Abstract
Platelet-derived microparticles (PMP) bind and modify the phenotype of many cell types including endothelial cells. Recently, we showed that PMP were internalized by human brain endothelial cells (HBEC). Here we intend to better characterize the internalization mechanisms of PMP and their intracellular fate. Confocal microscopy analysis of PKH67-labelled PMP distribution in HBEC showed PMP in early endosome antigen 1 positive endosomes and in LysoTracker-labelled lysosomes, confirming a role for endocytosis in PMP internalization. No fusion of calcein-loaded PMP with HBEC membranes was observed. Quantification of PMP endocytosis using flow cytometry revealed that it was partially inhibited by trypsin digestion of PMP surface proteins and by extracellular Ca2+ chelation by EDTA, suggesting a partial role for receptor-mediated endocytosis in PMP uptake. This endocytosis was independent of endothelial receptors such as intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 and was not increased by tumour necrosis factor stimulation of HBEC. Platelet-derived microparticle internalization was dramatically increased in the presence of decomplemented serum, suggesting a role for PMP opsonin-dependent phagocytosis. Platelet-derived microparticle uptake was greatly diminished by treatment of HBEC with cytochalasin D, an inhibitor of microfilament formation required for both phagocytosis and macropinocytosis, with methyl-β-cyclodextrin that depletes membrane cholesterol needed for macropinocytosis and with amiloride that inhibits the Na+/H+ exchanger involved in macropinocytosis. In conclusion, PMP are taken up by active endocytosis in HBEC, involving mechanisms consistent with both phagocytosis and macropinocytosis. These findings identify new processes by which PMP could modify endothelial cell phenotype and functions.
Keywords: endothelial cells, inflammation, internalization, macropinocytosis, microparticles, phagocytosis
Introduction
Microparticles (MP) are submicron membrane fragments released from many cell types upon activation or apoptosis. Their composition and functional properties vary with their cellular origin and the type of stimulus involved in their formation. Circulating MP mainly derive from platelets. Elevated levels of PMP have been associated with numerous pathological states such as atherothrombosis [1] or infectious diseases [2, 3]. Platelet-derived microparticle interactions with various cell types and their functional consequences have been described in many studies. Indeed, PMP modulate biological functions of endothelial cells, leucocytes or haematopoietic cells via antigens, bioactive lipids or chemokines that they transport [4-11].
Less attention has been paid to the fate of PMP and neither their life span nor the mechanisms of their clearance have been clearly determined yet. Several groups suggested that PMP were cleared from the circulation very quickly following infusion into animal [12, 13] and, more recently, lactadherin has been identified as one of the mediators of the clearance of phosphatidylserine (PS)-expressing PMP by splenic macrophages [14].
In a recent study, we showed that PMP were internalized by HBEC by an active process and induced changes in the surface phenotype of these cells that could have a pivotal role in the pathogenesis of cerebral malaria, an acute encephalopathy secondary to the infection by Plasmodium falciparum [15]. However, this internalization process had not been characterized. Here, using fluorescent markers for several endocytic compartments and various specific endocytosis inhibitors, we further characterize the intracellular sorting of PMP in HBEC and the mechanisms involved in this endocytosis. These analyses may help not only to elucidate the mechanisms by which PMP are taken up and degraded or recycled by cells, but also provide new insights in the regulation of the transport of biomolecules by PMP into target cells.
Materials and methods
Microvascular endothelial cells
Human brain endothelial cells, HBEC-5i, were derived by Dorovini-Zis et al. from small fragments of human cerebral cortex obtained from patients who had died of various causes [16]. These immortalized cells were then characterized and maintained in our laboratory, as described elsewhere [15, 17].
Platelet microparticles
Platelet-derived microparticles were purified from in vitro-activated platelet supernatants and labelled with red PKH26 or green PKH67 (Sigma-Aldrich, St. Louis, MO, USA) as previously described [15]. Briefly, washed platelets from human blood were incubated for 45 min. at 37°C in the presence of calcium ionophore A23187 (10 μmol/l). Platelets and cell debris were removed by serial centrifugations (6 min. at 2000 χ g and 2 min. at 13,000 χ g), PMP were pelleted by centrifugation 60 min. at 20,800 χ g and resuspended in PBS. Platelet-derived microparticles were then mixed volume to volume with PKH by gentle pipetting for 1 min. and washed with DMEM without phenol red (Gibco-Invitrogen-Life Technologies, Villebon sur Yvette, France). Alternatively, PMP were directly incubated with 10 μg/ml calcein AM (Invitrogen, Marseille, France) for 60 min. at 37°C and washed twice in PBS. Labelled PMP were counted by flow cytometry. Supernatants resulting from the final wash were used as a control.
