Abstract
Caveolin-1, an integral protein of caveolae, is associated with multiple cardiovascular signalling pathways. Caveolin-1 knockout (KO) mice have a reduced lifespan. As changes in artery structure and function are associated with ageing we have investigated the role of caveolin-1 ablation on age-related changes of small artery contractility and passive mechanical properties. Mesenteric small arteries isolated from 3 and 12-month wild-type (WT) and caveolin-1 KO mice were mounted on a pressure myograph and changes in passive and functional arterial properties were continuously monitored. In WT mice ageing was associated with a reduction in arterial contractility to noradrenaline which was reversed by inhibition of nitric oxide synthase with L-NNA. Similarly, in 3-month-old mice, caveolin-1 KO reduced contractility to noradrenaline by an L-NNA-sensitive mechanism. However, ageing in caveolin-1 KO mice was not associated with any further change in contractility. In WT mice ageing was associated with an increased passive arterial diameter and cross-sectional area (CSA), consistent with outward remodelling of the arterial wall, and a reduced arterial distensibility. Caveolin-1 ablation at 3 months of age resulted in similar changes in passive arterial properties to those observed with ageing in WT animals. However, ageing in caveolin-1 KO mice resulted in a reduced arterial CSA indicating different effects on passive structural characteristics from that observed in WT mice. Thus, caveolin-1 mice show abnormalities of small mesenteric artery function and passive mechanical characteristics indicative of premature vascular ageing. Moreover, caveolin-1 ablation modulates the age-related changes usually observed in mesenteric arteries of WT mice.
Keywords: caveolin-1, ageing, vascular properties
Introduction
Cardiovascular diseases are responsible for considerable mortality and morbidity in the developed world and ageing is a clear risk factor for the development of such disorders [1]. The WHO estimates a near tripling of people >60 years old by 2050 as well as an almost 30% increase in the number of people dying from cardiovascular disease [2, 3]. Although the underlying mechanisms responsible for the development and progression of these disorders are multiple, it is believed that changes in the structure, distensibility and contractile function of resistance arteries, with consequent alterations in vascular resistance and blood flow, are key contributory factors [4-7].
Important roles for caveolae, omega-shaped plasmalemmal invaginations, in the orchestration of vascular cell signalling events have become apparent [for reviews see 8–12]. Caveolae regulate many cellular processes including ion transport, signal transduction and, of particular relevance to the present study, vascular structure and function [13, 14]. Caveolin-1 has been long known to be a protein integral to caveolae formation and caveolin-1 knockout mice (caveolin-1 KO) lack identifiable caveolae in the vasculature [12, 14, 15]. Although these mice are viable and fertile they exhibit reductions in lifespan which suggests a role for caveolin-1 and/or caveolae in the ageing process [16, 17]. This is supported by the recent finding that caveolin-1 KO mice show changes in synaptic protein expression, localization and function synonymous with the brain ageing process [18].
Vascular contractile responses to adrenergic agonists, and vascular nitric oxide (NO) bioavailability, change with ageing [4, 19]. Coincidently, arteries from caveolin-1 KO mice have also been shown to exhibit altered contractile responses including impaired constriction to α-adrenergic agonists [15, 20]. This dampening of vasoconstrictor responses has been attributed to an enhanced bioavailability of NO. This, in turn, is believed to be due to increases in the activity of endothelial NO synthase (eNOS) upon caveolin ablation as, under native (WT) conditions, eNOS activity is thought to be tightly regulated by reversible binding to the scaffolding domain of caveolin-1 [21, 22]. Alterations in caveolin-1 expression have been demonstrated in certain disease states and may represent a mechanism for chronic regulation of eNOS activity [23, 24]. Thus, an intriguing prospect is that age-related changes in contractile function of resistance arteries may be influenced by ablation of caveolin-1.
Ageing has also been associated with changes in the passive characteristics of arteries: increases in arterial cross-sectional area (CSA), and reductions in distensibility, have been reported in conduit arteries and in rat small arteries [5, 6]. Indeed it is noteworthy that reduced arterial distensibility, albeit in large arteries, has been suggested as one of the earliest clinical manifestations of vascular ageing in humans [25]. In addition, changes in both the architecture and distensibility of resistance arteries are apparent in many disease states where they are correlated with elevated cardiovascular risk [26, 27]. Aside from one report of increased wall thickness in mesenteric resistance arteries from young caveolin-1 KO mice [14] the influence of caveolin-1 KO on passive structural characteristics has not been elucidated. Interestingly, alterations of sympathetic vasomotor tone and/or eNOS activity may contribute to arterial structural changes [27, 28]. This again focuses our attention on the novel possibility that caveolin-1 may influence the nature of age-related changes in arterial function and structure.
In the present study, therefore, we have compared the active functional responses to α-adrenergic stimulation with noradrenaline and the passive structural and mechanical characteristics of mesenteric small arteries from 3-month and 12-month-old mice. Moreover, we have investigated whether caveolin-1 deletion influences any age-related changes in mesenteric artery passive characteristics and contractile function.
Materials and methods
Mice
All procedures were covered by UK Home Office licence 40/3069. Pairs of wild-type (WT) and caveolin-1 KO mice [15] were kindly donated by Olivier Feron and Chantal Dessy (University of Louvain). Heterozygous breeding was established and used to maintain a colony of WT and caveolin-1 KO mice at the University of Manchester. Adult male mice were sacrificed by cervical dislocation at either 3 or 12 months of age. The latter timepoint was chosen as Park et al. [16] previously showed that the major decline in viability of caveolin-1 KO mice occurred between 27 and 65 weeks of age. Of note, and in agreement with other studies [29, 30], we observed no loss of life of caveolin-1 KO mice up to 12 months in the present study.
