Abstract
ATP-binding cassette transporter A1 (ABCA1) in pancreatic β cells influences insulin secretion and glucose homeostasis. This study investigates whether the long-acting agonist of the glucagon-like peptide 1, namely exendin-4, which mediates stimulatory effects on ABCA1 gene expression, could interfere with the Ca2+/calmodulin (CaM)-dependent protein kinase (CaMK) cascade. ABCA1 promoter activity was examined by reporter gene assay in rat insulin-secreting INS-1 cells incubated with exendin-4. CaMKIV activity was assessed by detection of activation-loop phosphorylation (Thr196) of CaMKIV. We investigated the influence of the constitutively active form (CaMKIVc) or CaMKIV knockdown on ABCA1 expression. Increased abundance of ABCA1 protein was noted in response to rising concentrations of exendin-4 with maximum induction at 10 nM. Exendin-4 also stimulated ABCA1 promoter activity, but failed to do so in the presence of STO-609, a CaMKK inhibitor. Up-regulation of CaMKIV phosphorylation (at Thr196) peaked after 10 min. of exposure to exendin-4. CaMKIVc enhanced or up-regulated ABCA1 promoter activity in INS-1 cells. Furthermore, exendin-4 induction of ABCA1 protein expression was significantly suppressed in cells treated with CaMKIV-siRNA. Activation of the CaMKK/CaMKIV cascade by exendin-4 stimulated ABCA1 gene transcription, indicating that exendin-4 plays an important role in insulin secretion and cholesterol ester content in pancreatic β cells.
Keywords: exendin-4, GLP-1, ABCA1, transcription, CaMKK/CaMKIV
Introduction
The insulin secretagogue hormone GLP-1 (glucagon-like peptide-1) and its long- acting agonist exendin-4 are new treatment agents for diabetes [1]. GLP-1 stimulates glucose-dependent insulin secretion and lowers blood glucose levels in type 2 diabetics. Previous studies demonstrate that GLP-1 activates multiple signalling pathways via GLP-1 receptor. These pathways involved protein kinase A (PKA), Ca2+/calmodulin (CaM)-dependent protein kinase (CaMK), mitogen-activated protein kinases (MAPK, ERK1/2), PI-3K, protein kinase B (PKB, Akt) and atypical protein kinase C-ζ (PKC-ζ) [2]. Subsequently, GLP-1’s multiple anti-diabetogenic functions were discovered, including stimulation of the proliferation of insulin-producing pancreatic β cells and inhibition of their apoptosis [1, 3].
ATP-binding cassette transporter A1 (ABCA1), a 254-kD cytoplasmic membrane protein, is a pivotal regulator of lipid efflux from cells to apolipoproteins and plays an important role in reverse cholesterol transfer [4]. Recent report indicates that mice with specific inactivation of ABCA1 gene in β cells had markedly impaired glucose tolerance and defective insulin secretion but normal insulin sensitivity. Pancreatic islets isolated from these mice demonstrated altered cholesterol homeostasis and impaired insulin secretion in vitro. These results establish a new role for ABCA1 gene in β cell cholesterol homeostasis and insulin secretion, indicating that cholesterol accumulation may contribute to β cell dysfunction in type 2 diabetes [5]. In the present study, we examined the effects of exendin-4 on ABCA1 expression in pancreatic β cells.
Material and methods
Transfection of cells and luciferase reporter gene assay
The reporter construct contained the ABCA1 gene sequence, which spanned the region from –919 to +224 as determined from the sequence that has been published [6]. The segment of interest was amplified using PCR and cloned into the luciferase reporter gene (pABCA1-LUC). Purified reporter plasmid was transfected into INS-1 (at 60% confluence) using a conventional cationic liposome transfection methods (Lipofectamine; Life Technologies, Gaithersbueg, MD, USA). One microgram of Rous sarcoma virus-β-galactosidase was added to the transfection mixture to monitor the efficiency of DNA uptake by the cells. All assays were corrected for β-galactosidase activity and the total amount of protein per reaction was identical. Both cDNA of Ca2+/CaM-independent mutant of CaMKIV (CaMKIVc, 305 HMDT to DEDD) and constitutively active CaMKK mutant (CaMKKc, residues 1–434) were constructed as described previously [7, 8].
