Abstract
Tissue engineering is an increasingly expanding area of research in the cardiovascular field that involves engineering, chemistry, biology and medicine. Cardiac tissue engineering (CTE) aims to regenerate myocardial damage by combining cells, matrix, biological active molecules and physiological stimuli. The rationale behind CTE applications is that in order to regenerate the ventricular wall after a myocardial infarction it is necessary to combine procedures that regenerate both cardiomyocytes and the extracellular matrix. The application of (stem) cells together with a matrix could represent an environment protected from the inflammatory and pro-apoptotic signals, a stemness/survival reservoir slowly releasing cells and factors promoting tissue regeneration and angiogenesis. This review will focus on the applications and advantages that CTE application could offer compared to conventional cell therapy.
Keywords: cell therapy, cardiac regeneration, cardiac tissue engineering, cardiac progenitor cells, cardiospheres
Introduction
Cardiovascular disease remains the leading cause for morbidity/mortality in the Western world. Cell transplantation into the damaged myocardium (cell therapy) has received extensive attention as a regenerative tool, and the accumulated evidence from both pre-clinical and clinical studies suggests that it has the potential to restore heart function. Currently, cellular cardiomyoplasty is being actively explored as therapy for regenerating the damaged myocardium, using different cell types, mostly derived from bone marrow or skeletal muscle. The clinical trials have highlighted the need to improve survival, engraftment and differentiation of the transplanted cells. Clearly a number of issues remain including the number of cells, the choice of an appropriate cell source that is able to provide all the main cell types of the heart and also the poor retention of the transplanted cells. In this review we will focus on cardiac tissue engineering (CTE), with particular attention to the improvements that this methodology can offer compared to conventional cell injection therapy.
Lessons from cell therapy
During the last few years, cellular therapy for the diseased heart has shown encouraging results on cardiac function in animal models of heart ischemia, using a variety of different cells, even without clear cardiovascular differentiation of the transplanted cells [1]. Several years after the first human clinical applications using non-cardiac stem cells as a therapy for acute myocardial infarction, heart failure or refractory angina, it should be recognized that the results are mixed, with benefits ranging from absent to transient, but marginal at most [1].
Pre-clinical studies demonstrated promising results and also short-term effects in patients were observed; however, in most trials long-term follow-up has shown that conventional pharmacological therapy often has the same late outcome as cellular therapy [1]. Cell therapy decreased the death rate of the endogenous myocytes, improved neoangiogenesis or positively affected ventricular remodelling in short-term follow-up, probably by secretion of paracrine factors. The small improvements in ventricular function, though, especially in the long-term follow-up, are probably because of the inability of the cells to actually form new cardiomyocytes.
The first limitation of direct cell injection is the considerable cell death that occurs in the first 7 days after infarction because of inflammation and ischemia. Zhang et al. found that high levels of cardiomyocyte death occurred within 4 days after implantation into injured hearts, and suggested ischemia as the main cause [2]. Similar data were reported in various animal models, thereby using different cell types and routes of administration. Generally more than 50% of the cells die within the first days after delivery, and only 5–10% of surviving cells could be reported in a pig and a rat model [3].
The ischemic environment induces cell death and needs vascularization because survival of transplanted cells is directly proportional to tissue perfusion. Injected cells show signs of irreversible ischemic injury after 1 day. The lack of matrix, particularly important during collection and preparation of the cells for transplantation, can also induce cell death and is mediated by the anoikis signalling pathway [4].
Different strategies have been developed in order to enhance cell survival upon transplantation, and include the use of bioactive cytoprotective molecules to prevent cell death. Studies with transgenic overexpression of Akt, BCL-2 or hypoxia inducible factor-1α, showed enhanced survival. In addition, in vitro pre-conditioning or simultaneous administration with insulin-like growth factor 1 (IGF-1), VEGF or free radical scavengers are all strategies that have demonstrated to increase the benefits of cell therapy [3].
However, co-administration of bioactive molecules results in a temporary benefit. Alternatively, genetic modification of transplantable cells, in order to enhance survival, has been tried, but remains risky for possible tumorigenic side effects.
