Abstract
Many patients suffer from chronic gastrointestinal diseases characterized by chronic inflammation, increased intestinal permeability and visceral pain in which there is no definitive treatment. Adult stem cells have recently been used in various disease states to contribute wound-healing processes. In the current study we investigated the ability of intra-colonic adult stem cells application to heal colonic inflammation in IL-10−/− mice with active colitis. The aims of this study were to determine whether intra-colonic infusion of adult colonic stem cells (CSCs) (local stem cell transplantation): (i) restores intestinal permeability; (ii) attenuates visceral hypersensitivity; (iii) heals murine colitis. IL-10−/− mice with active colitis were transplanted with adult stem cells. Mice received either a single intracolonic infusion of CSCs or colonic epithelial cells. Two weeks after transplantation, we measured visceral hypersensitivity and intestinal permeability and correlated these with histological improvement of colitis. IL-10−/− mice that received stem cell transplantation showed histopathologic evidence of recovery from colitis. Improvement in colitis as graded by pathology scores correlated with restoration of intestinal permeability and decreased visceral hypersensitivity. Intra-colonic administration of CSCs is a potential therapeutic method for treating refractory symptoms in patients with chronic gastrointestinal diseases associated with chronic inflammation and visceral hypersensitivity. This method may be safer and should have far fewer side effects than systemic stem cell administration.
Keywords: inflammatory bowel disease (IBD), intestinal permeability, stem cell, cytokines, TNF-α and IFN-γ, visceral hypersensitivity, IL-10−/− mice
Introduction
Inflammatory gastrointestinal disorders are characterized by chronic inflammation, abdominal pain, visceral hypersensitivity and diarrhoea. The pathophysiology of inflammatory injury to the gut is not clearly understood, but dysregulation of immunologic responses to mucosal antigens is likely to be involved [1]. As a result, most therapies involve immunosuppressive drugs that induce remission of gastrointestinal inflammation and associated symptoms. Currently, there are no therapeutic options that can definitely reverse gastrointestinal inflammation, abdominal pain and visceral hypersensitivity. To address this problem, we investigated stem cell therapy, which has been increasingly tried in the treatment of many previously intractable diseases. Recent advances in cellular therapy, regenerative medicine and advanced biomedicine have demonstrated a great potential for treating debilitating diseases with stem cell transplantation. Stem cells used for therapy have been isolated from numerous adult tissues and other non-embryonic sources. For example, bone marrow (BM)-derived stem cells transdifferentiate into mucosal compartments, their numbers are increased in damaged tissues, and they will function as stem cells in an engrafted organ [2-4]. Several therapeutic stem cell approaches involve the induction of circulating BM-derived stem cells following systematic transplantation. Such approaches may contribute to mucosal and/or tissue repair through stimulation of regenerative responses via differentiation of the implanted stem cells. The therapeutic approaches that use stem cells such as BM-derived stem cells may contribute to mucosal repair through stimulation of regenerative responses via differentiation of BM-derived stem cells. However, systemic stem cell transplantation may be associated with many side effects, which limit their clinical application. We therefore chose to determine if local application of adult stem cells can be beneficial during active colitis by increasing mucosal repair.
Recently, experimental stem cell therapy has been used in both humans with inflammatory bowel disease and animal models of intestinal inflammation [1, 3-8]. Okamoto and colleagues [4] were among the first to show the relevance of stem cell biology to treat chronic gastrointestinal inflammation by demonstrating the use of BM-derived stem cells. Another study showed that transplantation of immortalized CD34− stem cells isolated from mouse BM and peripheral blood can facilitate mucosal repair in a rodent model of colitis induced by dextran sodium sulphate (DSS) [9]. In another study, colon stem cells were enriched in LGR-5 cells revealing that colonic stem cells (CSCs) growing under self-renewal conditions [10] had substantially higher levels of these representative markers of self-renewal. However, despite these initial promising results, a major concern of these early studies is that they used systemic administration of stem cells, a method that may be associated with a significant number of side effects. Thus, it would be ideal if stem cell therapy could be targeted directly to the diseased tissues and thus avoid complications that may arise from systemic stem cell therapy.
In the current study, we investigated the benefits of delivering stem cells locally to the colon to treat inflammation and tissue injury in a murine model of IL-10−/− mice [11, 12]. IL-10−/− mice develop spontaneous enterocolitis associated with visceral hypersensitivity and pain, increased intestinal permeability and diarrhoea. The overall goal of the study was to investigate the pluripotency and self-renewal capacity of intra-colonic administration of adult CSCs to decrease colitis in IL-10−/− mice. Thus, the aims of the current study were to determine if intracolonic infusion of adult CSCs: (i) restores intestinal permeability; (ii) reduces visceral hypersensitivity and pain; (iii) reduces murine colitis through a reduction of local markers of inflammation.
Materials and methods
Stem cell preparation
Adult CSCs were derived from C57B6 mouse colon crypt tissues and obtained from Celprogen (Celprogen, Inc, San Pedro, CA, USA). Celprogen's adult CSC complete growth un-differentiating media was used to keep the stem cells in the undifferentiated state during cell culture (Table S1 and Fig. S1).
Animals’ preparation and experimental design
Male IL-10 knockout mice (IL-10−/−) (aged 9–13 weeks) and male wild-type control mice (C3H/HeJ) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in groups under constant temperature and humidity with 12-hr light—dark cycles, and were given free access to food and water (2018 Teklad Global 18% Protein Rodent Diet). Mice were monitored daily for changes in body weight, general condition, physical appearance and behaviour during the entire period following stem cell treatment. All procedures were approved by the University Institutional Animal Care and Use Committee.
A total of 71 mice were used in the current study including 55 IL-10−/− mice and 8 IL-10−/− mice control (wild-type mice) (C3H/HeJ). A total of 25 IL-10−/− mice received one dose intra-colonic transfusions of adult CSCs. There were several control groups. One control group (n= 8) consisted of IL-10−/− mice that received colonic infusion of colonic epithelial cells (CECs) in 50 μl of saline with 300 μl of matrix. Another control group (n= 8) consisted of IL-10−/− mice that received 50 μl of saline with 300 μl of matrix only (vehicle control). Two positive control groups, group one (n= 14) consisted of IL-10−/− mice that received no treatment at all; another group (n= 8) wild-type mice that received colonic TNBS infusion with 50% ethanol. A negative control group (n= 8) consisted of wild-type mice that received nothing.
The mice were killed using sodium pentobarbital (120 mg/kg, i.p.). Following killing, the colon (ascending, transverse and descending) was removed and processed for histopathology. Additional colonic tissue was used to assess intestinal permeability, as well as SuperArray qPCR assays, real-time PCR, Western blots, phosphatase assays and immuohistochemistry. We also had extra mice for characterizing the functional outcome of colitis abrogation following local stem cell infusion by using a flow cytometry analysis assay following isolation of lymphocytes from mouse colon.
Colonic stem cell transplantation
Recipient mice were fasted for 12 hrs before cell transplantation. The mice were then anaesthetized with 2–5% isoflurane and their colons were washed (by enema) with 0.5–1.0 ml saline via a 24 gauge plastic angiocatheter inserted into the lumen of the colon. Mice were then given 5 min. for their colon to empty. Then, 5×106 to 6×106 mouse CSCs or mouse CECs (Celprogen) were mixed with 0.3 ml matrix (ECM gel; Invitrogen, Carlsbad, CA, USA) and infused into the colon. Mice were held vertically for 3–5 min. while the gel mixture adhered to the colon. Animals were monitored until they fully recovered from the anaesthesia.
