Abstract
The aim of this article is to briefly review available data regarding changes in the structure of microvessels observed in patients with diabetes mellitus, and possible correction by effective treatment. The development of structural changes in the systemic vasculature is the end result of established hypertension. In essential hypertension, small arteries of smooth muscle cells are restructured around a smaller lumen and there is no net growth of the vascular wall, although in some secondary forms of hypertension, a hypertrophic remodelling may be detected. Moreover, in non-insulin-dependent diabetes mellitus a hypertrophic remodelling of subcutaneous small arteries is present. Indices of small resistance artery structure, such as the tunica media to internal lumen ratio, may have a strong prognostic significance in hypertensive and diabetic patients, over and above all other known cardiovascular risk factors. Therefore, regression of vascular alterations is an appealing goal of antihypertensive treatment. Different antihypertensive drugs seem to have different effect on vascular structure. In diabetic hypertensive patients, a significant regression of structural alterations of small resistance arteries with drugs blocking the renin–angiotensin system (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers) was demonstrated. Alterations in the microcirculation represent a common pathological finding, and microangiopathy is one of the most important mechanisms involved in the development of organ damage as well as of clinical events in patients with diabetes mellitus. Renin–angiotensin system blockade seems to be effective in preventing/regressing alterations in microvascular structure.
Keywords: remodelling, hypertension, diabetes mellitus, microcirculation, small arteries
Introduction
Microvascular complications are major contributors to morbidity, mortality and costs of both non-insulin-dependent (NIDDM) and insulin-dependent diabetes mellitus (IDDM) [1]. Damage of the small vessels in the kidney can lead to end-stage renal disease, structural alterations of the smaller vessels that supply nutrients and oxygen to peripheral nerves contribute to neuropathy and damage of the microvasculature of the eye is the leading cause of loss of vision in working-age adults. The clinical manifestations of microvascular disease are so characteristic of the disease that diabetes itself is defined primarily by the level of hyperglycaemia which causes microvascular complications.
Alterations in the microcirculation involve small resistance arteries, arterioles, capillaries and post capillary venules. Mechanisms possibly involved in the development of microvascular alterations are summarized in Table 1. A relevant role in the impairment of vascular distensibility may be played by advanced glycosylation end-products, which may be involved in the formation of collagen cross-links [2]. Changes in the mechanics of small vessels may also induce changes in the structure. Although there is a huge amount of data about microangiopathy (capillary and arterioles), very few data about morphology of small resistance arteries (diameter ranging from 100 to 350 μm) in diabetes mellitus are presently available. In one study [3], no difference in subcutaneous small artery structure was observed between control patients and patients with IDDM. On the contrary, it has been demonstrated that, in both hypertensive and normotensive patients with NIDDM, marked alterations in small artery structure are present [4], and that these alterations are more pronounced in hypertensive patients with NIDDM than in patients with essential hypertension or in normotensive diabetics (Fig. 1) [4]. In addition, in diabetic patients a clear increase in the media cross-sectional area of the vessels was observed, thus suggesting the presence of hypertrophic remodelling (vascular smooth muscle cells hypertrophy or hyperplasia) [4, 5] (Fig. 2). This was not the case of patients with essential hypertension, in which a eutrophic remodelling (re-arrangement of the same amount of wall material around a narrowed lumen) is usually observed [4, 5].
Table 1.
Mechanisms possibly involved in the genesis of microangiopathy
| Direct effect of growth factors, such as insulin or insulin-like growth factor-1 on vascular smooth muscle cells. |
| Glycosylation of collagen fibres with alterations of extracellular matrix, production of advanced glycosylation end-products, involved in vascular damage (stimulation of cytokines, complement activation, up-regulation of growth factor synthesis, induction of collagen cross-links), depletion of basement membrane glycosaminoglycanes. |
| Metabolic effects such as activation of polyol pathway, with consequent production of sorbitol and fructose which are unable to diffuse outside cells, thus inducing osmotic endothelial cell injury and protein kinase C activation. |
| Glycosylation of proteins, including haemoglobin, with reduction of its affinity to oxygen and thus induction of micro-ischemia and endothelial damage. |
| Formation of reactive oxygen species (hydrogen peroxide, superoxide). |
Fig 1.