To this end, CTE represents a possible solution to increase cell survival and cell retention in the heart. Different bioactive molecules can be linked to a specific matrix in order to provide their controlled release. Coupling PDGF-BB and IGF-1 to self-assembling peptide nanofibres demonstrated a prolonged delivery of the growth factors and resulted in an improved cardiac function compared to a control group that received only a single bolus injection [5, 6]. Interestingly, it was recently demonstrated that injection of rat cardiac progenitor cells (CPCs) together with IGF-1 modified peptide nanofibres (NF-IGF-1) enhanced cardiac function. The combination preserved left ventricular (LV) function and increased myocyte regeneration, as compared to saline, cells or NF-IGF-1 only [7].
Although it is possible to improve cell survival by increasing resistance to death stimuli, another important problem in cardiomyoplasty is related to low cell retention in the heart. Different studies demonstrated that a large number of cells are trapped in other organs, if injected systemically, or leaking from the site of myocardial injection [8, 9].
When cells are injected into an arrested heart by thiopental injection, cell retention is four times higher than injection into a beating heart. This indicates that cardiac contraction and/or myocardial perfusion are important in the early washout of cells [10]. In addition, an increase in cell retention is obtained by fibrin glue application on the site of injection [10]. Survival and retention can also be improved when cells are transplanted together with a matrix such as liquid collagen [11] or fibrin glue [12], resulting in improved cardiac function and remodelling.
It is evident that the first step to optimize the repair of a damaged myocardium is to increase cell retention and survival. This aspect is particularly important if we consider the absolute number of cardiomyocytes that are lost after MI and that one of the main limitations of cellular therapy is the number of cells that are theoretically needed to replace them.
However, an appropriate cell source is also fundamental. In order to obtain regeneration and to restore the damaged myocardium, cells should be used that are available in large numbers and capable of differentiating into cardiomyocytes and all the other cell types composing the heart. Cardiac stem/progenitor cells seem a logical cell source to exploit for cardiac regeneration therapy. Their presence in the heart, the frequent co-expression of early cardiac progenitor transcription factors and the capability for ex vivo and in vivo differentiation towards cardiac lineages, offer promise of enhanced cardiogenic potency compared to other cell sources.
Cardiac tissue engineering
In ischemic heart disease both cardiomyocytes and the extracellular matrix are pathologically disrupted or modified. Therefore, it could be important to exploit a combined procedure aiming at regenerating both myocardial cells and the extracellular matrix.
With the increased knowledge in (stem) cell biology, CTE is developing as a strategy to combine scaffold material and cells in order to provide a regenerative approach. The matrix should provide mechanical support to ventricular chamber integrity, in order to limit ventricular wall dilatation, and also provide a favourable environment for transplanted cells to enhance cell survival, proliferation and differentiation. The ideal matrix should: (i) be biodegradable, (ii) not induce any immune response by the host, (iii) support electro-mechanical properties of the heart and be replaced with newly synthesized ECM.
Recent advances in cell culture and CTE have facilitated the development of suitable cell-engineered, biodegradable grafts. The optimal biomaterials and cell types, however, have not been identified. Moreover, even a cell–scaffold combination ideal for a defined pathology (e.g. post-infarct limited scar) might not be adequate for another one (e.g. end-stage HF because of dilated cardiomiopathy or congenital malformation).
Two main approaches of CTE are performed: in vitro and in vivo CTE.
In vitro CTE focuses on seeding cells onto pre-formed porous scaffolds. The bio-complex is then cultivated in vitro and applied on the epicardial surface. In the in vivo CTE approach cells are injected with the matrix and formation of the biocomplex occurs at the site of injection.
In vivo CTE applications
In vivo or in situ CTE has recently emerged and involves injection of a mixture of biomaterials and cells. With this approach, cells and biomaterial are mixed and delivered by injection into the ventricular wall. This will improve survival and retention of the transplanted cells at the same time, and provide mechanical support to the damaged ventricle. The aim is to obtain regeneration and replacement of the damaged tissue in the natural milieu of the damaged myocardium. This approach is easy and feasible, but cell growth and differentiation cannot be controlled as tightly as in the in vitro model.