Phosphorylation of CaMKIV at Thr196
INS-1 cells were treated with 10 nM exendin-4 for 2 min. and harvested at predetermined time intervals. The cells were lysed and endogenous CaMKIV was immunoprecipitated using the anti-CaMKIV antibody. Western blotting analysis was carried out using anti-phospho-Thr196 antibody. The total cell lysate was also subjected to Western blotting analysis using CaMKIV antibody as control. Anti-phospho-CaMKIV Thr196 monoclonal antibodies were generated against the synthetic phosphopeptides corresponding to residues 189–203 of rat CaMKIV (CEHQVLMKT(p)VCGTPGY). Peptide was conjugated using keyhole limpet haemocyanin via the N-terminus cysteine and was injected into BALB/c mice as described previously [9].
Statistical analysis
Statistical comparisons were made using one-way anova and Student’s t-test, with a P-level of less than 0.05 considered as significant.
Results
Effects of exendin-4 on ABCA1 expression
The effect of exendin-4 on ABCA1 expression in INS-1 cells was examined by exposing the cells to varying concentrations (0–100 nM) of exendin-4. Results showed an increase in the abundance of the ABCA1 protein and mRNA in response to rising concentrations of exendin-4 with maximum induction observed at 10 nM (Fig. S1). We have confirmed the effect of exendin-4 using rat pancreatic islets. Figure 1A showed that exendin-4 also stimulated the expression of ABCA1 in rat pancreatic islets. Because exendin-4 increased both the protein and mRNA levels of ABCA1 in INS-1 cells, we speculated whether exendin-4 regulated the transcriptional activity of the ABCA1 promoter in INS-1 cells. To study this, we measured luciferase activity in the INS-1 cells transfected with pABCA1-LUC in the presence of various concentrations of exendin-4 (Fig. 1A). When INS-1 cells were exposed to 10 nM exendin-4, the insulin secretion or cholesterol ester content was increased or decreased, respectively, while the effects of exendin-4 on insulin secretion or cholesterol ester content were significantly reduced in the cells treated with the ABCA1-specific siRNA (Fig. S2). As a result, exendin-4 has a stimulatory effect on the activity of the ABCA1 promoter, which was consistent with the fact that it increased the ABCA1 protein and mRNA levels. To extend this observation further, we analysed the time course of ABCA1 mRNA expression and promoter activity (Fig. S3). Exposure of INS-1 cells to exendin-4 increased the ABCA1 mRNA following the activation of the promoter activity within 6 hrs.
Fig 1.
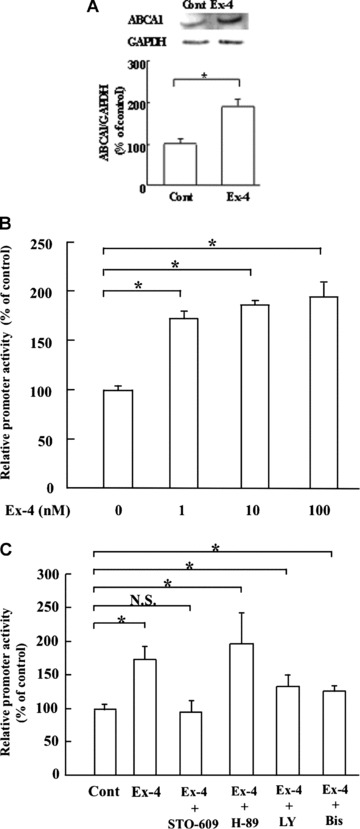
Effect of exendin-4 on ABCA1 expression in rat pancreatic islets and INS-1 cells. (A) Total cell lysate was purified from rat pancreatic islets treated with 10 nM of exendin-4 for 24 hrs. Western blot analysis was performed to examine ABCA1 expression. Expression of GAPDH was studied as the control, and the results are shown in the bottom lanes. The plot shows the ratio of ABCA1/GAPDH. Results are represented as mean ± S.E.M. of three experiments for each treatment group. The asterisk denotes a significant difference (P < 0.01). (B) Exendin-4 increases ABCA1 gene transcription. INS-1 cells were transfected with 1 μg pABCA1-LUC and treated with the indicated concentrations of exendin-4 for 24 hrs prior to cell harvesting. All assays were corrected for β-galactosidase activity, and the total amount of protein in each reaction was identical. The results were expressed as relative luciferase activity compared with that in the control cells arbitrarily set at 100. Each data point shows the mean ± S.E. of four separate transfections that were performed on separate days. The ‘*’ denotes the significant difference (P < 0.01). (C) Effects of the phosphatidylinositol 3-kinase inhibitor LY-294002, the PKC inhibitor bisindolylmaleimide I, PKA inhibitor H-89, and the CaMK inhibitor STO-609 on ABCA1 transcriptional activity in INS-1 cells with 10 nM exendin-4. Vehicle: 0.1% dimethyl sulphoxide. Each data point shows the mean ± S.E. of three separate transfections that were performed on separate days. The asterisk denotes a significant difference (P < 0.01).