In some experiments, to remove protease-sensitive glycoproteins from the surface of PMP, we performed digestion by 0.25% (w/v) trypsin (Gibco) for 30 min. at 37°C as described by Baj-Krzyworzeka et al. [18].
Identification of the endocytic compartments containing PMP
Human brain endothelial cells grown to confluence on gelatin-coated 8-well labtek slides (Nunc) were incubated for 90 min. total incubation time at 37°C with PKH67-labelled PMP and washed to remove unbound PMP. LysoTracker Red DND-99 (50 nM; Invitrogen) or ER-Tracker Red (100 nM; Invitrogen), for the labelling of lysosomes or endoplasmic reticulum respectively, were added 30 min. before washing and fixation. For early endosome labelling, HBEC were fixed with 4% (v/v) paraformaldehyde for 10 min. and permeabilized with Triton X-100 0.1% (v/v) in PBS 5 min. at room temperature. Non-specific binding was blocked by incubation for 30 min. in PBS containing 1% (w/v) BSA. Early endosome antigen 1 (EEA-1) was detected using a mouse monoclonal IgG1 to EEA-1 (clone 14, 1:1000; BD Transduction Laboratories, San Jose, CA, USA) revealed with Alexa-Fluor 555 conjugated goat antimouse secondary antibody (1:400; Invitrogen). Both primary and secondary antibodies were diluted in 1% (w/v) BSA in PBS and incubated for 1 hr at room temperature. Finally, cells were washed and kept in PBS for analysis. Samples were analysed by confocal laser scanning microscopy using an Axiovert 200 inverted microscope, equipped with a Zeiss LSM 510 scanning module and Zeiss LSM510 Software (Zeiss, Oberkochen, Germany).
PMP endocytosis by HBEC and inhibition assays
To quantify PMP endocytosis by HBEC, cells were grown to confluence in 96-well plates and were either left unstimulated or incubated overnight with 10 ng/ml tumour necrosis factor (TNF). Cell monolayers were then washed in serum-free medium and further incubated for 90 min. at 37°C with PKH67-labelled PMP (2000/μl) in serum-free DMEM.
To evaluate the effect of serum in PMP endocytosis, medium was supplemented with 10% foetal calf serum (FCS; Gibco) decomplemented by heating for 30 min. at 56°C.
In some experiments, the calcium-chelator EDTA was added to the medium to a final concentration of 2.5 mM during endocytosis assay to investigate the role of extracellular calcium in PMP endocytosis.
To evaluate the role of endothelial surface adhesion receptors in PMP internalization, HBEC were first pre-incubated for 30 min. at 4°C with mAbs against vascular cell adhesion molecule-1 (VCAM-1, CD106, clone 1G11, IgG1) and intercellular adhesion molecule-1 (ICAM-1, CD54, clone 84H10, IgG1) purchased from Beckman Coulter Immunotech. An irrelevant isotype-matched mAb (clone 679.1Mc7) was used as control.
To evaluate the effects of specific endocytosis inhibitors, HBEC were first pre-incubated with the following inhibitors in serum-free medium for 30 min. at 37°C: 10 μM cytochalasin D, 10 mM methyl-β-cyclodextrin and 250 μM amiloride. Cytochalasin D and amiloride were kept in the medium during the endocytosis assay. To avoid any effect of cholesterol depletion on PMP integrity, a washing step to remove methyl-β-cyclodextrin from the prior co-incubation medium was necessary before the addition of PMP and the endocytosis assay was carried out in the absence of this inhibitor. Cells were incubated with 0.5 mg/ml 70 kD-dextran (Sigma-Aldrich) at 37°C for 30 min. as a positive control for macropinocytosis.
Each inhibitor and EDTA were tested at different concentrations to establish working concentrations for maximum inhibition without toxic effects on cells. After incubation, HBEC were washed, detached by a short trypsin treatment and analysed for fluorescence by flow cytometry. To assess effective activation of HBEC by TNF, detached HBEC were analysed for surface up-regulation of VCAM-1 and ICAM-1 by flow cytometry as previously described [17]. For all these experimental conditions, flow cytometry analyses were performed with live cells using an Epics XL flow cytometer (Beckman Coulter Immunotech). Data were obtained and analysed using System2 software (Beckman Coulter Immunotech). Live cells were gated by forward/side scattering from a total of 10,000 events.
Statistical analyses
Continuous variables were tested for normal distribution with the Kolmogorov–Smirnov test and are presented as continuous variables as mean ± S.D. Means between two groups were compared using a two-tailed unpaired Student’s t-test. P values less than 0.05 were accepted as significant. All statistical analyses were performed with GraphPad Prism version 4.0 for Windows Software.