One of the first matrices applied with this approach was fibrin glue. Christman et al. transplanted myoblasts, fibrin glue or myoblasts in fibrin glue in rats at 1 week after MI and reported that, 24 hrs after injection, no differences in engrafted cells could be observed between cells transplanted with or without fibrin glue. However, after 4 weeks the percentage of engrafted cells was significantly higher with the combined treatment [13]. Both fibrin glue treatments displayed a small significant reduction of the scar size as compared with cell injection alone, probably because of improved neovascularization of the infarcted area [13]. When fibrin glue was injected directly into the scar in chronic heart failure (e.g. 5 weeks after MI), the treatment improved FS, increased wall thickness and LV internal diameter) already at 2 days after injection. Interestingly, 5 weeks after injection (10 weeks after MI), deterioration of FS was observed in both groups, although wall thickness was preserved in the fibrin-injected group [14]. One of the advantages of injectable biopolymers is the possibility to deliver the hydrogel percutaneously via catheters, thereby avoiding large surgical procedures and the associated risks and costs. Martens et al. studied different catheter delivery methods with variable concentrations of fibrinogen and thrombin, and demonstrated that this approach was feasible for future clinical application [12]. In addition, they demonstrated that, by using human MSC full, cardiac-specific retention was increased after transplantation in a rat model of acute MI when cells were transplanted with fibrin compared to saline. This resulted in a decrease in the presence of MSCs in liver and kidney, but not in the lungs [12].
Collagen can also be used in a liquid form thereby having the advantage that cells are better and more homogeneously distributed than in the preformed scaffold. The easy gelation at physiological temperature and the possibility to combine with other hydrogels (e.g. engineered heart tissue model [EHT]) are useful features of this protein. In addition to in vitro preparation tissue engineering, Huang et al. injected liquid collagen in a rat model of reperfusion injury. They demonstrated a higher number of capillaries 5 weeks after matrix injection when compared to saline group, but similar to fibrin or matrigel usage [15]. In a similar study, injection of collagen improved cardiac stroke volume and ejection fraction. However, in contrast with the previous study, no matrix infiltrating cells or induction of vascularization were observed in the histological analysis [16].
The application of alginate as a gel directly injected into the heart has been also reported. When alginate was injected directly in the myocardial wall of a 7-day-old MI, the alginate matrix significantly increased scar thickness, systolic and diastolic wall thickening, and cardiac function as compared to saline or cardiomyocyte injection alone. Similar beneficial effects in scar thickness and remodelling were obtained also when alginate was injected in a 2-month-old infarct [17].
Hao et al. used a VEGF-A or PDGF-BB modified alginate scaffold and injected this into a MI model [18]. The controlled release of the growth factors (alone or in combination) from the matrix was confirmed experimentally in vitro. Although the addition of growth factors induced vessel formation and increased smooth muscle cell infiltration, no differences in left ventricle diastolic diameter and ejection fraction could be observed [18]. Another intriguing approach is the use of a-cellular matrix as a gel that can be injected into the heart. Singelyn et al. recently reported the isolation of ECM from pig ventricle as a gel [19]. This cardiac ECM is a viscous liquid solution up to room temperature, and will become a gel at 37°C [19]. Preliminary studies demonstrated that the gel, when placed in a clinically compatible catheter, can be pushed through with minimal resistance, indicating the clinical applicability of the materials [19].
In vitro CTE applications
In vitro CTE consists of seeding cells into a pre-formed porous scaffold. Cells are cultivated under strict culture conditions in order to enhance survival, proliferation and differentiation. The formed patch is then applied onto the epicardial surface of the heart in order to provide mechanical support to the damaged heart and enhance cardiac performance. The matrix and cultivation parameters play an important role to guide and organize seeded cells in order to control and obtain a myocardial patch that is as similar to native heart tissue as possible. The advantage of the in vitro approach is the possibility to control the shape and size of the construct, as well as organization and differentiation rate of the seeded cells. The main limitation of this approach is the need for nutrient diffusion that limits the thickness of the construct itself. Therefore a vascularized tissue is required, together with mature contractile myocardial cells. The use of a perfusion-bioreactor can improve viability of the cultivated cells and/or enhance differentiation by applying mechanical stress. Different types of materials have been used to cultivate different cell types and then applied on the damaged ventricular wall to evaluate heart regeneration.
One of the first well-established 3D-myocardial in vitro models was created almost 10 years ago by Zimmerman et al., and called EHT. EHTs consist of a mixture of liquid collagen, growth factor-reduced matrigel and neonatal rat cardiomyocytes. The mixture is then casted into a circular mould, statically cultivated for 7 days and then subjected to mechanical stretch for seven more days. When transplanted into the heart the constructs integrated fully in the host myocardium 4 weeks after in vivo implantation, having a diameter of about 450 μm, and formed new capillaries connected with the recipient’s vessels. Engraftment and electrical coupling of the EHT patches was demonstrated by the propagation of electrical responses in remote myocardium and improvement of myocardial function [20].