Cells labelling with PKH26 staining
Mouse CSCs (∼20 million) or mouse CECs (∼20 million) were labelled with PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich, St. Louis, MO, USA) at 25°C according to the manufacturer's protocol. Cells were added to ECM gel and injected into the lumen of the colon as previously described above. Mouse colons were then removed, mounted and visualized using a Zeiss 510 META Laser Scanning Confocal microscope.
Nociceptive visceral hypersensitivity testing and measurement of EMG
Visceral pain testing was performed at baseline, 7 days and 2 weeks after stem cell treatment under blinded conditions and the order of testing was counterbalanced across groups. Testing was done following a 12-hr fast.
A Flat Bottom Holder (Kent Scientific Corp, Torrington, CT, USA) plastic restrainer was used to hold the unsedated animals during colonic distension. It was large enough to allow the mice to move inside of it. Because the holder is clear plastic, we were able to monitor all abdominal movement and contractions. The abdominal contractions in response to balloon distension were clearly distinct and readily recognizable compared to normal abdominal movements.
A 3-cm long, 1.5-cm maximal diameter balloon made of polyethylene was secured to tubing attached to an automated distension device (G & J Electronic Inc., Toronto, Canada) that was used to perform colonic distension. The balloon was lubricated and placed into the mouse's distal colon so that the tip of the balloon was 0.5–1 cm from the anus. Mice were restrained in a plastic containment device and allowed to acclimate for 15–20 min. before testing. Mice received phasic distension of the colon (to pressures of 0–60 mmHg in 5 mmHg ascending increments) until the first contraction of the testicles, tail, or abdominal musculature occurred, which was defined as the visceral nociceptive pain threshold and which was indicative of the first nociceptive response [13-16]. Colonic distensions were repeated two times with 5–10 min. inter-stimulus intervals and the mean pressures at the nociceptive threshold were recorded for each animal. The technicians involved in the study were blinded to the specific group the mice belonged to.
Surgical preparation [17-19]: Electrodes (Teflon-coated stainless steel wire, 5- to 10-mm tip separation; Cooner Wire Sales, Chatsworth, CA, USA) were sewn into the external oblique abdominal muscles after anaesthetization with 75 mg/kg i.p. pentobarbital sodium, in order to perform the electromyographic (EMG) recordings. The EMG electrodes were channelled subcutaneously below the dorsum of the neck and then externalized. After recovering for a minimum of 3 days, the animals were then tested. EMG recording: Colorectal distension–evoked (CRD-evoked) contraction of the abdominal musculature, termed the visceromotor response, was the behavioural response quantified. The EMG signal was filtered, amplified and recorded as has been described [17]. The balloon was connected to a pressure device that induced inflation of the balloon. Each distension trial lasted 40 sec. and EMG activity was quantified during 10 sec. before distension, 20 sec. during distension and 10 sec. after distension.
In vitro intestinal permeability
Mice were killed by intravenous pentobarbital (120 mg/kg, i.p.) injection. Approximately 2–3 cm of the distal colon was removed for in vitro permeability studies. The distal colon (2–3 cm) was mounted in an Ussing chamber exposing 0.725 cm2 of tissue (World Precision Instruments, Sarasota, FL, USA). Tissues were bathed on serosal and mucosal sides by Krebs buffer containing (in mmol/L) 121 NaCl, 5.95 KCl, 1.20 MgCl2, 1.35 NaH2PO4, 14.3 NaHCO3, 12.7 glucose, 11.58 CaCl2. Solutions were oxygenated (95% O2 to 5% CO2) and circulated in a volume of 10 ml in water-jacketed reservoirs maintained at 37°C. Short-circuit currents (Isc) were measured every 15 min. for 30 min. (EVC4000; World Precision Instruments, Sarasota, FL, USA). Isc values were measured using Krebs-agar bridges connected to silver chloride electrodes. Transepithelial electrical resistance (TER; Ω cm2) was calculated from the spontaneous or clamped Isc using Ohms’ law.
SuperArray qPCR assay and real-time PCR analysis
RNA was isolated from colon tissues using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The RNA was subsequently cleaned using Qiagen's RNeasy Kit (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol. The optional on-column DNase treatment was performed.
Reverse transcription was done using 1 μg RNA with SABiosciences's RT2 First Strand Kit (SABiosciences, Frederick, MD, USA) according to the manufacturer's protocol. Mouse colon tissue cDNA was analysed using SuperArray plates (Inflammatory Cytokines & Receptors PCR Array; SABiosciences, Frederick, MD, USA).
To validate the translational significance of these gene expression findings, mice colon samples were analysed using real-time PCR. SABiosciences's RT2 qPCR Primer Assays were used. Real-time PCR was performed using SABioscience's RT2 SYBR Green/ROX qPCR Master Mix for a Stratagene Mx3005P Real-Time PCR System according to the manufacturer's protocol. ROX was used as an endogenous reference and data were analysed using SABioscience's PCR Array Data Analysis Template. The comparative CT method (ΔΔCT) was used for quantification of gene expression. All samples were tested in triplicate, and average values used for quantification.
Western blot
Samples were homogenized in Nonidet-P40 lysis buffer (150 mM sodium chloride, 50 mM Tris, pH 8.0, 1% NP-40, 1% protease inhibitor cocktail) then agitated at 4°C for 2 hrs. Samples were centrifuged at 12,000 r.p.m. for 20 min. at 4°C and protein supernatants removed. Proteins were separated by 8–12% SDS-PAGE and transferred to nitrocellulose membranes. Protein expressions were visualized using the LI-COR Odyssey Infrared Imaging System (Lincoln, NE, USA).
Alkaline phosphatase assay
Alkaline phosphatase activity was determined with the SensoLyte™ pNPP Alkaline Phosphatase Colormetric Assay Kit (AnaSpec, Fremont, CA, USA) according to the manufacturer's instructions.
StemTAG™ alkaline phosphatase assay
After cultured cells reached 70–80% confluency, the culture medium was removed and cells were washed once with PBST before incubating in fixing solution at room temperature. Then, 0.4 ml per well of freshly prepared StemTAG™ AP Staining Solution (by mixing equal volume of StemTAG™ AP Staining Solution A and StemTAG™ AP Staining Solution B) was added.
ELISA assay
Protein was extracted from tissues using specific lysis buffer (20 mM Tris, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 1 mM EDTA and 0.1% SDS). TNF-α levels were determined using mouse and human Ready-SET-Go! ELISA Kits (eBiosciences, San Diego, CA, USA) and IFN-γ levels were determined using mouse and human BD OtpEIA ELISA Kits (BD Biosciences, San Jose, CA, USA) according to manufacturer's protocols. Plates were read at 450 nm and 570 nm, respectively. Wavelength subtraction was used for absorbance by subtracting values at 570 nm from 450 nm, and then data were analysed.
