Abstract
Evidence has been given that the adult heart contains a specific population of stromal cells lying in close spatial relationship with cardiomyocytes and with cardiac stem cells in sub-epicardial cardiogenic niches. Recently termed ‘telocytes’ because of their long cytoplasmic processes embracing the parenchymal cells, these cells have been postulated to be involved in heart morphogenesis. In our opinion, investigating the occurrence and morphology of telocytes during heart histogenesis may shed further light on this issue. Our findings show that typical telocytes are present in the mouse heart by early embryonic to adult life. These cells closely embrace the growing cardiomyocytes with their long, slender cytoplasmic processes. Hence, in the developing myocardium, telocytes may play nursing and guiding roles for myocardial precursors to form the correct three-dimensional tissue architectural pattern, as previously suggested.
Keywords: myocardial development, cardiomyocytes, cardiac stromal cells, telocytes, mouse heart
In most tissues and organs, form is imprinted in the stromal compartment, thus leading to the notion that the stroma is not just a packaging tissue, but may play important roles in the conformation of complex organ-specific architecture [1]. Stromal cells typically occur in the developing organs during pre-natal life, where they are referred to as mesenchymal cells and described as provided with long cytoplasmic processes which can favour the migration of parenchymal precursors towards their appropriate locations [2]. In this context, peculiar stromal cells provided with extended prolongations, originally called interstitial Cajal-like cells and now termed telocytes (telos, i.e. provided with long-distance cell projections) [3], have been identified in the adult human and rodent heart, both in the myocardium [4, 5] and in sub-epicardial cardiogenic niches, intermingled with immature cardiomyocytes (cardiomyoblasts) [6]. In particular, in the above study, the presence of close interactions between telocytes and cardiomyoblasts supports the hypothesis that the former cells may play a guiding and nursing role for myocardial precursors to achieve the correct three-dimensional myocardial organization [6]. This view also substantiates the assumption that the same mechanisms of prenatal development might also take place in the adult heart upon appropriate rousing signals and stimuli, with obvious consequences for regenerative medicine. In this perspective, we deemed necessary to investigate the pre- and post-natal heart, paying special attention to the morphological relationships between telocytes and cardiomyocytes during development.
Myocardial samples obtained from embryonic (E14, E17), newborn (P0, P6) and adult (2 months) CD1 mice were examined by immunohistochemistry and transmission electron microscopy. It was observed that, during cardiac development, stellate-shaped stromal cells featuring bona fide telocytes were present since the early developmental stages (Fig. 1A–F). They accompanied the proliferating myocardial buds moving from the epicardium towards the ventricular lumen and closely bordered the cardiomyocyte trabeculae for their entire length. At E14, short, thin columns of immature cardiomyocytes amidst stromal cells protruded from the sub-epicardial area towards the heart lumen (Fig. 1A). By E17 up to P6, the trabecular organization of the heart was maintained and the columns of cardiomyocytes were thick, interconnected and clearly bordered by telocytes (Fig. 1B, C, E). During the embryonic life, the putative telocytes were negative for c-kit, a marker for myocardial precursors [7], and CD34, a marker for adult telocytes [4] (Fig. 1A–E). During post-natal life, CD34 was expressed by a few putative telocytes at P0 and P6 (Fig. 1E), and by most of them in the adult hearts (Fig. 1F). By E17 to post-natal life, the epicardial lining cells and cells identifiable as endothelial cells also were CD34+ (Fig. 1D, E). In turn, c-kit-positivity was intense in both the epicardial lining cells and the cardiomyocytes of embryos and newborns (Fig. 1A–C), whereas it tended to decrease with age, being very faint in the cardiomyocytes and absent in the epicardium of the adult hearts. By transmission electron microscopy analysis, in the E14 and E17 hearts, cells featuring telocytes, i.e. provided with a small, oval-shaped body and long, thin processes, were observed in the sub-epicardial layer amidst the immature cardiomyocytes and in the myocardial trabeculae closely bordering the cardiomyocyte columns (Fig. 2A, B). In the early embryonic hearts, telocytes had immature features, as they had many free polyribosomes, and were surrounded by an electron-lucent matrix (Fig. 2A). In the newborn hearts, telocytes showed a more differentiated phenotype, with several rough endoplasmic cisternae located both in the cell body and processes (Fig. 2C), and were immersed in a loose extracellular matrix. Moreover, these cells established numerous interactions with the adjacent cardiomyocytes, in the form of focal plasma membrane contacts and intercellular bridges of flocculent, basal lamina-like material (Fig. 2D), similar to those observed in the cardiogenic niches of the adult heart [7].
Fig 1.