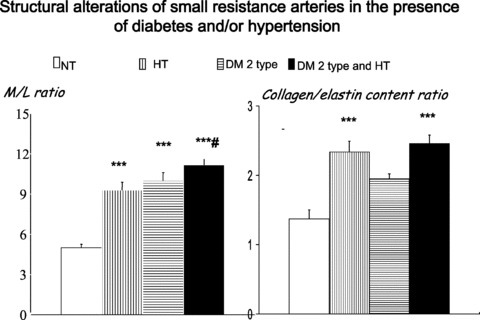
Left: Media to lumen ratio in subcutaneous small resistance arteries from normotensive patients (NT), essential hypertensive patients (HT), normotensive patients with NIDDM (DM 2 type) and hypertensive patients with NIDDM (DM 2 type and HT). A clear increase may be observed in all three pathologic groups, which is more evident in hypertensive patients with NIDDM. Right: collagen to elastin ratio (measured with electronic microscope) in the different groups. An increase was observed in essential hypertensive patients and in hypertensive patients with NIDDM. ***P < 0.001 versus normotensive patients; #P < 0.05 versus essential hypertensive patients. Mean ± S.E.M. (from [4]).
Fig 2.
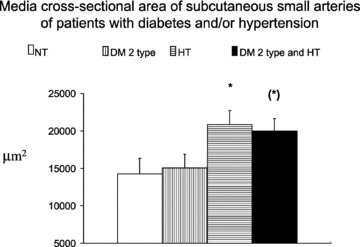
Medial cross-sectional area in subcutaneous small resistance arteries from normotensive patients (NT), essential hypertensive patients (HT), normotensive patients with NIDDM (DM 2 type) and hypertensive patients with NIDDM (DM 2 type and HT). An increase may be observed in the diabetic patients, which is more evident in normotensive patients with NIDDM. (*)P= 0.06, *P < 0.05 versus normotensives. Mean ± S.E.M. (from [4]).
A weak, but significant correlation between circulating levels of insulin and media to lumen ratio of subcutaneous small arteries was observed in diabetic patients, thus suggesting a possible role of insulin or insulin-like growth factor-1 in the genesis of hypertrophic remodelling in these patients [4]. However, an alternative explanation for the presence of hypertrophic remodelling in these vessels has been proposed [5]. In fact, a possible stimulus for hypertrophic remodelling could be the increased wall stress, as a consequence of the impaired myogenic response. Myogenic response is a pressure-induced vasoconstriction, which is the key component of blood flow autoregulation and stabilization of capillary pressure. The observation by Schofield et al. [5] of the lack of such a myogenic response in diabetic patients may therefore be responsible for the development of hypertrophic remodelling of small arteries (Fig. 3) [5]. Diabetic patients with NIDDM also show particularly evident alterations of the vascular extracellular matrix, as suggested by the observation of increased collagen to elastin ratio in their small arteries [4] (Fig. 1).
Fig 3.
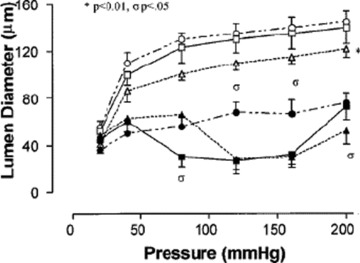
Myogenic response in subcutaneous small resistance arteries in patients with NIDDM. Passive pressure–lumen diameter relations for arteries from control patients (€), patients with EH (Δ) and hypertensive patients with NIDDM (°). *=P < 0.05, ANOVA, versus control. Active pressure–lumen diameter relations for arteries from control patients (▪), patients with essential hypertension (▴) and hypertensive patients with NIDDM (•). σ=P < 0.05 versus control vessels (from [5]).
Although small resistance arteries and arterioles may undergo a remodelling process and fibrosis in patholological conditions, capillaries may undergo a functional or structural rarefaction, with consequent reduction in the density per area units of tissue. This process of vascular rarefaction was previously observed in patients with hypertension [6] and also in patients with NIDDM, at least in the skeletal muscle [7].
On the contrary, in other vascular districts such as the retina, microvascular proliferation may be observed. In fact, diabetic retinopathy results either from capillary leakage or from new vessel formation (neovascularization, angiogenesis), caused by capillary closure and retinal ischemia. The capillaries leak lipid products and fluid in the area around the fovea and thicken the retina, which may lead to macular oedema. Angiogenesis is the result of retinal ischemia, and retinal haemorrhages are the consequence of the fragility of neovessels. The haemorrhage can enter the vitreous and cause sudden loss of vision. Several mechanisms and metabolic abnormalities, acting alone or in concert with each other, may lead to capillary death, leakage and occlusion and to the release of growth factors, finally resulting in new vessel formation and increased vascular permeability. A relevant role is played by vascular endothelial growth factor. Whereas vascular endothelial growth factor is involved in vascular leakage and angiogenesis, growth hormones and the insulin-like growth factor-1 are involved, as mediators, in angiogenesis. At present, inhibitors of these growth factors are under investigation in clinical trials in patients with diabetic retinopathy.