Isolation of mouse colon intra-epithelial lymphocytes
The method for isolation of colon intraepithelial lymphocytes was modified from ref [20]. The entire colon was removed from each mouse and was flushed with 40 ml (total) 4°C CMF to expel faecal matter. The colon was then cut longitudinally and laterally into 0.5 cm pieces that were placed into 40 ml of 4°C CMF. The colonic pieces were then transferred to a 50-ml conical centrifuge tube and vortex 15 sec. at maximum setting. The resulting supernatants obtained contained both intraepithelial lymphocytes and a few epithelial cells. For further details please see Supporting Information document 2.
Histopathological evaluation of colitis
Colon tissue was fixed in formalin and processed using standard techniques for haematoxylin and eosin staining. Microscopic evaluation of tissue was done in a blinded fashion by two pathologists. Damage seen on histopathology analysis was graded using an injury score system [21-23] with scores ranging from 0 to 15 (0 = no inflammation; 1–5 = mild; 6–10 = moderate; 11–15 = severe), also see Table S1.
Statistical analysis
All statistical analyses were done using R 2.7.0 and ASA software Version 9.1.3 of the ASA system and Prism version 6. Pearson correlation coefficients were calculated to explore the association between histopathologic evidence of recovery and intestinal permeability, as well as TNF-α and IFN-γ expression after local stem cell treatment. One-way and two-way ANOVAS were also used.
Results
Colonic stem cell pluripotency and self-renewal capacity
Colonic stem cells were isolated from mouse colon using specific markers (Provided by Celprogen). Colon stem cell gene expression profile further verified by quantitative real-time PCR assay and relative CT normalized with House Keeping gene (GAPDH) (Fig. S1) The self-renewal properties of CSCs and CECs were determined by Western blots and quantitative real-time PCR using different primer set (Fig. 1A). The results show that CSCs growing under self-renewal conditions or under differentiation conditions had substantially higher levels of the representative markers of self-renewal than did CECs. Alkaline phosphatase (AP) assays were done to measure self-renewal within these cells and revealed that the level of self-renewal in CSCs was higher (13.14 ± 2.58 fold greater) than in CECs (Fig. 1B). AP activities in CSCs under differentiation conditions were less than half of that in CSCs under self-renewal conditions. After 15 passages, CSCs under differentiating conditions were able to form a confluent monolayer similar to polarized CECs (Fig. 1C).
Fig 1.
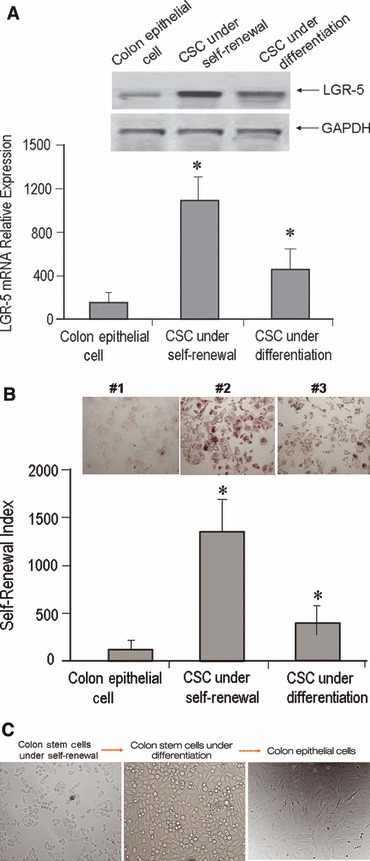
Colonic stem cell pluripotency and self-renewal capacity. (A) Lower panel shows real-time PCR analysis of gene expression of LGR-5. Colonic epithelial cells (CEC); CSC under self-renewal (colon stem cells under un-differentiating conditions); and CSC under differentiation [colon stem cells under differentiating conditions, also called colon progenitor cells (CPC)]. Two-way ANOVA indicated a significantly increased expression of LGR-5 in CSCs and CPCs compared to CEC (P < 0.001). In (A) upper panel shows LGR-5 expression in Western blot analysis. CSCs had greater LGR-5 expression when compared to the other two cell populations. The Western blot data further confirmed mRNA expression of LGR-5 was enriched in colon stem cells. (B) Use of alkaline phosphatase (PA) assays to detect levels of self-renewal. PA activity in CSCs was greater (13.14 ± 2.58 fold higher) than in CECs; the level of self-renewal in stem cells under differentiation conditions was less than half of that in CSCs under self-renewal conditions. In (B) upper panel, red stained cell colonies indicate #1 = phosphatase activity expression in colon epithelial cells; #2 = phosphatase activity expression in colonic stem cell under self-renewal; #3 = phosphatase activity expression in colonic stem cells under differentiation. (C) Mouse colon stem cells, mouse colon stem cells under differentiating conditions and differentiated colon epithelial cells.
Co-localization of CSCs in inflamed colon and evidence of CSCs transplanted into injured colon
Transplanted (via colon infusion) CSCs and CECs were labelled using PKH26 to identify the location of successfully implanted CSCs in the colons of IL-10−/− mice. The fluorescent signal from CSCs was predominantly detected in the mucosa and sub-mucosa of inflamed colon segments. These confocal microscope pictures were taken from an opened 1 cm piece of colon tissue (left panel of Fig. 2A–D) by using a Zeiss 510 META Laser Scanning Scope (Fig. 2A–E). There was a remarkable increased progression of fluorescent labelling which indicated CSC successfully implanted into inflamed colon, and also demonstrated that CSCs can survive up to 4 weeks after CSCs transfusion (Fig. 2E). We also noted that the PKH26 labelled stem cells only transplanted in inflamed (ulcerated) colon tissue. Thus, evidence of successful CSC transplantation in the inflamed colon was present as early as 1 day following CSC infusion. There was no fluorescent signal detected from transplanted CECs out to 14 days (Fig. 2F). Moreover, The PKH26 labelled stem cell signals were also exclusively detected in the inflamed surface epithelium (yellow coloured arrows) at 7 days following stem cell transplantation (Fig. 2G) when these pictures were taken from longitudinally sliced tissues. Double staining of the sliced mouse colon tissues with epithelial cell marker E-Cadherin (CDH1) was located in intracellular compartments (white coloured arrows) and the cell surface of crypts (red coloured arrows) (Fig. 2G). We have also provided more related cells survival and growth information analysed by using PKH26 as well as Ki67 antibody (which is a proliferation marker) to detect the cell proliferation condition, the data showed in Figure 2H demonstrated that a significant activation of cell proliferation started at 3 days following stem cell transplantation. A significant up-regulated of the Ki67 expression was detected from 7 days following CSCs infusion to 4 weeks.
Fig 2.