Results
Subcellular localization of internalized PMP
We previously demonstrated that PMP were internalized by HBEC in a time-dependent and temperature-sensitive process [15]. Once internalized, PMP were found to be surrounded by membranes labelled with Alexa-555 coupled wheat germ agglutinin, suggesting that PMP moved into large intracellular vesicles but did not stay along the plasma membrane.
To evaluate the potential role of endocytosis in PMP uptake, we first identified the intracellular vesicles in which PMP were processed after internalization. Using confocal microscopy, the cell distribution of PHK67-labelled PMP was compared to that of endosomal structures, as revealed by EEA1 and LysoTracker red staining. As shown in Figure 1, after 90 min. of co-incubation, 15% of PMP were found in EEA1-positive vesicles (top) and most PMP co-localized with LysoTracker red fluorescence in HBEC (middle). These data clearly indicate that PMP are internalized by an endocytic process and that, after uptake, PMP traffic along the endosomal compartment through early endosomes towards more acidic lysosomes. Endocytosed PMP showed no colocalization with ER-Tracker staining (bottom), indicating that endocytosed PMP did not enter the retrograde-transport pathway to the endoplasmic reticulum at that time.
Fig 1.
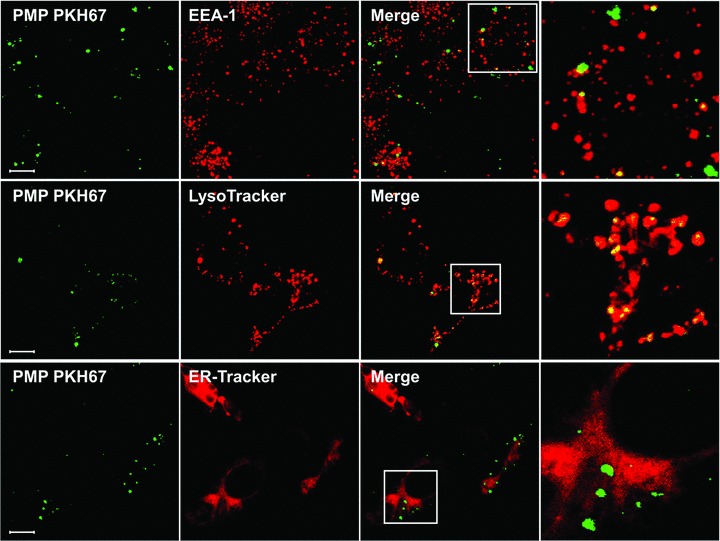
PMP are present in early endosomes and lysosomes. HBEC were incubated with PKH67-labelled PMP (green) or with PMP supernatants as a negative control for 90 min. at 37°C. HBEC were labelled with mouse anti-EEA-1 IgG revealed with Alexa 555-coupled goat antimouse IgG (top, red), with LysoTracker (middle, red) or with ER-Tracker (bottom, red). Cells were then analysed by confocal microscopy. For each selected sample, 15 optical sections separated by 0.6 nm steps were analysed. The lens used was a Zeiss Plan-Apochromat 63χ/1.40 oil immersion objective lens. Inserts of the merged images are represented in the right column. Bars: 10 μm.
Detection of PMP fusion with HBEC membrane by calcein release
To search for PMP fusion with the endothelial membrane and to detect a possible PMP fragmentation once internalized, PMP content was stained with soluble calcein AM (green) in addition to the membranous PKH26-labelling (red). After staining, 48% of PMP were double labelled for both calcein and PKH26 (Fig. 2A). Figure 2B shows that, after PMP internalization by HBEC, no PKH26 was transferred from PMP to endothelial membrane and no entrapped calcein was released within the cytosol, suggesting that PMP content did not diffuse inside the target cell but remained co-localized with PMP membranes. This observation clearly indicates that PMP internalization by HBEC does not involve the fusion of PMP lipids with the endothelial plasma membrane but, rather, an uptake of PMP material with subsequent localization in intracellular vesicles.
Fig 2.