DNA was extracted in lysis buffer (composition: 50 mM Tris pH8, 100 mM EDTA, 0.5% SDS, 10 mg/ml proteinase K; Promega, Southampton, UK) isopropanol precipitated, washed in ethanol and resuspended in distilled water. WT, heterozygous (Ht) or KO genotype was confirmed by PCR (Fig. 1) using primers specific for the WT (forward: TTTACCGCTTGTTGTCTACGA; reverse: TATCTCTTTCTGCGTGCTGA; product size 240bp) or KO genotype (forward: TATTCTGCCTTCCTGATGATAACTG; reverse: CCTGCGTGCAATCCATCTTGTTCAATG; product size 1500bp).
Fig 1.
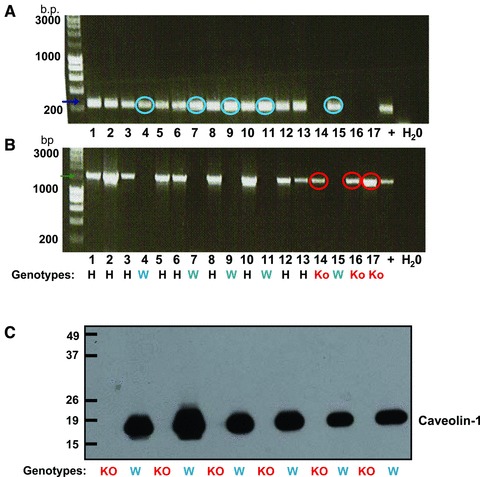
Determination of caveolin-1 knockout and wild-type genotype. Agarose gel electrophoresis of mouse PCR products of reactions using caveolin-1 knockout (A) or wild-type (B) primers. Samples positive only for knockout primers (red circles) were knockout mice (KO) and those positive only for wild-type primers (blue circles) were wild-type (W) mice. Samples positive for both primer sets were from heterozygous mice (H). (C) Western blotting of tissues with anti-caveolin-1 antibody confirmed ablation of caveolin-1 from KO mice.
Western blotting
Smooth muscle tissue from the gut of WT or caveolin-1 KO animals was snap frozen in liquid N2 and stored until required. Tissues were homogenized (150 mM NaCl, 1.0% Nonidet-P40, 0.5% sodium deoxycholate, 0.1% SDS 50 mM Tris pH8, with protease inhibitor cocktail, product number P8340; Sigma, Dorset, UK) with glass grinders and protein content measured in triplicate with a Bio-Rad DC protein assay kit. SDS-PAGE/Western blotting (10% acrylamide gels) was performed and PVDF membranes probed with rabbit polyclonal anti-caveolin-1 primary antibody (1:1000, sc894, Lot number B0409; Santa Cruz, Heidelberg, Germany) and goat anti-rabbit horseradish-peroxidase conjugated secondary antibody (1:2000, product number P0448; Dako UK Ltd., Camdridgeshire, UK). Protein was detected by enhanced chemiluminescence (ECL Plus, RPN2132; Amersham, Amersham, UK). At the end of the experiment, the PVDF membrane was stained by a 30 min. incubation with Napthol Blue Black solution (0.1% (w/v) Napthol Blue Black, 10% methanol, 2% acetic acid).
Tissue isolation and contractile measurements
Third order mesenteric arteries were prepared and mounted on a pressure myograph (Living Systems Instrumentation, VT, USA) as previously described [31, 32]. Arteries were pressurized to an intravascular pressure of 60 mmHg, superfused with physiological salt solution (PSS; 37°C, 95% air/5% CO2) and lumen diameters and wall thicknesses continuously measured using a video dimension analyser [31, 32]. Vessels were left to equilibrate for 30 min. before testing for viability (2 χ 100 mM high potassium PSS (KPSS), 1 χ 100 mM KPSS + 10 μM noradrenaline).
Cumulative concentration-response curves were constructed to the adrenergic agonist noradrenaline (10−9–10−5 M) in PSS. Following washout of noradrenaline and return to resting diameter, vessels were incubated with the nitric oxide synthase (NOS) inhibitor N-Nitro-L-arginine (L-NNA – 100 μM), or diluent, for 30 min. Cumulative concentration-response curves to noradrenaline were then repeated in the presence of L-NNA or diluent. Contractile responses were normalized as a percentage of the starting diameter for each vessel. Alterations in sensitivity were examined by comparing logEC50’s (calculated for each artery using non-linear regression GraphPad Prism (version 3.02; CA, USA) after ensuring correlation r2 >0.90 of the sigmoidal curve fit).
Passive arterial characteristics
Arteries (at 60 mmHg) were superfused with calcium-free PSS for 30 min. Intravascular pressure was then reduced to 5 mmHg and subsequently increased to 10 mmHg, 20 mmHg and then in 20 mmHg steps to 140 mmHg. Diameters were allowed to stabilize (5–7 min.) before proceeding to the next pressure step. Measurements of stable intralumenal diameter and left and right wall thicknesses (taken from the top, middle and bottom sections of each vessel and averaged) were obtained at each pressure.
CSA was calculated at each pressure from:
CSA μm2 = π (r + WT)2 −πr2
r = radius, WT = wall thickness (mean of left and right walls)
Stress and strain were calculated from:
Stress (dyn/μm2) = P x r/WT
P = intralumenal pressure (1 mmHg = 1334 dyn/μm2)
Strain = D-D0/D0
D = diameter at each pressure
D0 = diameter at 5 mmHg.
Elastic modulus (β) was determined from y = aeβx where a is constant and e is the base of natural logarithm.