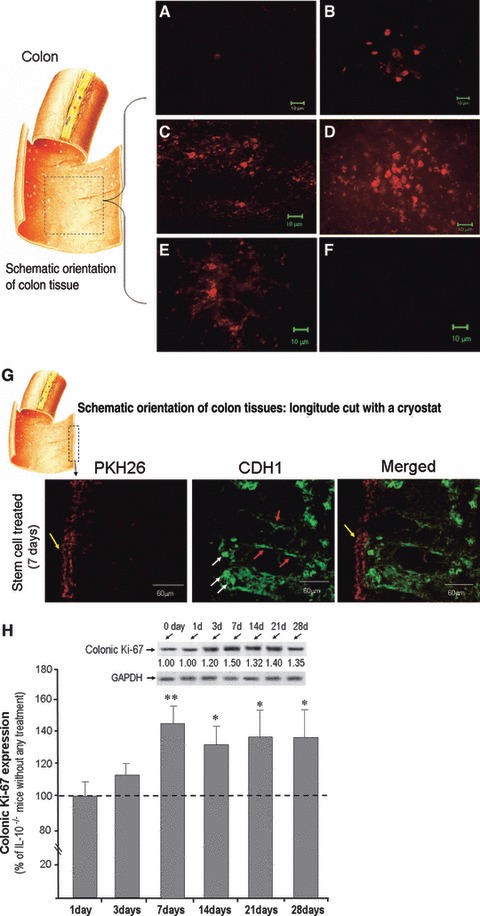
Co-localization of CSCs in inflamed colon: An additional group of mice was used for detecting stem cell location. Locally transplanted CSCs and CECs were labelled using PKH26 red fluorescent to detect stem cell migration to the inflamed colon in IL-10−/− mice. A piece of mouse colon (∼1 cm) was removed and the colon was opened (see left panel of colon picture), and then mounted flat with the mucosal surface facing up. The fluorescent signals from CSCs were predominantly detected in the mucosa of the inflamed colon. (A) 1 day after stem cells local infusion; (B) 3 days after stem cells local infusion; (C) 7 days after stem cells local infusion; (D) 14 days after stem cells local infusion; (E) 28 days after stem cells local infusion. No fluorescent signal was found after 14 days local infusion of CECs (F). The stem cell treated mouse colon with PKH26 label was also selected which was longitude cut with a cryostat, and then double labelled with E-cadherin (CDH1). As shown in (G), following a longitude cutting a colon tissue, the stem cell with PKH26 signal was presented in the surface of mucosa (yellow arrows) 3 days after stem cell transplantation, and CDH1 was showed in intracellular compartments (white arrows), and lateral membrane of crypts (red arrows). (H) shows Western blots indicated Ki-67 expression in CSCs treated mice (H, upper panel) compared to IL-10−/− mice without any treatment. There are six time points (1, 3, 7, 14, 21 and 28 days following CSCs infusion, each time point representative of 3 mice, and plus 3 IL-10−/− mice without any treatment as indicated). There was a significant up-regulated of the Ki67 expression was detected from 7 days following CSCs infusion to 4 weeks (H, lower panel).
Local stem cell transplantation reverses indicators of colitis
Histopathology analysis was performed using an injury score [21-23] with scores ranging from 0 (none) to 15 (severe) 2 weeks after local CSCs infusion (Fig. 3A–D). We also performed histopathology analysis up to 28 days after CSCs colon transfusion (data not shown) and similar results were obtained as those at day 14. IL-10−/− mice develop spontaneous enterocolitis associated with weight loss, passage of mucous, rectal prolapse and diarrhoea. Histological analysis of colonic tissue from IL-10−/− mice without any treatment exhibited an injury score between 11 and 15. There was pancolitis without any skip areas in the colon (data not shown). There were no improvements in the histological score in IL-10−/− mice 2 weeks after local transplantation of CECs or vehicle transfusion (Fig. 3A). In contrast, localized intra-colonic transfusion of a single dose of CSCs induced histological remission in IL-10−/− mice with active colitis (Fig. 3B–D). Following a single dose of CSCs, 16% of IL-10−/− mice (4 of 25) fully recovered (Fig. 3B), 60% (15 of 25) had only a mild degree of inflammation remaining (Fig. 3C), and 25% (6 of 25) showed a moderate degree of colitis remaining (Fig. 3D). The mean ± standard deviation for the pathology scores were 12.57 ± 1.59 for the untreated IL-10−/− mice group, 12.80 ± 1.92 for the CEC-treated group and 3.88 ± 2.63 for the CSC-treated group. No evidence of colitis was detected in any wild-type mice. Local stem cell therapy ameliorated histological colitis throughout the colon as there were no significant differences between the histological scores of the proximal and distal sections of the colon in stem cell treated mice. In the mild inflammation group (Fig. 3C), may notice many inflammatory cells and increased epithelial heights which further supports that IL-10−/− mice is a good model to study chronic inflammation.
Fig 3.
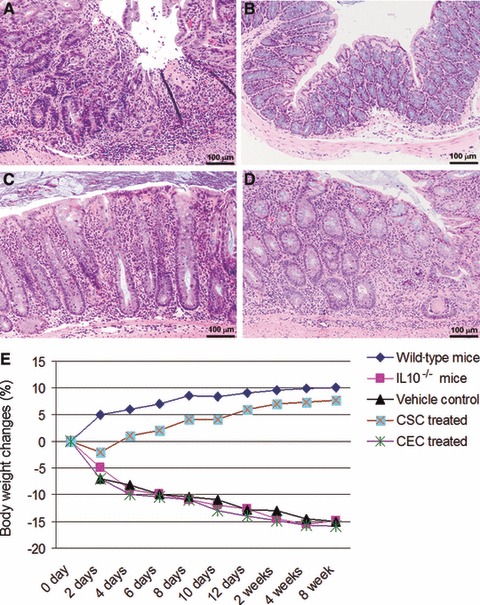
Local stem cell transplantation reverses indicators of colitis: Changes seen in colonic pathology were graded using a validated histological injury score. Histology scores ranging from 0 to 15 were ascribed to each specimen. (A) Colon from an untreated IL-10−/− mouse. Colitis was severe with enterocyte loss, crypt inflammation, the presence in the lamina propria of mononuclear cells and neutrophils, and epithelial hyperplasia. In contrast, CSCs transplantation induced histological remission in IL-10−/− mice with active colitis. Approximately 16% of CSC-treated IL-10−/− mice (4 of 25) showed complete resolution (B); approximately 60% of CSC-treated IL-10−/− mice (15 of 25) had only a mild degree of inflammation (injury scores between 1 and 5) (C), and approximately 25% of CSC-treated IL-10−/− mice (6 of 25) showed only moderate inflammation (injury scores between 6 and 10) (D). (E) IL-10−/− mice exhibit a significant increase in their body weight after a single dose of local stem cell transplantation compared to the other groups.
We monitored the IL-10−/− mice body weight up to 8 weeks after CSCs treatment compared to CECs treated mice. The IL-10−/− mice demonstrated a significant increase in body weight compared with CEC treated IL-10−/− mice (Fig. 3E). IL-10−/− mice receiving only cell matrix (ECM gel; Invitrogen, Carlsbad, CA, USA) or those that did not receive any treatment showed continual weight loss, and the individual mouse weight loss ratio was correlated with the pathology score.