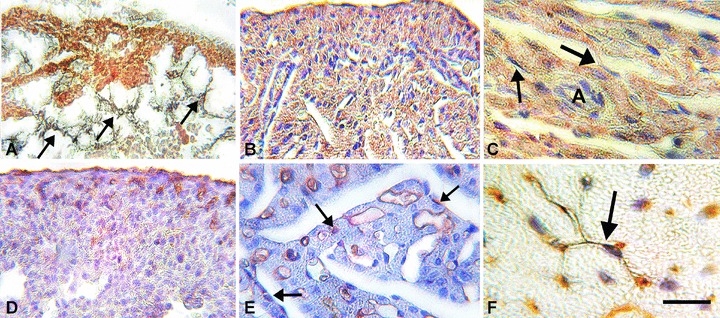
Immunohistochemistry. A–C: c-kit-immunolabelling. In (A), at E14 the lining epicardial cells and the immature cardiomyocytes are intensely c-kit+. Intermingled with these cells and towards the ventricular lumen there are several c-kit-negative stellate cells featuring the telocytes (arrows). In (B), at E17, and in (C), at P6, both immature and mature cardiomyocytes are c-kit+. The lining epicardial cells are still c-kit+ (upper part of B) and the putative telocytes (arrows) are c-kit–. These latter cells closely border the cardiomyocyte columns. D–F: CD34-immunolabelling. In (D), at E17, the lining epicardial cells and several sub-epicardial cells identifiable as endothelial cells are C34+. In (E), at P0, most of the endothelial cells as well as few interstitial cells (arrows), likely identifiable as telocytes, are CD34+. In (F), adult mouse heart, a typical telocyte (arrow) is CD34+. Bar: A, B, D= 40 μm; C, E= 20 μm; F= 15 μm.
Fig 2.
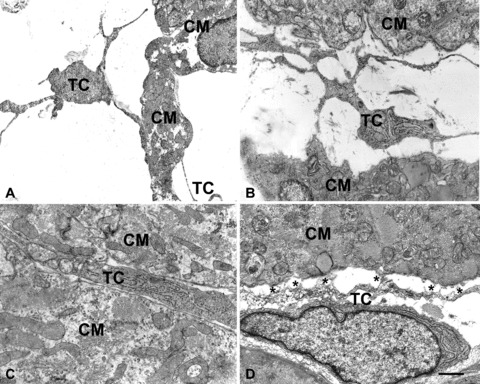
Electron microscopy. At E14 (A) and E17 (B), cells featuring telocytes are located in the wide space that separates the columns of immature cardiomyocytes. By their long, thin processes the telocytes come in contact and border the cardiomyocyte surface. In (C), at P0, a process of a telocyte showing a more differentiated phenotype, with several rough endoplasmic cisternae, is immersed in a loose extracellular matrix and occupies the interstitial space between two cardiomyocytes. The interstitium is now reduced in size. In (D), the process of a telocyte establishes numerous interactions (asterisks) with an adjacent cardiomyocyte, in the form of focal plasma membrane contacts and intercellular bridges of flocculent, basal lamina-like material. CM = cardiomyocytes; TC = telocytes. Bar: A= 1.6 μm; B= 1.3 μm; C= 1 μm, D= 0.6 μm.
Taken together, the above findings indicate that telocytes may actually play a fundamental role during heart development by setting up of a correct three-dimensional myocardial architecture and, possibly, nursing cardiomyocyte precursors until accomplishment of their differentiation programme. Conceivably, telocytes exert their task by multiple mechanisms, including not only the mechanical support for myocardial cell growth, differentiation and tissue compaction, but also the exchange of signals through cell–cell connections or microvesicular transfer of genetic information, as previously reported [8].
Besides contributing to a better comprehension of the complex process of cardiomyogenesis, the present findings may be relevant in the challenging field of cardiac repair. In fact, the endogenous cardiac stem/progenitor cells have a scarce regenerative potential [9, 10]. This fact poses a major barrier to the functional restoration of the diseased heart, especially after massive tissue loss as occurs upon ischemic infarction [11]. Such barrier may be outflanked by strategies able to stimulate the attitude of cardiac stem cells to proliferate, differentiate and functionally connect with pre-existing cardiomyocytes. In this context, the modulation of the nursing properties of telocytes to influence the developmental behaviour of cardiac stem cells could represent a promising target for cardiac regenerative medicine.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Doljanski F. The sculpturing role of fibroblast-like cells in morphogenesis. Perspect Biol Med. 2004;47:339–56. doi: 10.1353/pbm.2004.0048. [DOI] [PubMed] [Google Scholar]
- 2.McClay DR. The role of thin filopodia in motility and morphogenesis. Exp Cell Res. 1999;253:296–301. doi: 10.1006/excr.1999.4723. [DOI] [PubMed] [Google Scholar]
- 3.Popescu LM, Faussone-Pellegrini MS. Telocytes – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via Interstitial Cajal-like cells (ICLC) to telocytes. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinescu ME, Popescu LM. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J Cell Mol Med. 2005;9:972–5. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 8.Deregibus MC, Tetta C, Camussi G. The dynamic stem cell microenvironment is orchestrated by microvesicle-mediated transfer of genetic information. Histol Histopathol. 2010;25:397–404. doi: 10.14670/HH-25.397. [DOI] [PubMed] [Google Scholar]
- 9.Torella D, Ellison GM, M?ndez-Ferrer S, et al. Resident human cardiac stem cells: role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3:S8–13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 10.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun T, Martire A. Cardiac stem cells: paradigm shift or broken promise? A view from developmental biology. Trends Biotechnol. 2007;25:441–7. doi: 10.1016/j.tibtech.2007.08.004. [DOI] [PubMed] [Google Scholar]