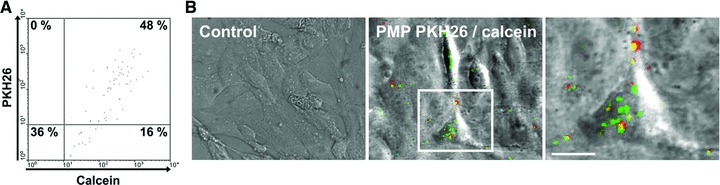
Co-localization of PMP cytoplasmic content and PMP-derived membranes inside the target cell. PMP were double-labelled with membrane dye PKH26 (red) and calcein AM that only becomes green fluorescent once hydrolysed by cytoplasmic esterases. (A) Analysis of PMP double-labelling by flow cytometry. Number in the upper right quadrant represents the percentage of double-labelled PMP. (B) PMP were then incubated with HBEC for 90 min. at 37°C. PMP supernatant was used as a negative control. Cells were washed, fixed with PFA 2% (v/v) and analysed by confocal microscopy. Merged images showing transmitted light and green and red channels are represented. Insert of the merged image is represented in the right column. Bar: 10 μm.
Mechanisms of PMP endocytosis
To further characterize PMP internalization by HBEC, we tested the effect of different modulators added just before or during the internalization assay and quantified the uptake of PKH67-labelled PMP by flow cytometry (Fig. 3A). Firstly, PMP internalization was only reduced by 38% after trypsin digestion (Fig. 3B), indicating that, in addition to the trypsin-sensitive protein components of PMP, other trypsin-resistant components such as lipids can be responsible for their interaction with HBEC. Secondly, pre-treatment of HBEC with EDTA resulted in a significant 33% decrease of PMP uptake (Fig. 3B), suggesting that this phenomenon is partially calcium dependent. Thirdly, we showed that PMP internalization was not affected when HBEC were first incubated with mAbs against VCAM-1 or ICAM-1. Moreover, PMP internalization was not increased in TNF-pre-stimulated HBEC as compared to resting cells (Fig. 3B). This suggests that PMP internalization is not mediated by TNF-up-regulated adhesion receptors such as VCAM-1 or ICAM-1. Surprisingly, in the presence of decomplemented FCS, there was a threefold increase in PMP uptake (Fig. 3B), suggesting a role for PMP opsonization by decomplemented FCS in their entry into HBEC.
Fig 3.
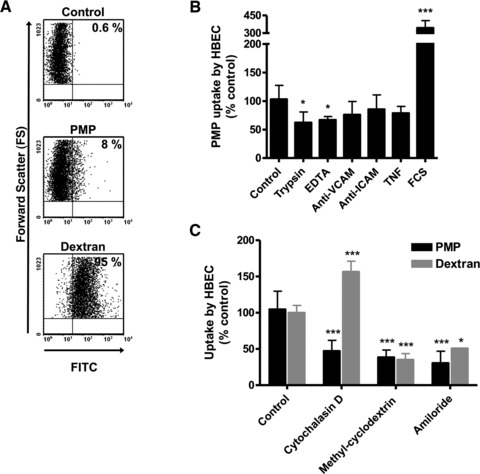
PMP endocytosis assay. HBEC were incubated with PKH67-labelled PMP or 70 kD FITC-dextran for 90 min. at 37°C. PMP supernatant was the negative control. Fluorescent cells were quantified by flow cytometry. (A) Numbers in the upper right quadrants represent the percentage of fluorescent cells. (B) HBEC were either co-incubated with trypsin-treated PMP, co-incubated with PMP in the presence of EDTA, pre-incubated with blocking mAbs against ICAM-1 or VCAM-1, pre-stimulated with TNF before PMP co-incubation or co-incubated with PMP in the presence of decomplemented FCS. (C) Effects of specific endocytosis inhibitors on PMP or 70 kD FITC-dextran uptake. HBEC were pre-treated with cytochalasin D, methyl-β-cyclodextrin or amiloride for 30 min. prior to adding PKH67-labelled PMP for 90 min. or 70 kD FITC-dextran for 30 min. (B, C) Results are presented as the percentage of PMP uptake (mean fluorescence intensity) compared to the 37°C-positive control (mean ± S.D.). A two-tailed, unpaired Student’s t-test was used for comparison of means; *P < 0.05, **P < 0.01 and ***P < 0.001.
To determine which of the several possible cellular uptake mechanisms were responsible for PMP internalization, the effects of three different endocytosis inhibitors were examined. These inhibitors were also tested on the entry of 70-kD FITC-dextran, a marker of macropinocytosis, for comparison. Cytochalasin D was used to inhibit actin elongation and the formation of microfilaments and microtubules, methyl-β-cyclodextrin to deplete membrane cholesterol and inhibit lipid raft-dependent pathways, and amiloride was tested to inhibit the Na+/H+ exchanger required for macropinocytosis [19]. In HBEC, 70 kD FITC-dextran endocytosis was reduced by methyl-β-cyclodextrin and amiloride, as expected for macropinocytosis (Fig. 3C, grey bars). Cytochalasin D was found to produce an unexpected increase in dextran entry into HBEC, indicating an alteration in macropinocytosis with this inhibitor. In contrast, all drugs tested were found to partially inhibit the entry of PMP into HBEC (Fig. 3C, black bars).