Local stem cell transplantation reverses visceral hypersensitivity and recovers intestinal permeability
Local stem cell transplantation resulted in decreased visceral hypersensitivity starting at 7 days in IL-10−/− mice following colonic infusion of CSCs compared to baseline and colonic epithelium cell treated mice, and significantly reversed visceral hypersensitivity 14, 21 and 28 days after local colon stem cells transplantation (Fig. 4A, lower panel). The EMG data also showed a significant reduction in EMG activity at 14, 21 and 28 days following CSCs treatment compared to CECs treated mice. Figure 4A upper panel demonstrates EMG tracings at 14 days after CSCs treatment compared to CECs treatment. IL-10−/− mice with active colitis exhibited increased intestinal permeability that positively correlated with higher pathology scores for colitis (Fig. 4B, left panel). The reduction in colitis severity with local CSC therapy in IL-10−/−mice correlated with restoration of intestinal permeability (increased resistance indicated decreased intestinal permeability). Analysis of mucosal permeability also showed that the progressive colitis observed in CECs treated IL-10−/− mice was associated with significantly higher intestinal permeability as reflected by decreased mucosal resistance compared to the CSCs treated IL-10−/− mice (Fig. 4B, right panel, P < 0.01).
Fig 4.

Local stem cell transplantation reverses visceral hypersensitivity and restores intestinal permeability. (A) Colonic distension pressures that elicited the nociceptive threshold for each IL-10−/− mice. Bar graph of colon distension pressures in mmHg versus time course in IL-10−/− mice following stem cell treated or colonic epithelium cell treatment. Error bars are expressed as means ± S.D. There was significantly decreased visceral hypersensitivity at 14, 21 and 28 days (*P < 0.05; **P < 0.01) following CSCs treatment compared to baseline and CECs treated IL-10−/−mice (A, lower panel). Upper plane of (A) shows EMG measurement at 14 days following CSCs infusion compared to CECs treated mouse with 35 mmHg distension pressure. There is a reduced EMG activity that demonstrated at IL-10−/− mouse its received CSCs treatment. (B, left side) IL-10−/− mice with active colitis exhibit increased intestinal permeability (decreased resistance of the intestinal barrier). Increased intestinal permeability (decreased resistance) was negatively correlated with increases in the colitis-related pathology scores (r=–0.75; P < 0.001). An analysis of in vitro intestinal permeability is shown in the right side of (B). IL-10−/− mice without any treatment and CECs treated IL-10−/− mice had increased intestinal permeability (decreased resistance) compared to normal control (wild-type) mice and CSC-treated mice (P < 0.01). No significant changes were detected in permeability between CECs treated IL-10−/− mice and untreated IL-10−/− mice.
Local transplantation of CSCs inhibits inflammatory responses through TNF-α and IFN-γ dependent mechanisms
We used Inflammatory Cytokines & Receptors PCR Array to investigate that what genes were modulated followed by CSCs translocation. Two sets of comparison were done using super-array analysis. One was a comparison of IL-10−/− mice (n= 3) and wild-type (normal) mice (n= 3). The other was a comparison of IL-10−/− mice with CSCs treatment compared to IL-10−/− mice with CECs treatment. Among the 82 known cytokine genes measured, there were 21 genes that were significantly increased in expression (P < 0.01) and in fold change ratio (three-fold and up in IL-10−/− mice compared with wild-type (control) mice (Table 1, left panel). There were 10 genes that were significantly diminished in expression (P < 0.05) after local stem cell infusion treatment when compared with CEC infusion treatment (Table 1, right panel). These data from cytokine profiling super-arrays further support the possible benefit of local transplantation of stem cells in murine colitis. More interestingly, there were only five genes (CCL6, CXCL15, IFN-γ, IL-20 and TNF-α) that overlapped and indicated significantly diminished expression after local stem cell treatment (Table 1). We further confirmed our findings for these five cytokines using real-time PCR. TNF-α and IFN-γ showed significantly elevated expression in IL-10−/− mice and significantly diminished expression after local stem cell treatment (Fig. 5A). Scatter and Volcano Plots are showed that IL-10−/− mice had significantly elevated colonic TNF-α and IFN-γ compared to wild-type animals (Fig. 5B). Two weeks following CSCs transplantation, IL-10−/− mice showed decreased levels of TNF-α and IFN-γ (Fig. 5C) compared the IL-10−/− mice that received CECs transplantation.
Table 1.
Cytokine Super-Array Analysis
| IL-10−/− mice versus wild type mice | Stem cell treated versus epithelial cell treated mice | |||||||
|---|---|---|---|---|---|---|---|---|
| Genes | Fold changes (log 10) | P Value | Genes | Fold changes (log 10) | P Value | |||
| CCL12 | 51.84 | < 0.01 | Caspl | −90.47 | 0.005 | |||
| CCL4 | 198.45 | < 0.01 | Ccl11 | −138.08 | 0.005 | |||
| CCL5 | 233.83 | < 0.005 | Ccl25 | −36.07 | 0.006 | |||
| CCL6 | 16.87 | < 0.007 | CCL6 | −69.36 | 0.005 | |||
| CCL8 | 545.97 | < 0.005 | CXCL15 | −19.24 | 0.01 | |||
| CCR2 | 85.39 | < 0.006 | IFN-γ | −118.00 | 0.04 | |||
| CCR9 | 8.24 | < 0.006 | IL18 | −16.48 | 0.01 | |||
| CXCL1 | 116.65 | < 0.004 | IL20 | −19.24 | 0.01 | |||
| CXCL10 | 37.17 | < 0.008 | IL5ra | −5.55 | 0.008 | |||
| CXCL11 | 131.23 | < 0.003 | TNF | −3.51 | 0.02 | |||
| CXCL15 | 8.22 | < 0.009 | ||||||
| CXCL9 | 396.91 | <0.01 | ||||||
| CXCR3 | 39.02 | < 0.006 | ||||||
| CCR10 | 16.07 | < 0.002 | ||||||
| IFN-γ | 430.34 | < 0.001 | ||||||
| IL1B | 79.67 | < 0.003 | ||||||
| IL1F6 | 83.63 | < 0.0001 | ||||||
| IL1R2 | 9.53 | < 0.008 | ||||||
| IL20 | 8.22 | < 0.009 | ||||||
| IL3 | 8.22 | < 0.009 | ||||||
| TNF | 3.91 | < 0.007 | ||||||
Fig 5.