Electron microscopy
Electron microscopy was undertaken to allow examination of arterial ultra-structure as described previously [31]. Briefly, isolated mesenteric arteries were mounted as described above and pressurized to a luminal pressure of 60 mmHg. Vessels were transferred rapidly, whilst pressurized, to fixative (2.5% glutaraldehyde in 0.1M sodium cacodylate buffer pH7.3) where they remained for 2 hrs before washing in 0.1M sodium cacodylate buffer. The vessels were gently removed from the cannulae and placed in a tube of 0.1M sodium cacodylate until required. Arteries were dehydrated and resin embedded as previously described [31]. Ultrathin (70 mn) sections were cut using a diamond knife, mounted on a copper grid and contrasted using uranyl acetate and lead citrate. A Philips 301 electron microscope was used to view sections at an accelerating voltage of 60 kV.
Statistics
Data were analysed by 2-way ANOVA and, when required, post hoc analysis by Bonferroni post-test. A Kolmogorov-Smirnov test was used to confirm normal distribution. LogEC50 values were compared using the unpaired Student’s t-test. In all cases significance was P < 0.05. All data are shown as mean ± SEM and n = number of arteries.
Results
Confirmation of caveolin-1 gene KO effects on expression of caveolin-1 protein or caveolae
The assignment of individual genotype based upon the above PCR expression profiles was confirmed in a subset of tissues wherein mice assigned by genotyping as caveolin-1 KO had, as anticipated, a complete absence of caveolin-1 protein in contrast to WT animals (Fig. 1C). Furthermore, whilst electron micrographs of arteries from WT arteries confirmed the presence of caveolae, there was no evidence of caveolae in arteries from caveolin-1 KO mice (Figs 2 and 3).
Fig 2.
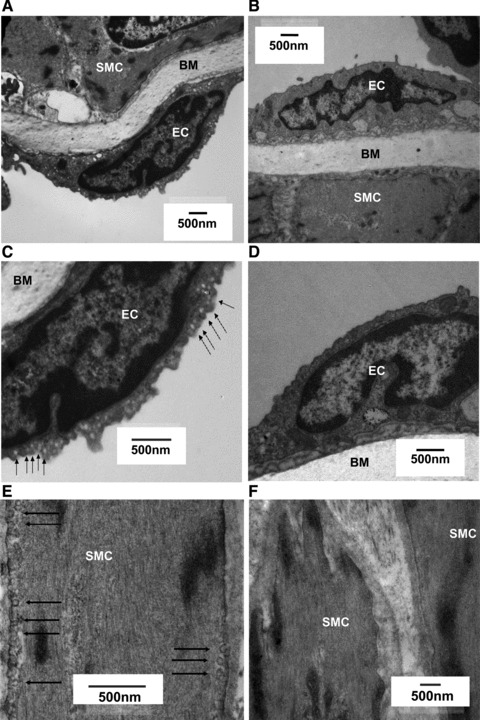
Electron micrographs of isolated mesenteric arteries from 3-month-old WT and caveolin-1 KO mice. Caveolae are indicated by arrowheads in images of vessels from WT mice (A, C, E), with the absence of caveolae in images of vessels from caveolin-1 KO mice (B, D, F). Smooth muscle cells (SMC), endothelial cells (EC) and the basal membrane (BM) are indicated.
Fig 3.
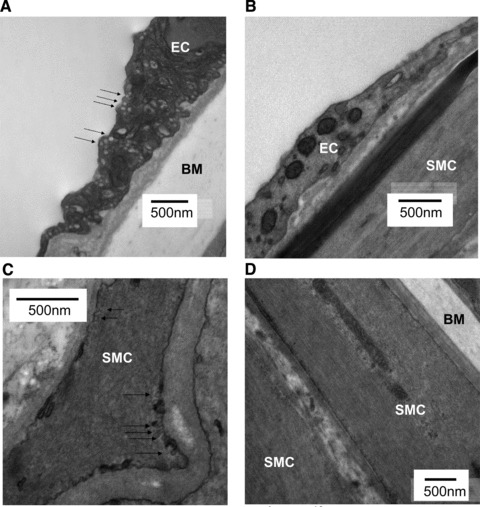
Electron micrographs of isolated mesenteric arteries from 12-month-old WT and caveolin-1 KO mice. Caveolae are indicated by arrowheads in images of vessels from WT mice (A, C), with the absence of caveolae in images of vessels from caveolin-1 KO mice (B, D). Smooth muscle cells (SMC), endothelial cells (EC) and the basal membrane (BM) are indicated.
Effect of ageing from 3 to 12 months on caveolae and caveolin-1 expression in WT animals
Arteries from both 3-month WT (n = 3) (Fig. 2A, C and E) and 12-month WT (n = 5) (Fig. 3A and C) mice displayed caveolae on smooth muscle and endothelial cells whereas those from caveolin-1 KO animals did not. Ageing from 3 months to 12 months was not associated with changes in the expression of caveolin-1 protein (Fig. 4).
Fig 4.
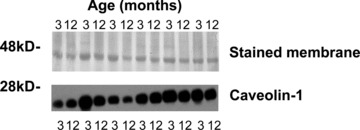
The effect of ageing from 3 months to 12 months on caveolin-1 expression in WT mice. Western blot (bottom panel) performed on 30 μg protein from homogenized samples of WT mice indicates no effect of ageing from 3 months to 12 months of caveolin-1 expression. Upper panel indicates actin expression in all samples as assessed by napthol blue black staining of PVDF membrane.