Discussion
A growing number of studies report the cellular uptake of MP by mononuclear haematopoietic cells, monocytes, neutrophils, plasmacytoid dendritic cells [18, 20-23] and by endothelial cells [15, 24-26]. However, the mechanisms involved in this process have not been clearly defined yet.
In this study, we have examined the intracellular localization of PMP internalized by brain microvascular endothelial cells and attempted to decipher the pathways involved in this phenomenon. Using markers for endosomal compartments, we demonstrated for the first time that internalized PMP were distributed within endosomes and lysosomes after cell entry, supporting the fact that endocytosis was the predominant route of PMP entry into endothelial cells. This major finding differs from the recent work by Terrisse et al. who did not find any co-localization of endocytosed endothelial-derived MP with markers of early or late endosomes after 2 hrs of co-incubation with HUVEC [25], suggesting that either kinetics or pathways for MP intracellular processing might vary between different endothelial cell types.
Although PMP were efficiently endocytosed by endothelial cells, they did not fuse with their plasma membrane because no diffusion of calcein-labelled PMP content occurred inside the target cell cytosol. This is consistent with the observation of Aharon et al. who found that monocyte-derived MP bound to and were ingested by HUVEC without any fusion of MP with the cell membrane [24] but contrasts with the fusion of monocyte-derived MP with activated platelets described by del Conde et al. [27]. These results suggest that MP interaction might involve different mechanisms depending on the target cell type.
Endocytosis may occur through several distinct mechanisms, usually divided into two broad categories: phagocytosis, a process restricted to specialized mammalian cells, and pinocytosis, which occurs in all mammalian cells and encompasses macropinocytosis, clathrin-mediated endocytosis, and caveolae-mediated endocytosis [28]. Although clathrin- and caveolin-dependent endocytosis involve the uptake of particles smaller than 100 nm in diameter, phagocytosis and macropinocytosis involve the uptake of larger volumes. Because MP are defined as particles with a diameter varying between 100 and 1000 nm [6], we focused on the potential role for phagocytosis or macropinocytosis in PMP uptake.
Although phagocytosis requires cell-surface recognition of particles and proceeds via sequential engagement of receptors, macropinocytosis occurs via non-selective membrane uptake. We thus tried to determine which type of MP surface components and endothelial receptors could be involved in MP endocytosis by HBEC. The partial inhibition of PMP internalization after treatment of PMP with trypsin indicates that protease-sensitive components of PMP, such as surface glycoproteins, play a minor role in PMP binding and further internalization but rather supports a role for other components such as lipids. PMP uptake was slightly inhibited by EDTA, suggesting that Ca2+-dependent (usually receptor-mediated) endocytosis was partly involved in PMP uptake. Because platelet adhesion and fusion to TNF-activated brain endothelial cells depends on ICAM-1 up-regulation on endothelial membranes and LFA-1 expression on platelets [29-31], we wanted to evaluate the role of ICAM-1 in PMP binding and internalization by HBEC. Pre-incubation of HBEC with mAb against ICAM-1 or VCAM-1 failed to inhibit PMP uptake. Furthermore, TNF activation of HBEC, which increases both ICAM-1 or VCAM-1 expression on HBEC surface [17], did not increase PMP internalization, implying that these adhesion receptors are not required for PMP endocytosis. On the other hand, the increased uptake of PMP in the presence of decomplemented FCS suggests that PMP can be opsonized by FCS components other than proteins from the complement cascade supporting a role for phagocytosis.
The composition of MP is related to their cellular origin and to the type of stimulus involved in their formation; therefore, the cellular events required for their internalization may differ between both target cells and MP cellular origins. Nonetheless, one common feature of MP is the exposure on their external leaflet of PS, also present on the external surface of apoptotic cells and apoptotic platelets. PS exposure [32, 33] and recognition by many receptors such as scavenger receptors present on macrophages [34] are crucial for phagocytosis and clearance of apoptotic platelets and are probably also relevant in the clearance of PMP. Indeed, three recent studies showed that MP endocytosis by either HUVEC [25, 26] or macrophages [35] was significantly inhibited in the presence of annexin V suggesting a major role for PS. Flaumenhaft proposed that PS exposure on PMP may support opsonization by C3b. C3b-bearing PMP could subsequently bind to erythrocytes through complement receptor CR1 and be delivered to scavenger endothelium in the liver and spleen by receptor-mediated endocytosis [13]. Scavenger receptors have been evoked to be involved in the removal from the circulation of red blood cell-derived MP by Kupffer cells in the liver [36]. Both scavenger receptors [37, 38] and PS receptor (PSR) [39] are expressed on microvascular endothelial cells but PSR is highly unlikely to be involved in PMP endocytosis under basal conditions because PSR does not appear to be constitutively expressed on the cell surface [39]. Other opsonins such as β2-glycoprotein I [35], IgM [40] or lactadherin [14] have been involved in MP phagocytosis by macrophages. Recently, Terrisse et al. showed that HUVEC internalized endothelium-derived MP through a process involving anionic phospholipids, lactadherin and αvβ3 integrin but not CD36 [25]. The importance of this potential interaction in our experimental model warrants further investigations especially as HBEC-5i express constitutive αvβ3 integrin but no CD36 on their surface [17].