Vascular structural alterations, end organ damage and cardiovascular events
As previously mentioned, the extent of structural alterations in small resistance vessels is more pronounced in patients with both diabetes mellitus and hypertension, thus suggesting that clustering of risk factors may have synergistic deleterious effects on the vasculature [4, 5]. An important pathophysiological and clinical consequence of the presence of structural alterations in small resistance arteries and arterioles may be an impairment of vasodilator reserve [8]. In fact, as mentioned in the previous section, remodelling of small resistance arteries is characterized by a narrowing of the lumen, which leads to an increase of flow resistance even at full dilatation, i.e. in the absence of vascular tone. A significant correlation between coronary flow reserve and subcutaneous small resistance artery remodelling has been observed in hypertensive patients, suggesting that structural alterations in small resistance arteries may be present at the same time in different vascular districts, including those of paramount clinical importance, such as coronary circulation [9]. An impaired microvascular hyperaemic response (which may reflect an altered flow reserve) has been observed in children with diabetes mellitus [10] as well as in adult patients with NIDDM [11]. Thus, alterations in the microcirculation may play an important role in the development of organ damage not only in hypertension but also in diabetes mellitus. In fact, a relevant prognostic role of an increased media to lumen ratio of subcutaneous small resistance arteries in a high-risk population (including normotensive and hypertensive diabetic patients) has been previously demonstrated [12]. More recently, these data have been re-evaluated, taking into account also the characteristics of the vascular remodelling. As previously mentioned, an increased media to lumen ratio in small arteries may be ascribed either to eutrophic or to hypertophic remodelling. For the same values of internal diameter, those patients who suffered cardiovascular events had a greater media cross-sectional area, in comparison with those without cardiovascular events [13], thus suggesting the presence of vascular smooth muscle cell growth. Therefore, it seems that, for the same size of the vessels explored, a greater amount of vascular wall material, suggesting the presence of hypertrophic remodelling, such as that observed in diabetic patients, could mean an even worse prognosis. It may be postulated that hypertrophic remodelling might be a ‘less physiological’ adaptation to increased blood pressure levels, thus playing a role in the development of target organ damage, both in hypertension and in diabetes mellitus. It has been also suggested, as previously reported, that an impairment of myogenic response may have a relevant role in the development of hypertrophic remodelling in patients at high cardiovascular risk. In addition, an impaired myogenic response in small vessels may also induce an increase of high blood pressure flow to target organs and downstream increase in capillary pressure, with consequent increased permeability and capillary leakage. Fluid extravasation may induce organ damage. Some data support the presence of an increased capillary pressure in patients with diabetes mellitus [14], especially if they have increased blood pressure values [15], although presently there is no general agreement about this issue. The increase in capillary pressure seems to be related to the extent of clinical complications as well as to metabolic control [16]. Also vascular rarefaction may have important consequences in terms of tissue perfusion. In fact, it has been demonstrated that in patients with NIDDM, the mechanisms through which insulin is able to increase total limb flow or achieve optimal microvascular perfusion is impaired [7].
Endothelial dysfunction
An impairment of the endothelial function, as evaluated by the vasodilator response to acetylcholine, has been detected in large and small arteries of patients with IDDM [17–21] as well as of those with NIDDM [4, 5, 22–24]. Endothelial cells are known to have important regulatory effects on the cardiovascular system through the release of vasodilator and vasoconstrictor factors. In addition, platelet aggregation as well as leucocyte extravasation through endothelium may be influenced by locally produced compounds. Therefore, endothelial dysfunction and damage may contribute to the inflammatory and thrombotic vascular lesions. We and others have previously demonstrated the presence of an impaired dilatation to acetylcholine and bradykinin in subcutaneous small resistance arteries of hypertensive and normotensive patients with NIDDM [4, 5, 24]. However, the concomitant presence of the two cardiovascular risk factors does not seem to induce a further worsening of endothelial function [4, 24]. A possible explanation is that, both in hypertension and in diabetes mellitus, oxidative stress, resulting from the vascular production of free radicals and/or cyclooxygenase-dependent substances, may reduce nitric oxide bioavailability. If the pathogenetic mechanism of endothelial dysfunction is similar in the two conditions, no additive effect should be expected.