Age-related changes in the contractile responses of isolated mesenteric arteries to noradrenaline and the influence of caveolin-1 ablation
Ageing of WT mice from 3 to 12 months was associated with a significant reduction in the constriction of mesenteric arteries to noradrenaline. (Fig. 5Ai). No changes in sensitivity were observed (log EC50 3-month WT = −6.40 ± 0.11, n = 10; 12-month WT = −6.12 ± 0.09, n = 13).
Fig 5.
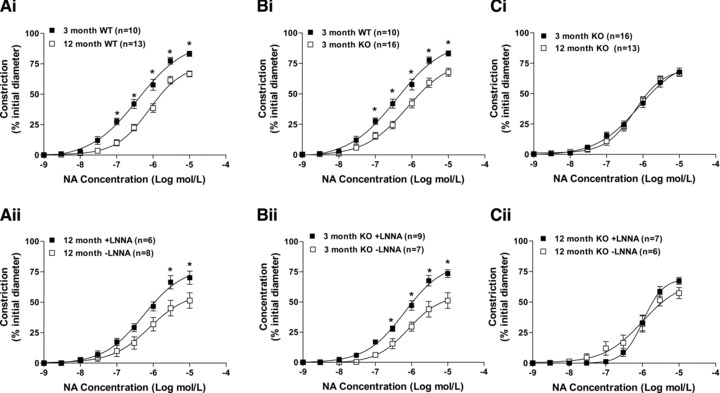
The effects of caveolin-1 ablation and ageing from 3 to 12 months on contractile responses of isolated mouse mesenteric small arteries to noradrenaline (NA). Normal ageing in wild-type (WT) mice reduced contractlity (Ai); effects which were reversed in the presence of L-NNA to inhibit nitric oxide synthase (Aii). Arteries from 3-month-old caveolin-1 knockout (KO) mice also exhibited reduced contractile responses when compared to those from age matched WT mice (Bi). Again this reduction was reversed by L-NNA (Bii). Ageing of caveolin-1 KO mice was not associated with any change in contractility to noradrenaline (Ci). Contractlity of arteries from 12-month-old caveolin-1 KO mice was not influenced by L-NNA (Cii).
Inhibition of NOS with L-NNA had no effect on absolute contractility of arteries from 3-month WT mice to noradrenaline nor was there an effect on sensitivity (log EC50 in L-NNA = −4.77 ± 1.15, without L-NNA = 6.12 ± 0.18, n = 5). However, L-NNA did significantly increase contractility of arteries from 12-month WT mice to levels similar to that seen in 3-month WT mice (Fig. 5Aii). This occurred without a change in sensitivity (log EC50 in L-NNA = −5.36 ± 0.69, without L-NNA = −5.99 ± 0.23, n = 6)). These data suggest that the age-related decrease in contractility to noradrenaline was due to an increased bioavailability of NO.
Caveolin-1 ablation caused similar changes to the contractility of arteries from 3-month-old mice as those observed with ageing from 3 months to 12 months in arteries from WT mice. A significant reduction in constriction to noradrenaline was observed in mesenteric arteries from 3-month caveolin-1 KO mice compared to WT (Fig. 5Bi). There were no changes in EC50 values (log EC50 3-month WT −6.40 ± 0.11, n = 10; 3-month KO −6.10 ± 0.10, n = 16). Similar to the effect of ageing from 3 months to 12 months in WT mice, the reduction in noradrenaline-dependent contractility of arteries from 3-month caveolin-1 KO mice was reversed in the presence of L-NNA (Fig. 5Bii). There were no changes in EC50 values (log EC50 in L-NNA = −6.12 ± 0.10 n = 9, without L-NNA = −6.12 ± 0.09, n = 7).
Arteries from caveolin-1 KO mice, however, showed a different response to ageing than those from WT animals. That is, caveolin-1 KO mice exhibited similar contractile responses to noradrenaline at both 3 and 12 month of age (Fig. 5Ci). Therefore, the blunting of contractility to noradrenaline observed with ageing in WT mice was not observed in ageing KO mice. Moreover, noradrenaline responsiveness of arteries from 12-month caveolin-1 KO mice were unaffected by inhibition of NOS (Fig. 5Cii). These differences were not specific to noradrenaline. Ageing from 3 to 12 months was also associated with a significant reduction of contractility of arteries from WT mice to KPSS (constrictions to, respectively, 75 ± 6% (n = 10) and 59 ± 9% (n = 13) of initial diameter; n = 13). Yet arteries from caveolin-1 KO mice exhibited similar responses to KPSS at 3 and 12 months of age (52 ± 9% (n = 16) and 38 ± 6% (n = 13) respectively of initial diameter).
Age-related changes in the passive characteristics of isolated mesenteric arteries: the influence of caveolin-1 ablation
Ageing from 3 to 12 months increased the passive diameter of mesenteric arteries from WT mice which was associated with an increase in CSA (Fig. 6Ai and ii). An increase in diameter and an increase in CSA are consistent with age-related outward remodelling of arteries. Ageing from 3 to 12 months of WT mice was associated with a significant decrease in the strain:pressure relationship and no change in the stress:pressure relationship (Fig. 7Ai and ii). Ageing was also associated with a leftward shift of the stress-strain curve of mesenteric arteries from WT mice indicating a reduction in distensibility (Fig. 7Aiii). This was evinced by an increased elastic modulus (β) of arteries from 12-month-old WT mice (β = 4.74 ± 0.19; n = 11) versus 3-month WT mice (β = 3.50 ± 0.20; n = 14). Thus ageing from 3 to 12 months was associated with outward remodelling and a reduction in distensibilty of mesenteric arteries from WT mice.
Fig 6.