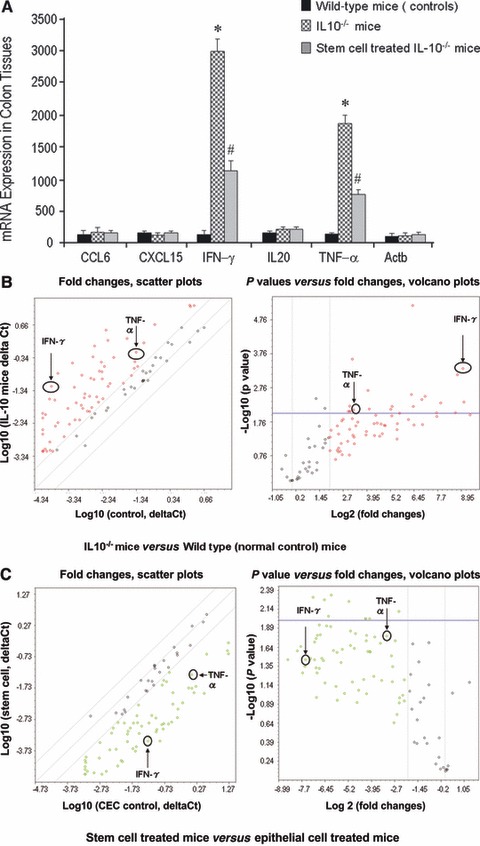
Local transplantation of CSCs inhibits inflammatory responses through TNF-α and IFN-γ dependent mechanisms. (A) Following Super-Array assay (from Table 1), CCL6, CXCL15, IFN-γ, IL20 and INF-α were tested by using quantitative real-time PCR. The data further confirmed the enhanced expression of IFN-γ and TNF-α in IL-10−/− mice (P < 0.05), whereas stem cell treated IL-10−/− mice showed significantly reduced expression of these two cytokines (P < 0.05). (B) Alterations in inflammatory cytokines, chemokines and receptor expression in IL-10−/− mice colon compared with normal control (wild-type) mice colon. Total RNA from each sample was characterized in triplicate, and the relative expression level and P values for each gene in the related samples are plotted against each other in the Scatter and Volcano Plots, depicting the relative expression levels (log 10) for selected genes in IL-10−/− mice versus normal control mice (left panel of B). In the right panel of (B), P values are plotted against fold changes. The genes for which the difference in expression levels were greater than a factor of three (fold) and/or P < 0.01 are shown to lie outside of the cut-off lines on the graph. TNF-α and IFN-γ are among the most up-regulated genes in the IL-10−/− mice group when compared to normal control (wild-type) mice. (C) Comparison of IL-10−/− mice with local stem cell treatment versus IL-10−/− mice with local epithelial cell treatment using PCR Array kits for inflammatory cytokines, chemokines and receptor. The relative expression level and P values for each gene in the related samples are plotted against each other in the Scatter and Volcano Plots, depicting the relative expression levels (log 10) for selected genes in stem cell treated mice versus epithelial cell treated IL-10−/− mice (left panel of C), In the right panel of (C), these two genes also show down-regulation after stem cells treatment compared to the epithelial cells treated group, P < 0.05.
We have also used an induced colitis group (TNBS-colitis) as a positive control group to compare to wild-type mice and IL-10−/−mice. The data indicated that both IL-10−/− mice and induced colitis mice demonstrated higher TNF-α and INF-γ expression from their colon tissues compared to (wild-type mice) normal control mice (Fig. S2).
Therapeutic mechanisms of transplanted stem cells
Next, we explored the mechanisms for the therapeutic action of transplanted local CSCs in IL-10−/− mice compared with IL-10−/− mice that received local infusion of CEC or vehicle only (ECM gel) infusion. As depicted in Figure 6A, RNA expression levels of TNF-α and IFN-γ in colonic tissue analysed by qPCR arrays were significantly increased in IL-10−/− mice compared to wild-type mice. Following CSCs treatment, TNF-α and IFN-γ expression were decreased in IL-10−/− mice compared to mice treated with CECs and/or vehicle controls (Fig. 6B). To further demonstrate the benefits of local stem cell therapy, we compared TNF-α and IFN-γ release from IL-10−/− mice and wild-type (control) mice (same mice used in the above experiments) to test levels of TNF-α and IFN-γ protein expression. The TNF-α and IFN-γ released from wild-type (control) mice was below the sensitivity of the ELISA for all animals. As expected, IL-10−/− induced significantly higher levels of release of TNF-α and IFN-γ from colon tissue homogenates harvested from IL-10−/− mice than levels from wild-type (control) mice (Fig. 6C, P < 0.01). Treatment of IL-10−/− mice with local stem cell transplantation significantly inhibited TNF-α and IFN-γ release from IL-10−/− mice colon tissues. Levels of release of TNF-α and IFN-γ were closely associated with the degree of recovery (Fig. 6C). A statistical analysis by Pearson correlation (using the SAS System) further indicated that the pathology score is positively correlated with TNF-α and IFN-γ expression in both real-time PCR (Fig. 6D) and Elisa assays (Fig. 6E).
Fig 6.
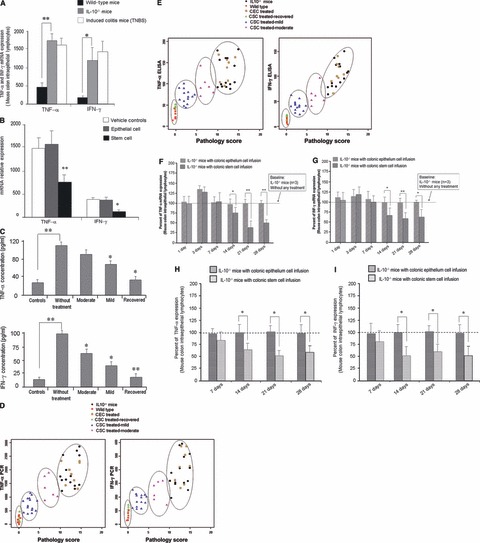
Therapeutic mechanisms of transplanted stem cells. Real-time PCR was used to detect TNF-α and IFN-γ expression in mouse colonic tissues. (A) IL-10−/− mice have significantly higher levels of TNF-α and IFN-γ expression compared to normal control (wild-type) mice (P < 0.01). On the other hand, IL-10−/− mice had significantly decreased local TNF-α and IFN-γ release 2 weeks after stem cell transplantation (compared to epithelial cell treated IL-10−/− mice and vehicle control IL-10−/− mice (B:P < 0.001 and P < 0.05, respectively). To confirm the changes in TNF-α and IFN-γ release from mouse colon, ELISA assays were used (C). They showed that levels of release of TNF-α and IFN-γ were closely associated with the degree of resolution of colitis that was induced by local CSC therapy (Control = wild-type mice; without treated = IL-10−/− mice without treatment; moderate = IL-10−/− mice after CSC treatment, which showed moderate inflammation histologically; mild = IL-10−/− mice after CSC treatment, which showed mild inflammation; Recovered = IL-10−/− mice after CSC treatment, which showed no inflammation. There was a significant difference in TNF-α concentrations between the control and without treatment group (*P < 0.01). Moreover, there was also a significant difference in TNF-α concentrations between the without treated and the mild and recovered groups (*P < 0.05). A substantial difference in IFN-γ concentrations was also observed between the control and without treatment group (P < 0.01). There was also a notable alteration in IFN-γ concentrations and between the moderate, mild and recovered groups when compared to the without treated group (P < 0.05, 0.05 and 0.01). (D and E) Pearson correlation analysis (using SAS) showed that the pathology score positively correlated with TNF-α (r= 0.81; P < 0.001) and IFN-γ (r= 0.74; P < 0.001) expression in both real-time PCR (D) and ELISA assays, TNF-α (r= 0.77; P < 0.001) and IFN-γ (r= 0.82; P < 0.001) (E). (F and G) both represent TNF-α/INF-γ expression within the intraepithelial lymphocytes (each time point representing three mice). There was decreased expression which started at day 14 day of TNF-α and INF-γ that continued to 4 weeks following local CSCs transfusion compared to CECs treated mice. Flow cytometry analysis further demonstrated decreased TNF-α and INF-γ expression within colonic lymphocytes from day 14 up to day 28 following CSCs treatment compared to CECs treated mice (H and I).