A second important point relates to the mechanisms of endothelial dysfunction in small resistance arteries of hypertensive and diabetic patients. In essential hypertensive patients, the relatively small vasodilator responses to acetylcholine and bradykinin infused into the brachial artery are not usually blocked by inhibitors of nitric oxide synthase (i.e. L-NMMA), whereas in normotensive patients the inhibitory effect of L-NMMA is preserved. In subcutaneous small arteries of patients with essential hypertension, and also in those with NIDDM, inhibitors of nitric oxide synthase are able to block about 50% of the vasodilator effect of acetylcholine or bradykinin, whereas the remaining vasodilatation is blocked by ouabain, thus suggesting that production of both nitric oxide and endothelium-derived hyperpolarizing factor may be involved [4, 24]. No effect has been observed when indomethacin was added to the organ bath; therefore, the production of cyclooxygenase-dependent substances seems to be of minor importance [4, 24].
It has been also proposed that insulin and insulin resistance may be involved in the genesis of endothelial, and in general, microvascular dysfunction in diabetes mellitus. Insulin is able to induce a vasodilating effect in the microcirculation (which is, at least in part, endothelium dependent) in normal patients [25, 26]. In addition, insulin may recruit skeletal muscle capillaries in vivo by a nitric oxide dependent action, and this increased capillary recruitment may contribute to the subsequent glucose uptake [27]. However, part of these effects is lost in diabetes mellitus [25]. Hyperinsulinaemia, as a result of insulin resistance may have detrimental effects on microvascular function also in the pre-diabetic state [28]. On the other hand microvascular structural alterations may contribute to an impaired delivery of insulin to skeletal muscles [29]. Therefore, a complex interplay of structural and functional alterations of the microcirculation may, at least in part, explain the detrimental consequences of diabetes mellitus in terms of organ perfusion [30], and, ultimately, in terms of increased incidence of cardiovascular events.
Effect of treatment
There are relatively few data about the effect of treatment on structural and functional alterations in the microcirculation of patients with diabetes mellitus. In the United Kingdom Prospective Diabetes Study, a large randomized controlled trial that included almost 5000 patients, it has been demonstrated that a tight haemodynamic and metabolic control is associated with a lower incidence of microvascular disease [31], and, in general, of clinical end-points related to microvascular disease [32].
Even fewer data are presently available about the effects of antihypertensive drugs on small artery structure in hypertensive diabetic patients. Despite effective antihypertensive treatment, resistance arteries from hypertensive diabetic patients showed marked remodelling, greater than that of vessels from untreated, non-diabetic, hypertensive patients, in agreement with the high cardiovascular risk of patients suffering from both diabetes and hypertension [33]. Recently, a study has compared the effects of 1-year treatment with the angiotensin-converting enzyme (ACE) inhibitor (enalapril) or the angiotensin II receptor blocker (candesartan), on subcutaneous small artery structure in hypertensive patients with NIDDM [34]. The two drugs were equally effective in reducing media to lumen ratio of small arteries (Fig. 4); however, candesartan was more effective than enalapril in normalizing vascular collagen content, probably through a more pronounced stimulation of the local production of metalloproteinase 9 (a collagen-degrading enzyme). At variance to what observed in the majority of studies in normoglycaemic hypertensive patients, media to lumen ratio of small arteries in treated diabetic patients did not reach the values observed in normotensive controls, therefore suggesting that a complete regression of vascular hypertrophic remodelling is probably more difficult to obtain [34]. Angiotensin II receptor blockers seem to be effective in diabetic hypertensive patients also when given on top of an ACE inhibitor treatment [35]. In addition, angiotensin II receptor blockers seem to be also particularly effective in terms of improvement of endothelial function in small resistance arteries [36] (Fig. 5). It has been recently proposed that drugs that may stimulate PPAR-α or PPAR-γ receptors (such as fibrates of glitazones) may be useful in terms of vascular protection and regression of structural alterations in the microcirculation. However, no data are presently available in human beings. In addition we do not know whether a regression of vascular structural or functional alterations in diabetic patients may be prognostically relevant, i.e. whether it is associated to a real protection from cardiovascular events [37].