Both phagocytosis and micropinocytosis are characterized by extensive membrane reorganization and are reduced by cytochalasin D treatment [28]. Thus, the reduction of PMP entry into cells by cytochalasin D does not allow us to identify one of the two processes as a major pathway for PMP entry into HBEC. The decrease of PMP uptake by the cholesterol depleting agent methyl-β-cyclodextrin shows that PMP endocytosis depends on cholesterol content and suggests a role for macropinocytosis because cholesterol is required for membrane ruffling and macropinocytosis [41]. It should be stressed out that, in addition to cholesterol depletion, methyl-β-cyclodextrin has pleiotropic effects on different membrane components, such as phospholipids, that could have a potential role in PMP endocytosis. The incomplete inhibition of PMP entry by the Na+/K+ transporter inhibitor amiloride indicates that endocytic uptake of PMP occurs partially by non-selective macropinocytosis [19]. The unique susceptibility of macropinocytosis to inhibitors of Na+/H+ exchange has been extensively used to differentiate it from other types of endocytosis. However, caution should be made in interpreting these data because the inhibition of macropinocytosis by amiloride could also result from its effects on cell pH [42].
Phagocytosis of particles, apoptotic bodies and aged erythrocytes is predominately attributed to macrophages, neutrophils and dendritic cells. However, several studies have described the uptake of large objects by endothelial cells, including apoptotic bodies [43-46] and MP [24-26]. More surprising is the endocytic activity of endothelial cells derived from the brain. Indeed, the most important function of endothelial cells that line the brain capillaries is to form the blood–brain barrier (BBB), which physically separates blood components from the brain and the cerebrospinal fluid. This barrier is based, in part, on a paucity of vesicular activity within the brain microvascular endothelial cells that limits endocytosis. However, endocytosis can be modulated by vasoactive substances, such as angiotensin and bradykinin [47], and cytokines, such as TNF [48], and may dramatically increase in disease states, rendering the BBB less restrictive for blood-borne proteins and cells.
Understanding PMP endocytotic process could provide essential clues that may reveal how endothelial cells can differentially regulate the transport of different components across the BBB. This is likely to shed light on the processes underlying numerous neurological disorders during which an increased production of PMP at the interface of the BBB occurs. For example, PMP carry agents implicated in neurodegenerative disorders such as amyloid β-protein precursor present in amyloid plaques in Alzheimer’s disease [49] and PMP could actively transport these agents across the BBB [50].
Overall, our findings provide new mechanisms by which MP interact with endothelial cells and could modify their phenotype and functions. This could help understand the role of MP in the broad range of physiological and pathological processes during which MP levels are increased. MP uptake by endothelial cells could also participate in MP clearance from the circulation.