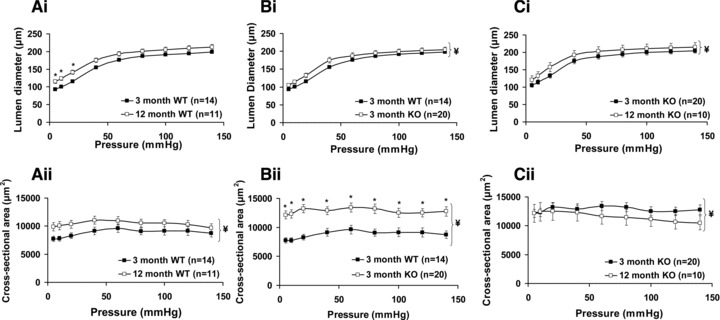
The effects of caveolin-1 ablation and ageing from 3 to 12 months on passive mesenteric arterial properties. Normal ageing in wild-type (WT) mice increased passive arterial diameter (Ai) and increased arterial cross sectional area (Aii). Arteries from 3-month-old caveolin-1 knockout mice (KO) also had an increased passive diameter (Bi) and increased cross sectional area (Bii) when compared to those from age-matched WT mice. Ageing in caveolin-1 KO mice was associated with an increase in passive diameter (Ci) and a reduction in cross sectional area (Cii).
Fig 7.
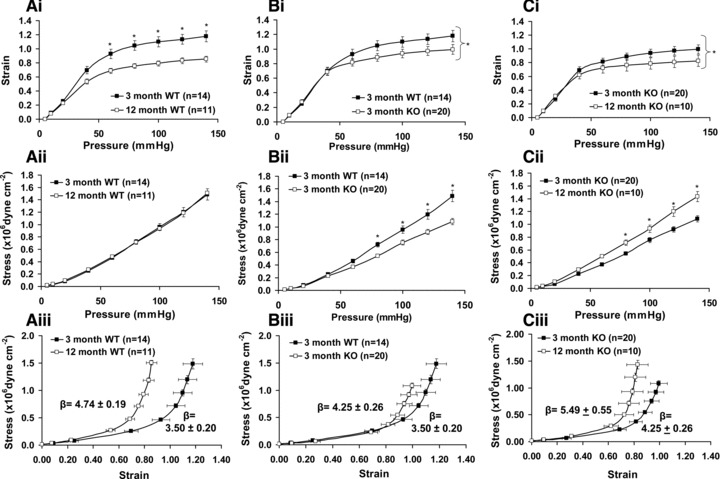
The effects of caveolin-1 KO and ageing on mesenteric arterial strain, stress and distensibility. Ageing in WT mice (Ai-iii) was associated with reductions in the strain:intraluminal pressure relationship and reduced distensibility but no change in the stress:pressure relationship. Arteries from 3-month KO mice (Bi-iii) also had reduced strain:pressure curves and distensibility and reduced stress:pressure curves. Ageing in KO mice (Ci-iii) was again associated with reductions in strain:pressure and distensibility.
Caveolin-1 KO caused similar changes in the measured passive structural and mechanical characteristics of arteries from 3-month-old mice as those observed with ageing from 3 months to 12 months in arteries from WT mice. That is, there were increases of passive diameter and wall CSA of mesenteric arteries from 3-month-old caveolin-1 KO mice versus WT indicative of outward remodelling (Fig. 6Bi and ii). Caveolin-1 ablation was also associated with a significant reduction of the strain:pressure relationship (Fig. 7Bi), the stress:pressure curve (Fig. 7Bii) and distensibility (Fig. 7Biii; β = 3.50 ± 0.20 (n = 14) and 4.25 ± 0.26 (n = 20) for arteries from, respectively, 3-month WT and caveolin-1 KO mice). Thus, caveolin-1 ablation induced changes in mesenteric arterial diameter, CSA and intrinsic distensibility which were similar to those observed with ageing in arteries from WT mice. These observations are consistent with the notion that caveolin-1 ablation is associated with premature vascular ageing.
Arteries from 12-month caveolin-1 KO mice exhibited changes in passive structural characteristics to 3-month KO mice albeit these diverged somewhat from the effects of ageing observed on arteries from WT mice. In caveolin-1 KO mice ageing was associated with an increase in arterial diameter (Fig. 6Ci), and a reduction in intrinsic distensibility (β = 4.25 ± 0.26 (n = 20) for 3-month and 5.49 ± 0.55 (n = 10) for 12-month caveolin-1 KO mice; Fig. 7Ciii), as observed with ageing in WT mice. However, ageing in caveolin-1 KO mice resulted in a reduction in CSA (Fig. 6Cii) not observed in ageing of WT mice. Differences were also seen in the stress:pressure relationship; this was reduced with ageing in arteries from caveolin-1 KO mice (Fig. 7ii) but not WT mice. At 12 months of age the diameters of arteries from WT and caveolin-1 KO mice were similar as was distensibility (β = 4.74 ± 0.19 (n = 11) and 5.49 ± 0.55 (n = 10) for 12-month WT and caveolin-1 KO mice respectively).
Discussion
In this work we report that ageing from 3 to 12 months is associated with changes to both the functional and passive structural and mechanical properties of small mesenteric arteries from mice. Similar changes are seen from 3-month-old caveolin-1 KO mice suggesting that ablation of caveolin-1 induces premature ageing of small mesenteric arteries. Furthermore, the absence of caveolin-1 also modulates the normal mesenteric artery ageing process such that different effects of ageing on both passive and functional properties are observed between arteries from WT and caveolin-1 KO mice. Our main observations are depicted in Figure 8.
Fig 8.