Fig 4.
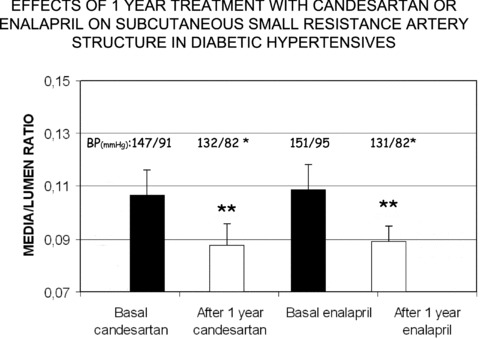
Media to lumen ratio in subcutaneous small resistance arteries from hypertensive patients with NIDDM, before and after 1-year treatment with the ACE inhibitor enalapril or the angiotensin II receptor blocker candesartan. A significant and similar reduction was observed with both drugs. BP = blood pressure **=P < 0.01 versus Basal (from [34]).
Fig 5.
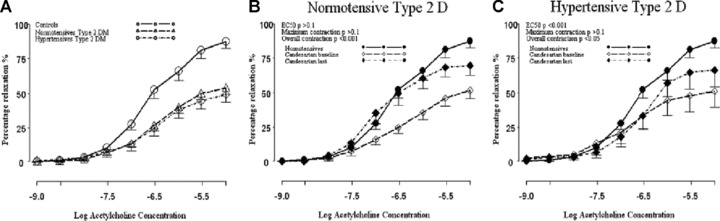
Dilatation of vessels comparing control patients with normotensive and hypertensive type 2DM (A) and response assessed by change in EC50, maximal relaxation and overall relaxation to candesartan in normotensive (B) and hypertensive (C) type 2 DM exposed to increasing concentrations of acetylcholine after pre-constriction with norepinephrine (from [36]).
Few data about patients with type 1 diabetes mellitus are presently available. Recently, it was observed that small arteries from patients with type 1 diabetes mellitus show hypertrophic growth in response to elevated blood pressure, similar to that seen in type 2 diabetes mellitus [38]. However, metabolic improvements enable eutrophic remodelling to occur in response to an increase in blood pressure [38].
Development in the topic in the next years
The relative invasiveness of the fat tissue biopsy, the limited availability of the technique (performed in few laboratories) and the lack of prognostic data about regression of microvascular alterations, ‘makes this approach unsuitable for general use’[39]. We are presently waiting for non-invasive approaches, which may provide us with further important insight about the effect of antihypertensive drugs, and, more importantly, about the possible prognostic impact of the regression of vascular structural alterations in hypertension and diabetes. The recently proposed evaluation of the media to lumen ratio of retinal arteries seems to represent a promising approach in this regard [40].
Conclusions
Alterations in the microcirculation represent a common finding, and microangiopathy is one of the most important mechanisms involved in the development of organ damage as well as of clinical events in patients with diabetes mellitus. Both patients with essential hypertension and those with NIDDM are characterized by alterations in the resistance vasculature, i.e. an increased media to lumen ratio, which in diabetics is the consequence of the so-called hypertrophic remodelling. Structural alterations of small arteries are associated with an increased cardiovascular risk in hypertensive and diabetic patients, perhaps as a consequence of an impaired organ flow reserve in several vascular districts, including the coronary vascular bed. In fact, it has been observed that the presence of an increased wall to lumen ratio in the subcutaneous resistance arteries is associated with a worse prognosis in high-risk patients. Hypertrophic remodelling, such as that observed in diabetic patients, seems to be associated with an even worse prognosis. Data about the effect of therapy on microvascular structure in diabetic patients are scarce; however, renin–angiotensin system blockade seems to be effective in regressing, at least in part, the microvascular structure, although we do not know whether this improvement is associated with a better clinical prognosis.
Conflict of interest
The authors confirm that there are no conflicts of interests.