Birla et al. mixed neonatal cardiomyocytes and fibrin gel in a small silicone tube, made a longitudinal slit and placed the tube around the femoral artery of a recipient rat, to generate an intrinsic supply. Three weeks after implementation, the explanted construct was full of vascularized tissue with ‘cardiac-like’ contractility properties and a positive chronotropic response to epinephrine and a positive inotropic response to Ca2+[21]. Another matrix that has been extensively studied in in vitro CTE applications is collagen.
Preformed porous dry collagen has been already used in clinical settings for 30 years as a haemostatic agent to repair tissue injuries and prevent haemorrhages. Kofidis et al. utilized preformed collagen matrix for the first time to cultivate neonatal rat cardiomyocytes and presented a model of in vitro contractile cardiac tissue. When these constructs were attached to the epicardial surface of infarcted rat hearts, they induced neovascularization and reduced LV dilatation up to 3 weeks [22].
We seeded human umbilical cord derived stem cells in the same collagen matrices and transplanted them into 7-day-old infarcts in a mouse model. The cell-matrix treatment prevented myocardial wall thinning, limited post-ischemic remodelling and displayed a lower end-diastolic volume and improved ejection fraction, compared to single treatments [23]. So this approach seems to improve the efficiency of cell treatment at the time-points studied (1 and 6 weeks), corresponding to an improvement in an early post-infarct cardiac remodelling, vascularization and angiogenesis, but the data are not demonstrating real myocardial regeneration.
The clinical relevance of this matrix was also tested in a small non-randomized clinical trial with patients having a chronic scar, an EF < 35% and indication of concomitant single off-pump coronary artery bypass graft surgery. Ten patients received direct injection of bone marrow mononuclear cells and ten patients received cells seeded into a scaffold of 7 × 5 × 0.6 cm and sutured over the infarct and peri-infarct areas. After 1 year follow-up (10 ± 3.5 months), no adverse events were reported, suggesting feasibility and safety of the treatment. In addition, improved deceleration time, decreased LV end-diastolic volume and reduced scar area thickness could be observed. Although promising, further studies are warranted in order to better evaluate cardiac function improvement through this scaffold, because of the concomitant coronary artery bypass graft surgery mediated revascularization [24].
Further modifications of this pre-formed collagen matrix were studied by Schussler et al. They coupled an RGD peptide to the collagen material in order to increase viability, contractile performance and differentiation of seeded cardiomyocytes [25]. When compared to non-modified collagen, RGD modified scaffold increased viability of transplanted cardiomyocytes up to 1 month in vitro. In addition, the cells were better aligned and elongated, with the presence of cross striations, and contractile performance was increased threefold as compared with the non-modified scaffolds.
As mentioned before, the choice of an appropriate cell source is fundamental in order to obtain improvement in cardiac function, not only by secretion of paracrine factors and by improving vasculogenesis, but also by regeneration of cardiomyocytes. To this end we cultivated CPCs in the form of cardiospheres [26], and cultivated them in a preformed commercially available collagen scaffold or in gelatine-based scaffolds [27, 28].
CPCs grown as cardiospheres represent per se a real cardiac microtissue, in which the more differentiated cells are located in the external layers whereas those proliferating and expressing stemness and angiogenic markers are in the core.
Moreover our previous observations [26, 29, 30] suggest that the CSps microtissue might fulfil important criteria of tissue engineering technology for cardiac diseases, that is:
Long-term maintenance of differentiation capacity, in vitro and in vivo,
Multicellular type cultivation,
Potential for self-organization, polarization and microstructure formation between different cells,
Production of an extracellular matrix,
Vascularization, including induction of microvessels development, and connection to the host capillary system after implantation (after heterotopic injection),
Development of inter-tissue super-structures and
Compatibility with high-efficiency stable gene transfer technologies to engineer complementary cell phenotypes or provide therapeutic interventions (after both lenti/adeno/adeno-associated virus infection).