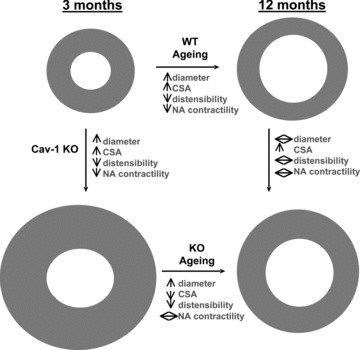
Summary diagram depicting the major changes in mouse mesenteric arteries with ageing between 3 and 12 months in WT and caveolin-1 KO mice.
Contractile changes with ageing of WT mice
Ageing from 3 to 12 months was associated with a significantly reduced contractile response of mesenteric arteries from WT mice to noradrenaline. This reduced contractility was completely reversed in the presence of L-NNA to inhibit NOS suggesting that it was attributable to an age-related increase in the bioavailability of NO. Similar age-related changes in arterial contractility and NO sensitivity have previously been demonstrated [33-36] although not ubiquitously [19, 37-42]. Mouse mesenteric artery eNOS expression has been reported to be unchanged with ageing suggesting that alterations in eNOS activity may underlie the changes in NO-sensitivity [43]. Here we show by electron microscopy that caveolae are present in mesenteric arteries from 12-month WT mice and that smooth muscle caveolin-1 expression is not modulated by ageing from 3 to 12 months. However, to directly ascertain if there is a link between caveolae number, and/or caveolin-1 expression/localization, and the functional changes with ageing it will be important in future microscopic studies of serial arterial sections to quantify the effects of ageing on these parameters in endothelial and smooth muscle cells.
Similar contractile changes with caveolin-1 KO in 3-month-old mice
Mesenteric arteries from 3-month-old caveolin-1 KO mice also exhibited a reduced contractile response to noradrenaline. This was similar to that reported previously for aortae and pulmonary arteries from young adult caveolin-1 KO mice [11, 44, 45]. This reduced response was again normalized in the presence of L-NNA. The noradrenergic contractile responses of arteries from 3-month-old caveolin-1 KO mice, therefore, were similar to that observed in arteries from WT mice that had aged to 12 months. This is consistent with the notion of caveolin-1 ablation resulting in premature ageing of small mesenteric arteries.
Different contractile changes with ageing of caveolin-1 KO mice
While arteries from aged WT mice exhibited a NO-dependent reduction in contractile responses to noradrenaline, ageing from 3 to 12 months in caveolin-1 KO mice was not associated with any changes in noradrenergic contractility nor did NOS inhibition influence matters.
Passive structural and mechanical changes with ageing of WT mice
Ageing of WT mice between 3 and 12 months was also associated with changes to the passive characteristics of arteries, namely an increased diameter and CSA, consistent with outward remodelling of the arterial wall, and a reduced arterial distensibility. The latter is concomitant with a reduction in the strain:pressure curves with ageing. Studies on rat mesenteric arteries have demonstrated similar age-related changes in passive arterial structure [6, 46] and, in murine mesenteric arteries, Gros et al. [7] reported age-related increases in luminal diameter. Age-related reductions in distensibility have been shown in mesenteric arteries, carotid arteries and aorta of rats suggesting phenomenological commonality between many vessel types [5, 22, 47].
Similar passive structural and mechanical changes with caveolin-1 KO in 3-month-old mice
Arteries from 3-month caveolin-1 KO mice also exhibited increases in arterial diameter and CSA. We examined luminal dimensions in live arteries across a wide range of intravascular pressures and thereby extended previous studies suggesting this from the examination of fixed non-pressurized mesenteric arteries [14]. Reductions in the strain:pressure curve and distensibility of arteries of 3-month caveolin-1 KO mice when compared to 3-month WT mice were also observed. All these changes are similar to the remodelling that is evident with ageing from 3 to 12 months in WT mice.
Different passive structural and mechanical changes with ageing of caveolin-1 KO mice
Notwithstanding these findings, there are some differences of note. There is a reduced stress:pressure curve in 3-month caveolin-1 KO mice versus WT mice that is not observed with ageing in WT mice. Moreover, the effect of ageing on certain passive mesenteric arterial characteristics in caveolin-1 KO mice is also different from WT mice. Whilst ageing in WT mice was associated with no change in the stress:pressure curve yet an increased CSA, in caveolin-1 KO mice it was associated with an increased stress:pressure curve and reduced CSA. Consequently, although the three conditions of ageing in WT mice, caveolin-1 KO in young mice and ageing in caveolin-1 KO mice each exhibited reductions in distensibility, the structural remodelling mechanisms underlying this common output may differ: for example, in the arrangements of extracellular matrix protein deposition in the internal elastic lamina (IEL) or intercellular spaces. Interestingly, ageing-mediated increases in aortic stiffness have been correlated to enhanced collagen/elastin fibre content [48] and caveolin depletion has been linked to a variety of tissue fibrosis scenarios including coronary vascular collagen deposition [49]. Qualitatively, the IEL of mesenteric arteries from 3-month WT and KO mice appear similar (Fig. 2A and B) and, in sheep aorta, remodelling in the medial wall layer rather than the IEL correlates with age-dependent increases in vessel stiffness [48]. Nonetheless future studies are required to establish the exact molecular nature of small artery remodelling events with ageing and/or caveolin-1 KO. To this end, it will be important to employ microscopic examination of serial arterial sections to quantify any alterations in cellular and extracellular components between the three conditions.