References
- 1.Blonde L. State of diabetes care in the United States. Am J Manag Care. 2007;13:S36–40. [PubMed] [Google Scholar]
- 2.Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 3.McNally PG, Watt PAC, Rimmer T, et al. Impaired contraction and endothelium-dependent relaxation in isolated resistance vessels from patients with insulin-dependent diabetes mellitus. Clin Sci. 1994;87:31–6. doi: 10.1042/cs0870031. [DOI] [PubMed] [Google Scholar]
- 4.Rizzoni D, Porteri E, Guelfi D, et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non insulin dependent diabetes mellitus. Circulation. 2001;103:1238–44. doi: 10.1161/01.cir.103.9.1238. [DOI] [PubMed] [Google Scholar]
- 5.Schofield I, Malik R, Izzard A, et al. Vascular structural and functional changes in type 2 diabetes mellitus. Evidence for the role of abnormal myogenic responsiveness and dyslipidemia. Circulation. 2002;106:3037–43. doi: 10.1161/01.cir.0000041432.80615.a5. [DOI] [PubMed] [Google Scholar]
- 6.Antonios TF, Singer DR, Markandu ND, et al. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33:998–1001. doi: 10.1161/01.hyp.33.4.998. [DOI] [PubMed] [Google Scholar]
- 7.Clark MG, Barrett EJ, Wallis MG, et al. The microvasculature in insulin resistance and type 2 diabetes. Semin Vasc Med. 2002;2:21–31. doi: 10.1055/s-2002-23506. [DOI] [PubMed] [Google Scholar]
- 8.Folkow B. Physiological aspects of primary hypertension. Physiol Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 9.Rizzoni D, Palombo C, Porteri E, et al. Relationships between coronary vasodilator capacity and small artery remodeling in hypertensive patients. J Hypertens. 2003;21:625–32. doi: 10.1097/00004872-200303000-00030. [DOI] [PubMed] [Google Scholar]
- 10.Shore AC, Price KJ, Sandeman DD, et al. Impaired microvascular hyperhaemic response in children with diabetes mellitus. Diab Med. 1991;8:619–23. doi: 10.1111/j.1464-5491.1991.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 11.Strain WD, Chaturvedi N, Nihoyannopoulos P, et al. Differences in the association between type 2 diabetes and impaired microvascular function among Europeans and African Caribbeans. Diabetologia. 2005;48:2269–77. doi: 10.1007/s00125-005-1950-9. [DOI] [PubMed] [Google Scholar]
- 12.Rizzoni D, Porteri E, Boari GEM, et al. Prognostic significance of small artery structure in hypertension. Circulation. 2003;108:2230–5. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 13.Izzard AS, Rizzoni D, Agabiti-Rosei E, et al. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23:247–50. doi: 10.1097/00004872-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Parving HH, Viberti GC, Keen H, et al. Hemodynamic factors in the genesis of diabetic microangiopathy. Metabolism. 1883;32:943–49. doi: 10.1016/0026-0495(83)90210-x. [DOI] [PubMed] [Google Scholar]
- 15.Fegan PG, Tooke JE, Gooding KM, et al. Capillary pressure in subjects with type 2 diabetes and hypertension and the effect of antihypertensive therapy. Hypertension. 2003;41:1111–7. doi: 10.1161/01.HYP.0000068200.09187.1E. [DOI] [PubMed] [Google Scholar]
- 16.Sandeman DD, Shore AC, Tooke JE. Relation of skin capillary pressure in patients with insulin-dependent diabetes mellitus to complications and metabolic control. N Engl J Med. 1002;327:760–4. doi: 10.1056/NEJM199209103271103. [DOI] [PubMed] [Google Scholar]
- 17.McNally PG, Watt PAC, Rimmer T, et al. Impaired contraction and endothelium-dependent relaxation in isolated resistance vessels from patients with insulin-dependent diabetes mellitus. Clin Sci. 1994;87:31–6. doi: 10.1042/cs0870031. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone MT, Creager SJ, Scales KM, et al. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1994;88:2510–6. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 19.Clarkson P, Celermajer DS, Donald AE, et al. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol. 1996;28:573–9. doi: 10.1016/0735-1097(96)82380-1. [DOI] [PubMed] [Google Scholar]
- 20.Zenere BM, Arcaro G, Saggiani F, et al. Noninvasive detection of functional alterations of the arterial wall in IDDM patients with and without microalbuminuria. Diabetes Care. 1995;18:975–82. doi: 10.2337/diacare.18.7.975. [DOI] [PubMed] [Google Scholar]