Thus even if CSps alone appear to provide an optimal cardiogenic environment, preliminary data shows that CSps can grow within and populate different kinds of scaffolds currently in use for tissue engineering. In particular CSps seeded in collagen or gelatine-based scaffolds have shown prolonged viability during culture (up to 7 days or more) (Fig. 1a). Some differences in the spreading out and production of extracellular matrix were observed, with a more consistent proliferation in the commercial collagen scaffold.
Fig 1.
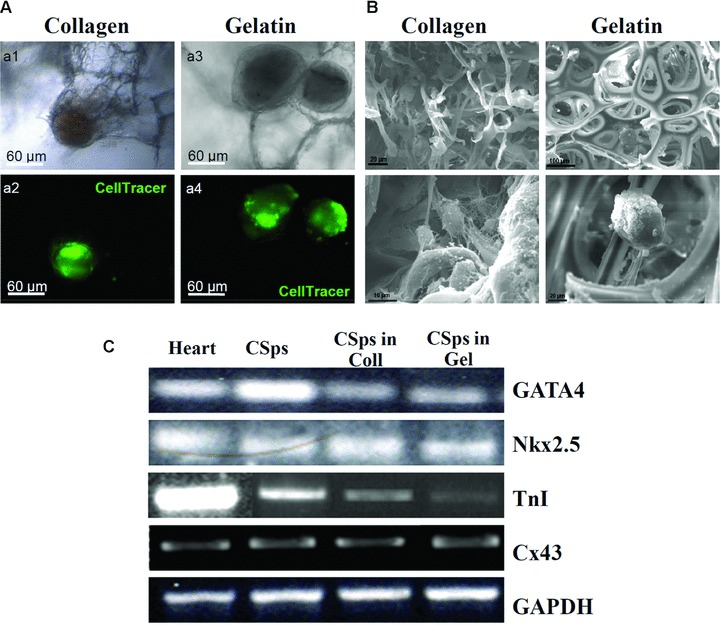
Cardiospheres cultivated in collagen or gelatine scaffold. (a) CSps pre-labelled with the fluorescent vital, seeded in collagen (a1 and a2) or gelatine (a3 and a4) matrix and cultivated for 1 week; (b) scanning electron microscope analysis of CSps in collagen and gelatine scaffolds; (c) RT-PCR analysis of CSps cultivated in the matrices.
CSps displayed very good integration with both matrices, as demonstrated by scanning electron microscope analysis (Fig. 1b). In addition RT-PCR analysis for the expression of cardiac markers was performed, and demonstrated that after 7 days in culture CSps seeded in collagen and gelatine scaffolds retained their cardiogenic commitment, as demonstrated by expression of Nkx 2.5, GATA-4, Connexin-43 and TnI (Fig. 1c).
Our hypothesis is that autologous cardiac stem/progenitor cells, in combination with the optimal biodegradable biomaterial, can be used to build tissue-engineered cardiac patches, which could preserve survival, growth and differentiation potential of the embedded cells. Moreover, this biocomplex could even serve as a cellular reservoir, allowing their slow migration along the epicardial surface to the damaged cardiac tissue, where they could exert a paracrine function, encouraging both local angiogenic/anti-apoptotic activity and resident progenitors recruitment and activation [30].
We also isolated Sca-1+ expressing cells [31] from human foetal heart biopsies. These cells, named cardiomyocytes progenitor cells appeared to be CPCs, restricted to the cardiac lineage that is able to differentiate into cardiomyocytes under precise conditions [32]. Preliminary data indicate that cardiomyocytes progenitor cells seeded in preformed collagen matrix are able to proliferate and to colonize the scaffold and could represent another cell source for CTE.
Conclusions
CTE is a rapidly evolving field that is receiving increased attention as a promising approach to repair the damaged myocardium. Cardiac cell therapy has been extensively studied in the last decade and different clinical trials were performed with several cell types. Despite the sometimes conflicting results, feasibility and safety of cellular therapy was demonstrated, although the benefits, in terms of long-term improvement of cardiac function, are still doubtful.
CTE offers the advantage to shelter transplanted cells by providing a physiological environment and by protecting them from death stimuli, overcoming two of the main problems of direct cell injection. In addition, transplantation of cells together with a matrix provides mechanical support to the infarcted ventricular wall and can prevent post-infarction ventricular dilatation.