We then evaluated potential mechanism(s) related to abrogation of the colitis by isolating lymphocytes from mouse colon and performing RT-PCR and flow cytometry to detect TNF-α and INF-γ expression after CSCs transplantation compared to CECs transplantation. Figure 6F and G both represent TNF-α/INF-γ expression within the intraepithelial lymphocytes which were both slightly increased at 3 days. However, there was decreased expression of TNF-α and INF-γ, which started at day 14 and continued to 4 weeks following local CSCs transfusion. The temporary increased expression of TNF-α and INF-γ may due to local immune effects after local cell transplantation. Flow cytometry analysis further demonstrated decreased TNF-α and INF-γ expression within colonic lymphocytes from day 14 and up to day 28 following CSCs treatment compared to CECs treated mice (Fig. 6H and I).
Thus, our data indicate that local stem cell therapy treats chronic inflammatory gastrointestinal disorders not only through self-renewal capacity, but also decreases TNF-α and IFN− as the result of decreased overall inflammation.
Discussion
In the current study, we evaluated the potential therapeutic benefits of localized transplantation of adult CSC therapy in healing murine colitis and in reducing intestinal permeability and visceral hypersensitivity. By using IL-10−/− mice, we found that: (1) local stem cell transplantation facilitates recovery of IL-10−/− mice from chronic colitis through self-renewal mechanisms; (2) the mechanisms mediating recovery may involve blocking TNF-α and IFN-γ dependent pathways; (3) additional mechanisms may involve normalization of intestinal permeability, reduction of visceral hypersensitivity, inhibition of apoptosis, faster cell cycle progression and increased epithelial barrier function. Thus, local stem cell therapy may be a new and innovative modality with which to treat chronic gastrointestinal disorders with intestinal inflammation, increased intestinal permeability and visceral hypersensitivity [24, 25].
The delivery of CSCs locally by intracolonic infusion directly to the site of the effected colonic tissue contrasts with previous studies that used systemic administration of stem cells to induce remission in murine colitis [1, 7, 9, 26]. Systemic stem cell therapy has been used to treat various diseases including cancer, aplastic anaemia and sickle cell disease. The cells may be given fresh or they may be preserved first with a chemical called DMSO and frozen for later use. Some stem cell transplant procedures include infusion of red blood cells along with the stem cells. The side effects of systemic stem cell transplantation, such as invasive fungal infections, increased blood pressure and heart rate, decreased kidney function, have been reported [27, 28]. A unique feature of our study is that we used local delivery of stem cells to activate self-renewal signalling properties. Local delivery of stem cells may be associated with fewer and less severe adverse effects than systemic delivery of stem cells, which may also increase the willingness of patients to use stem cells based on this strategy. Our new strategy can potentially be used to reduce gastrointestinal symptoms through restoring intestinal permeability; healing colitis; as well as decreasing visceral hypersensitivity and pain.
In earlier studies, stem cell therapy was carried out by treating IL-10−/− mice with chronic colitis [1]. However, in that study, IL-10−/− mice were first treated with whole body irradiation to ablate the BM. Mice were then rescued with tail vein injections of 106 male whole BM cells to replace immune system components that do not develop in IL-10−/− mice. There was significant histological improvement in the colitis and the contribution of BM cells to colonic subepithelial myofibroblasts was increased in inflamed mucosa compared to normal mucosa. In addition, there was decreased mRNA expression of several pro-inflammatory cytokines, including TNF-α and IFN-γ. However, the reductions in tissue injury could have been caused, in part or in whole, by the whole body irradiation, and not just from the stem cells.
Another group of investigators used DSS-treated rats with busulphan-induced hypoplastic marrow [26]. Mesenchymal stem cells were then transplanted into the rats via tail vein injection. Animals treated with mesenchymal stem cells had increased epithelial barrier integrity. Although the author pointed out that busulphan has minimal immunosuppressive properties, it is possible that it suppressed the local host immune response and in that way contributed to normalization of the intestinal epithelial barrier.
A recent study demonstrated that TNBS-induced colitis in mice was ameliorated by using human umbilical cord mesenchymal stem cells [29]. Another study reported the effects of human adult stem cells from adipose tissue on reversal of colitis induced in mice by DSS [7]. Following 7 days of DSS, mice were injected intraperitoneally with human adipose stem cells or murine adipose stem cells. There was reduction in the severity of the colitis and decreased Th1-driven inflammatory responses. In addition, there was decreased expression of several pro-inflammatory cytokines including TNF-α and IFN-γ.
Unlike the approaches mentioned above, our study focused on using intra-colonic, local, stem cell transplantation to treat murine colitis. The colitis improved after successful local engraftment of CSCs and the transcriptional level of the pro-inflammatory cytokines examined decreased in the inflamed colonic tissues. At least in the intestine, where the cell turnover is most rapid among all body organs, the priority is to maintain transepithelial resistance by enhancing epithelial cell linings as a primary barrier. In our studies, we used syngeneic cells, which were genetically identical, but derived from a different mouse. Use of syngeneic cells is similar to application of autologous stem cells collected from a patient and then infused back into the patient. The current study raises several additional questions that may be the focus of future investigation. First, it would be interesting to determine if a similar outcome could be obtained using allogenic stem cells? Another interesting point that can be raised by our study is whether repeated stem cell therapy might have additive effects versus a single treatment as we have done in this current study. Also, although our study was carried out to 28 days, future studies are needed to determine if the beneficial effects are persistent well beyond the 28-week time point.
It was interesting that CSC transplantation ameliorated colitis throughout the colon despite the fact that stem cells were infused into the left colon of the mice during the initial treatment. One explanation as to why remote sites of the colon were also healed may be due to physiologic colonic motility. In the normal state, most of colonic peristalsis is retrograde or away from the rectum. Thus, colonic contents are mixed back and forth between the right and left colon. This serves to increase the efficiency of the colon in absorbing water and electrolytes from the liquid faeces via a mixing effect. Thus, one would anticipate that stem cells would be delivered to remote areas of the colon as a result of this peristaltic mixing. Other potential mechanisms involved in local CSC transplantation ameliorates colitis at remote sites of the injection place include wound-healing signalling pathway, such as message RNA and miRNA associated with their targets for recovery; or through compressing inflammation cytokine, such as decreased TNF-α and INF-γ expression at colon; or through cell migration effects. These proposed potential mechanisms may necessitate further studies in the future.
Another interesting question raised by this study is whether local CSC infusion induced a protective systemic immune response that underlies or contributes to the beneficial effect on colitis? Certainly, one cannot exclude the possibility that the implanted CSCs may have had a minor systemic effect. The major effects of CSC engrafts were clearly local and structural as they effectively healed the injured colonic mucosa. However, it is possible that there was a systemic effect that was limited to a reduction in systemic inflammatory cytokines as a result of healing of the colonic mucosal layer, which then no longer released cytokines into the systemic circulation. To exclude extracolonic systemic effects, we performed systemic cytokine profiles 3 days after stem cell treatment on spleens from three groups of mice (CSC treated IL-10−/−; CEC treated IL-10−/−; and IL-10−/− without treatment). There was no significant difference between the three groups of mice indicating that the systemic effects on cytokine levels was directly related to the implanted CSCs at least for acute time points (data not shown). Our current study suggests that stem cell therapy can reverse experimental colitis, decrease colonic proinflammatory cytokines and restore epithelial barrier permeability. Also, local administration of adult stem cells is a potentially safer approach that should cause many fewer side effects than systemic administration. In addition, unlike several of the earlier studies described, our approach did not require immunosuppression before stem cell transplantation [1, 26]. It is also likely that in the other studies there was modulation of the innate immune system following stem cell transplantation. Our approach has the advantage that colitis, local inflammatory cytokines and the epithelial barrier are all improved with only minimal effects on the innate immune system.