Acknowledgments
The authors are indebted to the Plate-forme d’Imagerie Commune Scientifique de Luminy for expert technical assistance. This work was supported by grants from the National Health and Medical Research Council of Australia (NHMRC Project Grant No. 464893; www.nhmrc.gov.au), the Australian Research Council (ARC Discovery Project No. DP0774425; www.arc.gov.au) and from the European Union-Health and Medical Research Council of Australia (EU-NHMRC Project Grant No. 512101; europa.eu), the Rebecca L. Cooper Foundation (www.cooperfoundation.org.au), the University of Sydney Major Equipment Grant scheme (www.usyd.edu.au) and the AL Kerr Bequest, Sydney Medical School. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authors’ contributions
DF performed the research. DF, FEA, AM, GEG and VC designed the research, analysed the data and wrote the paper. MCA, GC and TF provided material and analytical tools.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Tan KT, Lip GY. The potential role of platelet microparticles in atherosclerosis. Thromb Haemost. 2005;94:488–92. doi: 10.1160/TH05-03-0201. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–5. [PubMed] [Google Scholar]
- 3.Pankoui MfonkeuJB, Gouado I, Fotso KuateH, et al. Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS One. 2010;5:e13415. doi: 10.1371/journal.pone.0013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry OP, Pratico D, Lawson JA, et al. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99:2118–27. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barry OP, Pratico D, Savani RC, et al. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–44. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jy W, Mao WW, Horstman L, et al. Platelet microparticles bind, activate and aggregate neutrophils in vitro. Blood Cells Mol Dis. 1995;21:217–31. doi: 10.1006/bcmd.1995.0025. ; discussion 231a. [DOI] [PubMed] [Google Scholar]
- 7.Forlow SB, McEver RP, Nollert MU. Leukocyte-leukocyte interactions mediated by platelet microparticles under flow. Blood. 2000;95:1317–23. [PubMed] [Google Scholar]
- 8.Salanova B, Choi M, Rolle S, et al. Beta2-integrins and acquired glycoprotein IIb/IIIa (GPIIb/IIIa) receptors cooperate in NF-kappaB activation of human neutrophils. J Biol Chem. 2007;282:27960–9. doi: 10.1074/jbc.M704039200. [DOI] [PubMed] [Google Scholar]
- 9.Janowska-Wieczorek A, Majka M, Kijowski J, et al. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98:3143–9. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 10.Mause SF, von Hundelshausen P, Zernecke A, et al. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1512–8. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 11.Mause SF, Ritzel E, Liehn EA, et al. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 12.Rand ML, Wang H, Bang KW, et al. Rapid clearance of procoagulant platelet-derived microparticles from the circulation of rabbits. J Thromb Haemost. 2006;4:1621–3. doi: 10.1111/j.1538-7836.2006.02011.x. [DOI] [PubMed] [Google Scholar]
- 13.Flaumenhaft R. Formation and fate of platelet microparticles. Blood Cells Mol Dis. 2006;36:182–7. doi: 10.1016/j.bcmd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta SK, Abdel-Monem H, Niravath P, et al. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113:1332–9. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faille D, Combes V, Mitchell AJ, et al. Platelet microparticles: a new player in malaria parasite cytoadherence to human brain endothelium. Faseb J. 2009;23:3449–58. doi: 10.1096/fj.09-135822. [DOI] [PubMed] [Google Scholar]
- 16.Dorovini-Zis K, Prameya R, Bowman PD. Culture and characterization of microvascular endothelial cells derived from human brain. Lab Invest. 1991;64:425–36. [PubMed] [Google Scholar]
- 17.Wassmer SC, Combes V, Candal FJ, et al. Platelets potentiate brain endothelial alterations induced by Plasmodium falciparum. Infect Immun. 2006;74:645–53. doi: 10.1128/IAI.74.1.645-653.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baj-Krzyworzeka M, Majka M, Pratico D, et al. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30:450–9. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu NQ, Lossinsky AS, Popik W, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76:6689–700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mack M, Kleinschmidt A, Bruhl H, et al. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med. 2000;6:769–75. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 21.Ray DM, Spinelli SL, Pollock SJ, et al. Peroxisome proliferator-activated receptor gamma and retinoid X receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. doi: 10.1160/TH07-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–8. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 23.Angelot F, Seilles E, Biichle S, et al. Endothelial cell-derived microparticles induce plasmacytoid dendritic cell maturation: potential implications in inflammatory diseases. Haematologica. 2009;94:1502–12. doi: 10.3324/haematol.2009.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100:878–85. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- 25.Terrisse AD, Puech N, Allart S, et al. Internalization of microparticles by endothelial cells promotes platelet/endothelial cell interaction under flow. J Thromb Haemost. 2010;8:2810–9. doi: 10.1111/j.1538-7836.2010.04088.x. [DOI] [PubMed] [Google Scholar]
