, et al. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–73. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- 21.Franke WW, Kapprell HP, Cowin P. Immunolocalization of plakoglobin in endothelial junctions: identification as a special type of Zonulae adhaerentes. Biol Cell. 1987;59:205–18. doi: 10.1111/j.1768-322x.1987.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 22.Claycomb WC, Lanson NA, Stallworth BS, et al. HL-1 cells: A cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci. 1998;95:2979–84. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peitsch WK, Grund C, Kuhn C, et al. Drebrin is a widespread actin-associating protein enriched at junctional plaques, defining a specific microfilament anchorage system in polar epithelial cells. Eur J Cell Biol. 1999;78:767–78. doi: 10.1016/S0171-9335(99)80027-2. [DOI] [PubMed] [Google Scholar]
- 24.Lieber M, Smith B, Szakal A, et al. A continuous tumour-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 25.Flügel RM, Crefeld T, Munk K. Detection of SV40 T antigen with labelled antibodies: radioimmunoassay and autoradiography. Int J Cancer. 1977;19:656–63. doi: 10.1002/ijc.2910190509. [DOI] [PubMed] [Google Scholar]
- 26.Boukamp P, Petrussevska RT, Breitkreutz D. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–71. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt CJ, Franke WW, Goerdt S, et al. Homo- and heterotypic cell contacts in malignant melanoma cells and desmoglein 2 as a novel solitary surface glycoprotein. J Invest Dermatol. 2007;127:2191–206. doi: 10.1038/sj.jid.5700849. [DOI] [PubMed] [Google Scholar]
- 28.Achtstätter T, Hatzfeld M, Quinlan RA, et al. Separation of cytokeratin polypeptides by gel electrophoretic and chromatographic techniques and their identification by immunoblotting. Methods Enzymol. 1986;34:355–71. doi: 10.1016/0076-6879(86)34102-8. [DOI] [PubMed] [Google Scholar]
- 29.Borrmann CM, Grund C, Kuhn C, et al. The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol. 2006;85:469–85. doi: 10.1016/j.ejcb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Barth M, Schumacher H, Kuhn C, et al. Cordial connections: molecular ensembles and structures of adhering junctions connecting interstitial cells of cardiac valves in situ and in cell culture. Cell Tiss Res. 2009;337:63–77. doi: 10.1007/s00441-009-0806-x. [DOI] [PubMed] [Google Scholar]
- 31.Pieperhoff S, Borrmann CM, Grund C, et al. The area composita of adhering junctions connecting heart muscle cells of vertebrates. VII. The different types of lateral junctions between the special cardiomyocytes of the conduction system of ovine and bovine hearts. Eur J Cell Biol. 2010;89:365–78. doi: 10.1016/j.ejcb.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Schmelz M, Moll R, Kuhn C. Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: II. Different types of lymphatic vessels. Differentiation. 1994;57:97–117. doi: 10.1046/j.1432-0436.1994.5720097.x. [DOI] [PubMed] [Google Scholar]
- 33.Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeiffer F, Kumar V, Butz S, et al. Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur J Immunol. 2008;38:2142–55. doi: 10.1002/eji.200838140. [DOI] [PubMed] [Google Scholar]
- 35.Ebata N, Nodasaka Y, Sawa Y, et al. Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res. 2001;61:40–8. doi: 10.1006/mvre.2000.2280. [DOI] [PubMed] [Google Scholar]
- 36.Kowalczyk AP, Navarro P, Dejana E, et al. VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J Cell Sci. 1998;111:3045–57. doi: 10.1242/jcs.111.20.3045. [DOI] [PubMed] [Google Scholar]
- 37.Valiron O, Chevrier V, Usson Y, et al. Desmoplakin expression and organization at human umbilical vein endothelial cell-to-cell junctions. J Cell Sci. 1996;109:2141–9. doi: 10.1242/jcs.109.8.2141. [DOI] [PubMed] [Google Scholar]
- 38.Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128:929–41. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X, Stuart A, Dettin LE, et al. Desmoplakin is required for microvascular tube formation in culture. J Cell Sci. 2004;117:3129–40. doi: 10.1242/jcs.01132. [DOI] [PubMed] [Google Scholar]
- 40.Palatinus JA, O'Quinn MP, Barker RJ, et al. ZO-1 determines adherens and gap junction localization at intercalated disks. Am J Physiol Heart Circ Physiol. 2011;300:H583–94. doi: 10.1152/ajpheart.00999.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palatinus JA, Gourdie RG. Xin and the art of intercalated disk maintenance. Am J Physiol Heart Circ Physiol. 2007;293:H2626–8. doi: 10.1152/ajpheart.00954.2007. [DOI] [PubMed] [Google Scholar]
- 42.Wiche G, Krepler R, Artlieb U, et al. Occurrence and immunolocalization of plectin in tissues. J Cell Biol. 1983;97:887–901. doi: 10.1083/jcb.97.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rezniczek GA, Janda L, Wiche G. Plectin. Methods Cell Biol. 2004;78:721–55. [PubMed] [Google Scholar]