Summary and conclusions
Many patients suffer from chronic gastrointestinal diseases characterized by chronic inflammation, increased intestinal permeability, and visceral hypersensitivity and pain in which there is no definitive treatment. We have recently shown that patients with irritable bowel syndrome (IBS) have increased intestinal permeability that is associated with visceral hypersensitivity and pain [24, 25]. Adult stem cells have recently been used in various disease states to contribute wound-healing mechanisms. In our current study, IL-10−/− mice that received stem cell transplantation showed histopathologic evidence of recovery from colitis that correlated with restoration of intestinal permeability and decreased visceral hypersensitivity. Future studies need to be done to determine if intra-colonic administration of CSCs could be used in patients with chronic inflammatory bowel diseases. This localized approach to stem cell therapy might lead to new and innovative techniques to treat patients with inflammatory bowel disease who are refractory to medical therapy.
Acknowledgments
This study was supported by NIH grants (NS053090, AT005291) and by a VA Merit Review Award from the Medical Research Service of the Department of Veterans Affairs.
Conflict of interest
None of the authors have a financial or other relationship that might lead to a conflict of interest.
Supporting Information
Histological Pathology Score.
Mouse colon stem cell gene expression profile compared with mouse differentiated colon epithelial cells. There is an increased gene expression of SSEA-3, SSEA-4, HHF-B1 BMI-1, CDX2 and Prominin-1 in mouse colon stem cells compared to differentiated colon epithelial cells.
TNF-α and INF-γ message RNA expression. The TNBS induced colitis group (n = 6 mice) as positive control to compare to wild-type mice (n = 6) and IL-10−/− mice (n = 7). Both IL-10−/− mice and induced colitis mice (3 day after TNBS) demonstrated higher TNF-α and INF-γ expression from their colon tissues compared to normal control mice.
References
- 1.Bamba S, Lee C-Y, Brittan M, et al. Bone marrow transplantation ameliorates pathology in interleukin-10 knockout colitis mice. J Pathol. 2006;209:265–73. doi: 10.1002/path.1967. [DOI] [PubMed] [Google Scholar]
- 2.Direkze NC, Forbes SJ, Brittan M, et al. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow transplanted mice. Stem Cells. 2003;21:514–20. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 3.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–7. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 5.Ando Y, Inaba M, Sakaguchi Y, et al. Subcutaneous adipose tissue-derived stem cells facilitate colonic mucosal recovery from 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats. Inflamm Bowel Dis. 2008;14:826–38. doi: 10.1002/ibd.20382. [DOI] [PubMed] [Google Scholar]
- 6.Andoh A, Bamba S, Fujiyama Y, et al. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005;40:1089–99. doi: 10.1007/s00535-005-1727-4. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–89. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 8.Korbling M, Katz RL, Khanna A, et al. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738–46. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 9.Khali PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–54. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 11.Bristoil IJ, Farmer MA, Cong Y, et al. Heritable susceptibility for colitis in mice induced by IL-10 deficiency. Inflamm Bowel Dis. 2000;6:290–302. doi: 10.1002/ibd.3780060407. [DOI] [PubMed] [Google Scholar]
- 12.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10 mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 13.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–69. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 14.Wesselmann U, Czakanski PP, Affaitati G, et al. Uterine inflammation as a noxious visceral stimulus: behavioral characterization in the rat. Neurosci Lett. 1998;246:73–6. doi: 10.1016/s0304-3940(98)00234-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Q, Price DD, Caudle RM, et al. Visceral and somatic hypersensitivity in TNBS-induced colitis in rats. Dig Dis Sci. 2008;53:429–35. doi: 10.1007/s10620-007-9881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Price DD, Verne GN. Reversal of visceral and somatic hypersensitivity in a subset of hypersensitive rats by intra-colonic lidocaine. Pain. 2008;139:218–24. doi: 10.1016/j.pain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamp EH, Jones CW, III, Tillman SR, et al. Quantitative assessment and characterization of visceral nociception and hyperalgesia in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G434–44. doi: 10.1152/ajpgi.00324.2002. [DOI] [PubMed] [Google Scholar]
- 18.Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nat Protoc. 2007;2:2624–31. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- 19.Jones CW, III, Gebhart GF. Models of visceral pain: colorectal distension (CRD) Curr Protoc Pharmacol. 2004 doi: 10.1002/0471141755.ph0536s25. ; 5.36.1-17.doi:10.1002/0471141755. [DOI] [PubMed] [Google Scholar]
- 20.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr Protoc Immunol. 1996;173(19):1–16. doi: 10.1002/0471142735.im0319s17. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy RJ, Hoper M, Deodhar K, et al. Interleukin 10-deficient colitis: new similarities to human inflammatory bowel disease. Br J Surg. 2000;87:1346–51. doi: 10.1046/j.1365-2168.2000.01615.x. [DOI] [PubMed] [Google Scholar]
- 22.Madsen KL, Doyle JS, Jewell LD, et al. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116:1107–14. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 23.Saverymuttu S, Hodgson HJF, Chadwick VS. Controlled trial comparing prednisolone with an elemental diet plus non-absorbable antibiotics in active Crohn's disease. Gut. 1985;26:994–8. doi: 10.1136/gut.26.10.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain. 2009;146:41–6. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Q, Souba WW, Croce CM, et al. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–84. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yabana T, Arimura Y, Tanaka H, et al. Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol. 2009;218:350–9. doi: 10.1002/path.2535. [DOI] [PubMed] [Google Scholar]
- 27.Keung YK, Lau S, Elkayam U, et al. Cardiac arrhythmia after infusion of cryopreserved stem cells. Bone Marrow Transplant. 1994;14:363–7. [PubMed] [Google Scholar]
- 28.McCoy D, Depestel DD, Carver PL. Primary antifungal prophylaxis in adult hematopoietic stem cell transplant recipients: current therapeutic concepts. Pharmacotherapy. 2009;29:1306–25. doi: 10.1592/phco.29.11.1306. [DOI] [PubMed] [Google Scholar]
- 29.Liang L, Dong C, Chen X, et al. Human umbilical cord mesenchymal stem cells ameliorate mice trinitrobenzene sulfonic acid (TNBS)-induced colitis. Cell Transplant. 2011;20:1395–408. doi: 10.3727/096368910X557245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological Pathology Score.
Mouse colon stem cell gene expression profile compared with mouse differentiated colon epithelial cells. There is an increased gene expression of SSEA-3, SSEA-4, HHF-B1 BMI-1, CDX2 and Prominin-1 in mouse colon stem cells compared to differentiated colon epithelial cells.
TNF-α and INF-γ message RNA expression. The TNBS induced colitis group (n = 6 mice) as positive control to compare to wild-type mice (n = 6) and IL-10−/− mice (n = 7). Both IL-10−/− mice and induced colitis mice (3 day after TNBS) demonstrated higher TNF-α and INF-γ expression from their colon tissues compared to normal control mice.