The choice of the appropriate cell source and matrix plays a fundamental role in a successful tissue engineering application. For cardiac use, cardiomyocytes have been extensively studied as a possible cell source for heart regeneration. However, their use is not clinically applicable and other cell sources need to be considered. CPCs seem to be the most promising cell source because of their intrinsic capacity to proliferate, thereby providing suitable cell numbers, but most importantly for their natural commitment and differentiation potential to the cardiac lineages [1].
Acknowledgments
This work was supported by Pasteur Institute/Cenci-Bolognetti Foundation and PRIN 2007 from Italian MIUR.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Gaetani R, Barile L, Forte E, et al. New perspectives to repair a broken heart. Cardiovasc Hematol Agents Med Chem. 2009;7:91–107. doi: 10.2174/187152509787847128. [DOI] [PubMed] [Google Scholar]
- 2.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 3.Robey TE, Saiget MK, Reinecke H, et al. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–81. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zvibel I, Smets F, Soriano H. Anoikis: roadblock to cell transplantation. Cell Transplant. 2002;11:621–30. doi: 10.3727/000000002783985404. [DOI] [PubMed] [Google Scholar]
- 5.Davis ME, Hsieh PC, Takahashi T, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsieh PC, Davis ME, Gannon J, et al. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–48. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padin-Iruegas ME, Misao Y, Davis ME, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–87. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller-Ehmsen J, Krausgrill B, Burst V, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–84. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 9.Hou D, Youssef EA, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–6. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 10.Terrovitis J, Lautamaki R, Bonios M, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–26. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kutschka I, Chen IY, Kofidis T, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–73. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 12.Martens TP, Godier AF, Parks JJ, et al. Percutaneous cell delivery into the heart using hydrogels polymerizing in situ. Cell Transplant. 2009;18:297–304. doi: 10.3727/096368909788534915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christman KL, Vardanian AJ, Fang Q, et al. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Christman KL, Chin E, et al. Restoration of left ventricular geometry and improvement of left ventricular function in a rodent model of chronic ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2009;137:180–7. doi: 10.1016/j.jtcvs.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 15.Huang NF, Yu J, Sievers R, et al. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–6. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 16.Dai W, Wold LE, Dow JS, et al. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 17.Landa N, Miller L, Feinberg MS, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 18.Hao X, Silva EA, Mansson-Broberg A, et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–85. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Singelyn JM, DeQuach JA, Seif-Naraghi SB, et al. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmermann WH, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 21.Birla RK, Borschel GH, Dennis RG, et al. Myocardial engineering in vivo: formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Eng. 2005;11:803–13. doi: 10.1089/ten.2005.11.803. [DOI] [PubMed] [Google Scholar]
- 22.Kofidis T, Akhyari P, Wachsmann B, et al. Clinically established hemostatic scaffold (tissue fleece) as biomatrix in tissue- and organ-engineering research. Tissue Eng. 2003;9:517–23. doi: 10.1089/107632703322066697. [DOI] [PubMed] [Google Scholar]
- 23.Cortes-Morichetti M, Frati G, Schussler O, et al. Association between a cell-seeded collagen matrix and cellular cardiomyoplasty for myocardial support and regeneration. Tissue Eng. 2007;13:2681–7. doi: 10.1089/ten.2006.0447. [DOI] [PubMed] [Google Scholar]
- 24.Chachques JC, Trainini JC, Lago N, et al. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): one year follow-up. Cell Transplant. 2007;16:927–34. doi: 10.3727/096368907783338217. [DOI] [PubMed] [Google Scholar]
- 25.Schussler O, Coirault C, Louis-Tisserand M, et al. Use of arginine-glycine-aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardium-like tissue graft. Nat Clin Pract Cardiovasc Med. 2009;6:240–9. doi: 10.1038/ncpcardio1451. [DOI] [PubMed] [Google Scholar]
- 26.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 27.Barbetta A, Rizzitelli G, Bedini R, et al. Porous gelatin hydrogels by gas-in-liquid foam templating. Soft Matter. 2010;6:1785–92. [Google Scholar]
- 28.Barbetta A, Gumiero A, Pecci R, et al. Gas-in-liquid foam templating as a method for the production of highly porous scaffolds. Biomacromolecules. 2009;10:3188–92. doi: 10.1021/bm901051c. [DOI] [PubMed] [Google Scholar]
- 29.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 30.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 106:971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Vliet P, Roccio M, Smits AM, et al. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–9. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smits AM, Van Vliet P, Metz CH, et al. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–43. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]