- 21.Lambert J, Aarsen M, Donker AJ, et al. Endothelium-dependent and –independent vasodilation of large arteries in normoalbuminuric insulin-dependent diabetes mellitus. Arteriosc Thromb and Vasc Biol. 1996;16:705–11. doi: 10.1161/01.atv.16.5.705. [DOI] [PubMed] [Google Scholar]
- 22.Goodfellow J, Ramsey MW, Luddington LA, et al. Endothelium and inelastic arteries: an early marker of vascular dysfunction in non-insulin dependent diabetes. BMJ. 1996;312:744–5. doi: 10.1136/bmj.312.7033.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parving HH, Nielsen FS, Bang LE, et al. Macro-microangiopathy and endothelial dysfunction in NIDDM patients with and without diabetic nephropaty. Diabetologia. 1996;39:1590–7. doi: 10.1007/s001250050619. [DOI] [PubMed] [Google Scholar]
- 24.Rizzoni D, Porteri E, Guelfi D, et al. Endothelial dysfunction in small resistance arteries of patients with non-insulin dependent diabetes mellitus. J Hypertens. 2001;19:913–9. doi: 10.1097/00004872-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Rossi M, Cupisti A, Ricco R, et al. Skin vasoreactivity to insulin iontophoresis is reduced in elderly subjects and is absent in treated non-insulin-dependent diabetes patients. Biomed Pharmacother. 2004;58:560–5. doi: 10.1016/j.biopha.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Clerk LH, Vincent MA, Lindner JR, et al. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev. 2004;20:3–12. doi: 10.1002/dmrr.414. [DOI] [PubMed] [Google Scholar]
- 27.Vincent MA, Clerk LH, Lindner JR, et al. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–23. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 28.Jaap AJ, Shore AC, Tooke JE. Relationship of insulin resistance to microvascular dysfunction in subjects with fasting hyperglycaemia. Diabetologia. 1997;40:238–43. doi: 10.1007/s001250050669. [DOI] [PubMed] [Google Scholar]
- 29.Vincent MA, Clerk LH, Rattigan S, et al. Active role for the vasculature in the delivery of insulin to skeletal muscle. Clin Exp Pharmacol Physiol. 2005;32:302–7. doi: 10.1111/j.1440-1681.2005.04188.x. [DOI] [PubMed] [Google Scholar]
- 30.Scognamiglio R, Negut C, De Kreutzenberg SV, et al. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation. 2005;112:179–84. doi: 10.1161/CIRCULATIONAHA.104.495127. [DOI] [PubMed] [Google Scholar]
- 31.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 33.Endemann DH, Pu Q, De Ciuceis C, et al. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension. 2004;43:399–404. doi: 10.1161/01.HYP.0000112029.03691.e7. [DOI] [PubMed] [Google Scholar]
- 34.Rizzoni D, Porteri E, De Ciuceis C, et al. Effect of treatment with candesartan or enalapril on subcutaneous small artery structure in hypertensive patients with non insulin-dependent diabetes mellitus. Hypertension. 2005;45:659–65. doi: 10.1161/01.HYP.0000153308.91043.97. [DOI] [PubMed] [Google Scholar]
- 35.Savoia C, Touyz RM, Endemann DH, et al. Angiotensin receptor blocker added to previous antihypertensive agents on arteries of diabetic hypertensive patients. Hypertension. 2006;48:271–7. doi: 10.1161/01.HYP.0000230234.84356.36. [DOI] [PubMed] [Google Scholar]
- 36.Malik RA, Schofield IJ, Izzard A, et al. Effects of angiotensin type-1 receptor antagonism on small artery function in patients with type 2 diabetes mellitus. Hypertension. 2005;45:264–9. doi: 10.1161/01.HYP.0000153305.50128.a1. [DOI] [PubMed] [Google Scholar]
- 37.Rizzoni D, Agabiti Rosei E. Small artery remodeling in diabetes mellitus. Nutr Metab Cardiovasc Dis. 2009;19:587–92. doi: 10.1016/j.numecd.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Greenstein AS, Price A, Sonoyama K, et al. Eutrophic remodeling of small arteries in type 1 diabetes mellitus is enabled by metabolic control: a 10-year follow-up study. Hypertension. 2009;54:134–41. doi: 10.1161/HYPERTENSIONAHA.109.129718. [DOI] [PubMed] [Google Scholar]
- 39.Task Force for the Management of Arterial Hypertension of the European Society of Hypertension and of the European Society of Cardiology. 2007 Guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 40.Harazny JM, Ritt M, Baleanu D, et al. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension. 2007;50:623–9. doi: 10.1161/HYPERTENSIONAHA.107.090779. [DOI] [PubMed] [Google Scholar]