Possible interactions between contractile and passive structural and mechanical changes of ageing and/or caveolin-1 KO mice
Arterial outward remodelling – that is, increases in luminal diameter and CSA as observed in ageing WT mice and 3-month-old caveolin-1 KO mice – has been reported to accompany pathological conditions associated with reduced myogenic tone [27]. It is noteworthy, therefore, that reduced mesenteric artery myogenic tone has been previously reported for young caveolin KO mice and aged normal mice [7, 50]. An increase in NO bioavailability would also be expected to reduce arterial tone and may therefore impact on structural parameters. In the present study it is also notable that conditions of increased NO bioavailability (assumed from the ability of L-NNA incubation to enhance noradrenaline contractions) observed in the aged WT mice and the 3-month-old caveolin-1 KO mice were associated with enhanced CSA. In contrast, the contractile responses to noradrenaline of arteries from 12-month caveolin-1 KO mice were insensitive to L-NNA and a decrease in CSA was observed.
Summary
The results of the present study suggest that caveolin-1 deletion is associated with premature ageing of mesenteric arteries both in terms of passive mechanical characteristics and noradrenergic contractile function and, furthermore, modulates the normal effects of ageing on these vessels. In future studies it will be important to establish three things. First, whether similar effects are observed in small arteries serving other vascular beds. Second, to examine the effects of caveolin-1 ablation, and of vascular ageing, over a longer time course. Third, to microscopically determine the direct effects of caveolin-1 ablation, and/or ageing, on arterial morphology and microstructure.
Acknowledgments
This work was supported by a British Heart Foundation studentship (FS/06/045) and a Wellcome Trust VIP award. We thank Drs. O. Feron and C. Dessy (Université catholique de Louvain) for the supply of caveolin-1 KO/WT mice breeding pairs, E. Cartwright and F. Baudoin-Stanley for assistance with genotyping and C. J. P. Jones (Manchester University) for electron microscopy.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Cupples LA, D’Agostino RB. Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. In: Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Study: an epidemiological investigation of cardiovascular disease. Bethesda, MD: US Department of Health and Human Services; 1987. pp. 9–20. [Google Scholar]
- 2. Available at http://www.who.int/features/factfiles/ageing/en/index.html.
- 3.Taggart M. Vascular function in health and disease review series. J Cell Mol Med. 2010;14:1017. doi: 10.1111/j.1582-4934.2010.01050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen H, Hasenkam JM, Pilegaard HK, et al. Age-dependent changes in α-adrenoceptor-mediated contractility of isolated human resistance arteries. Am J Physiol. 1992;263:H1190–6. doi: 10.1152/ajpheart.1992.263.4.H1190. [DOI] [PubMed] [Google Scholar]
- 5.Briones AM, Salaices M, Vila E. Mechanisms underlying hypertrophic remodeling and increased stiffness of mesenteric resistance arteries from aged rats. J Gerontol A Biol Sci Med Sci. 2007;62:696–706. doi: 10.1093/gerona/62.7.696. [DOI] [PubMed] [Google Scholar]
- 6.Moreau P, D’Uscio LV, Luscher TF. Structure and reactivity of small arteries in aging. Cardiovasc Res. 1998;37:247–53. doi: 10.1016/s0008-6363(97)00225-3. [DOI] [PubMed] [Google Scholar]
- 7.Gros R, Van Wert R, You X, et al. Effects of age, gender, and blood pressure on myogenic responses of mesenteric arteries from C57BL/6 mice. Am J Physiol. 2002;282:H380–8. doi: 10.1152/ajpheart.2002.282.1.H380. [DOI] [PubMed] [Google Scholar]
- 8.Pani B, Singh BB. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium. 2009;45:625–33. doi: 10.1016/j.ceca.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman A, Swörd K. The role of caveolin-1 in cardiovascular regulation. Acta Physiol. 2009;195:231–45. doi: 10.1111/j.1748-1716.2008.01907.x. [DOI] [PubMed] [Google Scholar]
- 10.Thomas CM, Smart EJ. Caveolae structure and function. J Cell Mol Med. 2008;12:796–809. doi: 10.1111/j.1582-4934.2008.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razani B, Engeman JA, Wang XB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 12.Scherer PE, Lewis RY, Volonte D, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–46. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RG. The caveolae membrane system. Ann Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 14.Albinsson S, Shakirova Y, Rippe A, et al. Arterial remodeling and plasma volume expansion in caveolin-1-deficient mice. Am J Physiol. 2007;293:R1222–31. doi: 10.1152/ajpregu.00092.2007. [DOI] [PubMed] [Google Scholar]
- 15.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 16.Park DS, Cohen AW, Frank PG, et al. Caveolin-1 null (−/−) mice show dramatic reductions in life span. Biochemistry. 2003;42:15124–31. doi: 10.1021/bi0356348. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Timme TL, Naruishi K, et al. Mice with cav-1 gene disruption have benign stromal lesions and compromised epithelial differentiation. Exp Mol Pathol. 2008;84:131–40. doi: 10.1016/j.yexmp.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Head BP, Peart JN, Panneerselvam M, et al. Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS ONE. 2011;s12:e15697. doi: 10.1371/journal.pone.0015697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–9. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 20.Shakirova Y, Bonnevier J, Albinsson S, et al. Increased Rho activation and PKC-mediated smooth muscle contractility in the absence of caveolin-1. Am J Physiol. 2006;291:C1326–35. doi: 10.1152/ajpcell.00046.2006. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Gα subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–90. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel JB, Feron O, Sacks D, et al. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–6. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- 23.Schwencke C, Schmeisser A, Walter C, et al. Decreased caveolin-1 in atheroma: loss of antiproliferative control of vascular smooth muscle cells in atherosclerosis. Cardiovasc Res. 2005;68:128–35. doi: 10.1016/j.cardiores.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Razani B, Altschuler Y, Zhu L, et al. Caveolin-1 expression is down-regulated in cells transformed by the human papilloma virus in a p53-dependent manner. Replacement of caveolin-1 expression suppresses HPV-mediated cell transformation. Biochem. 2000;39:13916–24. doi: 10.1021/bi001489b. [DOI] [PubMed] [Google Scholar]