- 26.Rautou PE, Leroyer AS, Ramkhelawon B, et al. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–43. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- 27.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 28.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 29.Grau GE, Tacchini-Cottier F, Vesin C, et al. TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. Eur Cytokine Netw. 1993;4:415–9. [PubMed] [Google Scholar]
- 30.Lou J, Donati YR, Juillard P, et al. Platelets play an important role in TNF-induced microvascular endothelial cell pathology. Am J Pathol. 1997;151:1397–405. [PMC free article] [PubMed] [Google Scholar]
- 31.Mannel DN, Grau GE. Role of platelet adhesion in homeostasis and immunopathology. Mol Pathol. 1997;50:175–85. doi: 10.1136/mp.50.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira J, Palomo I, Ocqueteau M, et al. Platelet aging in vivo is associated with loss of membrane phospholipid asymmetry. Thromb Haemost. 1999;82:1318–21. [PubMed] [Google Scholar]
- 33.Maugeri N, Rovere-Querini P, Evangelista V, et al. Neutrophils phagocytose activated platelets in vivo: a phosphatidylserine, P-selectin, and {beta}2 integrin-dependent cell clearance program. Blood. 2009;113:5254–65. doi: 10.1182/blood-2008-09-180794. [DOI] [PubMed] [Google Scholar]
- 34.Brown SB, Clarke MC, Magowan L, et al. Constitutive death of platelets leading to scavenger receptor-mediated phagocytosis. A caspase-independent cell clearance program. J Biol Chem. 2000;275:5987–96. doi: 10.1074/jbc.275.8.5987. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Monem H, Dasgupta SK, Le A, et al. Phagocytosis of platelet microvesicles and beta2- glycoprotein I. Thromb Haemost. 2010;104:335–41. doi: 10.1160/TH09-12-0849. [DOI] [PubMed] [Google Scholar]
- 36.Willekens FL, Werre JM, Kruijt JK, et al. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–5. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 37.Goti D, Hrzenjak A, Levak-Frank S, et al. Scavenger receptor class B, type I is expressed in porcine brain capillary endothelial cells and contributes to selective uptake of HDL-associated vitamin E. J Neurochem. 2001;76:498–508. doi: 10.1046/j.1471-4159.2001.00100.x. [DOI] [PubMed] [Google Scholar]
- 38.Husemann J, Loike JD, Anankov R, et al. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40:195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 39.Setty BN, Betal SG. Microvascular endothelial cells express a phosphatidylserine receptor: a functionally active receptor for phosphatidylserine-positive erythrocytes. Blood. 2008;111:905–14. doi: 10.1182/blood-2007-07-099465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litvack ML, Post M, Palaniyar N. IgM promotes the clearance of small particles and apoptotic microparticles by macrophages. PLoS One. 2011;6:e17223. doi: 10.1371/journal.pone.0017223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimmer S, van Deurs B, Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol. J Cell Sci. 2002;115:2953–62. doi: 10.1242/jcs.115.14.2953. [DOI] [PubMed] [Google Scholar]
- 42.Koivusalo M, Welch C, Hayashi H, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188:547–63. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fens MH, Mastrobattista E, de Graaff AM, et al. Angiogenic endothelium shows lactadherin-dependent phagocytosis of aged erythrocytes and apoptotic cells. Blood. 2008;111:4542–50. doi: 10.1182/blood-2007-06-094763. [DOI] [PubMed] [Google Scholar]
- 44.Hess KL, Tudor KS, Johnson JD, et al. Human and murine high endothelial venule cells phagocytose apoptotic leukocytes. Exp Cell Res. 1997;236:404–11. doi: 10.1006/excr.1997.3745. [DOI] [PubMed] [Google Scholar]
- 45.Dini L, Pagliara P, Carla EC. Phagocytosis of apoptotic cells by liver: a morphological study. Microsc Res Tech. 2002;57:530–40. doi: 10.1002/jemt.10107. [DOI] [PubMed] [Google Scholar]
- 46.Kirsch T, Woywodt A, Beese M, et al. Engulfment of apoptotic cells by microvascular endothelial cells induces proinflammatory responses. Blood. 2007;109:2854–62. doi: 10.1182/blood-2006-06-026187. [DOI] [PubMed] [Google Scholar]
- 47.Guillot FL, Audus KL. Angiotensin peptide regulation of fluid-phase endocytosis in brain microvessel endothelial cell monolayers. J Cereb Blood Flow Metab. 1990;10:827–34. doi: 10.1038/jcbfm.1990.139. [DOI] [PubMed] [Google Scholar]
- 48.Defazio G, Trojano M, Ribatti D, et al. ICAM 1 expression and fluid phase endocytosis of cultured brain microvascular endothelial cells following exposure to interferon beta-1a and TNFalpha. J Neuroimmunol. 1998;88:13–20. doi: 10.1016/s0165-5728(98)00064-2. [DOI] [PubMed] [Google Scholar]
- 49.Nomura S, Komiyama Y, Miyake T, et al. Amyloid beta-protein precursor-rich platelet microparticles in thrombotic disease. Thromb Haemost. 1994;72:519–22. [PubMed] [Google Scholar]
- 50.Horstman LL, Jy W, Minagar A, et al. Cell-derived microparticles and exosomes in neuroinflammatory disorders. Int Rev Neurobiol. 2007;79:227–68. doi: 10.1016/S0074-7742(07)79010-4. [DOI] [PubMed] [Google Scholar]