- 25.Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–26. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosei EA, Rizzoni D. Small artery remodelling in diabetes. J Cell Mol Med. 2010;14:1030–60. doi: 10.1111/j.1582-4934.2010.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heagerty AM, Heerkens EH, et al. Small artery structure and function in hypertension. J Cell Mol Med. 2010;14:1037–43. doi: 10.1111/j.1582-4934.2010.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soucy KG, Ryoo S, Benjo A, et al. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–59. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 29.Le Saux O, Teeters K, Miyasato S, et al. The role of caveolin-1 in pulmonary matrix remodeling and mechanical properties. Am J Physiol. 2008;295:L1007–17. doi: 10.1152/ajplung.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trushina E, Du Charme J, Parisi J, et al. Neurological abnormalities in caveolin-1 knock out mice. Behav Brain Res. 2006;172:24–32. doi: 10.1016/j.bbr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Shaw L, Sweeney MA, O’Neill SC, et al. Caveolae and sarcoplasmic reticular coupling in smooth muscle cells of pressurised arteries: the relevance for Ca2+ oscillations and tone. Cardiovasc Res. 2006;69:825–35. doi: 10.1016/j.cardiores.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 32.Shaw L, Taggart MJ, Austin C. Mechanisms of 17-β-oestradiol induced vasodilatation in isolated pressurized rat small arteries. Br J Pharmacol. 2000;129:555–65. doi: 10.1038/sj.bjp.0703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka Y, Funabiki M, Michikawa H, et al. Effects of aging on α1-adrenoceptor mechanisms in the isolated mouse aortic preparation. J Smooth Musc Res. 2006;42:131–8. doi: 10.1540/jsmr.42.131. [DOI] [PubMed] [Google Scholar]
- 34.Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res. 2005;66:374–83. doi: 10.1016/j.cardiores.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Vila E, Vivas NM, Tabernero A, et al. α1-adrenoceptor vasoconstriction in the tail artery during ageing. Br J Pharmacol. 1997;121:1017–23. doi: 10.1038/sj.bjp.0701193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohi Y, Thiel MA, Buhler FR, et al. Activation of endothelial L-arginine pathway in resistance arteries. Effect of age and hypertension. Hypertension. 1990;16:170–9. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- 37.Toda N, Okamurta T, Miyazaki M. Age-dependent changes in the response of isolated beagle coronary arteries to transmural electrical stimulation and catecholamines. J Pharmacol Exper Ther. 1986;238:319–26. [PubMed] [Google Scholar]
- 38.Karaki H, Nakagawa H, Urakawa N. Age-related changes in the sensitivity to verapamil and sodium nitroprusside of vascular smooth muscle of rabbit aorta. Br J Pharmacol. 1985;85:223–8. doi: 10.1111/j.1476-5381.1985.tb08850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duckles SP, Carter BJ, Williams CL. Vascular adrenergic neuroeffector function does not decline in aged rats. Circ Res. 1985;56:109–16. doi: 10.1161/01.res.56.1.109. [DOI] [PubMed] [Google Scholar]
- 40.Matz RL, Andriantsitohaina R. Preservation of vascular contraction during ageing: dual effect on calcium handling and sensitization. Br J Pharmacol. 2003;138:745–50. doi: 10.1038/sj.bjp.0705104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–66. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 42.Sun D, Huang A, Yan EH, et al. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol. 2004;286:H2249–56. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang YM, Huamg A, Kaley G, et al. eNOS uncoupling and endothelial dysfunction in aged vessels. Am J Physiol. 2009;297:H1829–36. doi: 10.1152/ajpheart.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murata T, Lin MI, Huang Y, et al. Re-expression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–82. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pojoga LH, Yao TM, Sinha S, et al. Effect of dietary sodium on vasoconstriction and eNOS-mediated vascular relaxation in caveolin-1 deficient mice. Am J Physiol. 2008;294:H1258–65. doi: 10.1152/ajpheart.01014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adrian M, Laurant P, Berthelot A. Effect of magnesium on mechanical properties of pressurised mesenteric small arteries from old and adult rats. Clin Experim Pharmacol Physiol. 2004;31:306–13. doi: 10.1111/j.1440-1681.2004.03992.x. [DOI] [PubMed] [Google Scholar]
- 47.Qiu H, Depre C, Ghosh K, et al. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation. 2007;116:669–76. doi: 10.1161/CIRCULATIONAHA.107.689208. [DOI] [PubMed] [Google Scholar]
- 48.Graham HK, Akhtar R, Kridiotis C, et al. Localised micro-mechanical stiffening in the ageing aorta. Mech Ageing Dev. 2011 doi: 10.1016/j.mad.2011.07.003. ; doi:10.1016/j.mad.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krieger MH, Di Lorenzo A, Teutsch C, et al. Telmisartan regresses left ventricular hypertrophy in caveolin-1-deficient mice. Lab Invest. 2010;90:1573–81. doi: 10.1038/labinvest.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubroca C, Loyer X, Retailleau K, Loirand G, et al. RhoA activation and interaction with Caveolin-1 are critical for pressure-induced myogenic tone in rat mesenteric resistance arteries. Cardiovasc Res. 2007;73:190–7. doi: 10.1016/j.cardiores.2006.10.020. [DOI] [PubMed] [Google Scholar]