CaMKK mediated and exendin-4-induced expression of the ABCA1 gene
To further examine how exendin-4 stimulated ABCA1 gene expression, we decided to study the potential involvement of signalling pathways. Therefore, we tested the effects of known signalling cascade inhibitors on exendin-4-induced stimulation of ABCA1 promoter activity. In this study, INS-1 cells were exposed to 10 nM exendin-4 plus either a phosphatidylinositol 3-kinase (PI3K) inhibitor (LY; 10 μM LY-294002), a PKC inhibitor (Bis; 10 μM bisindolylmaleimide I), a PKA inhibitor (H-89; 1 μM, KT5720; 100 nM) or a CaMKK inhibitor (STO; 1 μg/ml STO-609). The results showed that the stimulatory effect of exendin-4 on ABCA1 promoter activity persisted in the presence of PKC, PI3K, PKA inhibitors (Fig. 1B), but not in the presence of the CaMKK inhibitor. To confirm this observation, we measured the levels of endogenous ABCA1 expression with those inhibitors by Western blot analysis (Fig. S4). These findings suggest that the CaMKK-mediated signalling cascade may involve in exendin-4-induced stimulation of ABCA1 promoter activity.
Phosphorylation of CaMKIV by exendin-4 in INS-1 cells
Our study showed that ABCA1 expression in INS-1 cells was enhanced by exendin-4 that was inhibited by STO-609, CaMKK inhibitor. It has been shown that CaMKK is capable of activating multiple protein kinases including CaMKI, CaMKIV, PKB, AMP-kinase and SAD-B kinase. To determine which downstream effecter kinase of CaMKK is involved in this mechanism, we first examined the ability of exendin-4 to stimulate the CaMKIV activity in INS-1 cells by using anti-phospho-Thr196 monoclonal antibodies because phosphorylation of Thr196 in the activation loop of CaMKIV by CaMKK has been shown to greatly induce its kinase activity. INS-1 cells were treated with exendin-4 before they were harvested at predetermined time intervals and were subjected to Western blotting analysis using either anti-phospho-Thr196 antibodies (Fig. 2A, upper insert) or anti-CaMKIV antibodies (Fig. 2A, lower insert). Compared to untreated cells, cells treated with exendin-4 showed an increase in CaMKIV phosphorylation. Up-regulation of CaMKIV phosphorylation peaked after 10 min. of exendin-4 treatment and returned to the basal level after 60 min.
Fig 2.
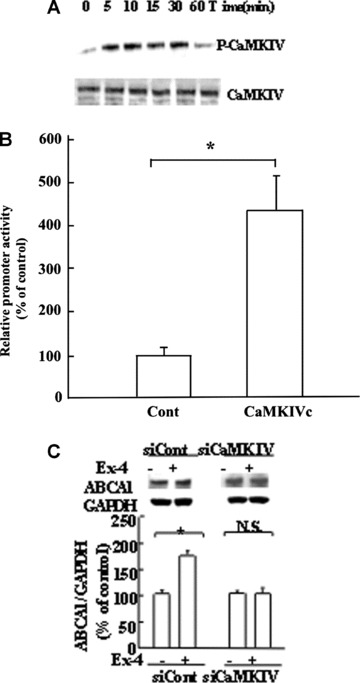
CaMKK cascade stimulates the ABCA1 promoter activity in response to exendin-4. (A) Phosphorylation of CaMKIV by exendin-4 in INS-1 cells. INS-1 cells were exposed to 10 nM exendin-4 for 2 min. before harvest at the predetermined time intervals. The total cell extracts were subjected to immunoprecipitation using anti-CaMKIV antibodies and SDS-PAGE, followed by Western blotting analysis using anti-phospho-Thr196 antibodies (upper insert). Total cell lysates were also blotted using anti-CaMKIV antibodies as a control (lower insert). (B) Effect of CaMK cascade on ABCA1 promoter activity. Cells were transfected with pABCA1-LUC and an empty vector, or CaMKIVc expression vectors. The cells were incubated for 24 hrs after transfection. Each data point shows the mean ± S.E. of three separate transfections that were performed on separate days. The asterisk denotes a significant difference (P < 0.01). (C) Effects of CaMKIV knockdown on ABCA1 expression in INS-1 cells. SiRNA of CaMKIV (siCaMKIV) or scrambled siRNA (siCont) was transfected into INS-1 cells, and then treated with exendin-4 (Ex-4). At 24 hrs after transfection, the abundance of ABCA1 protein level was measured using Western blot analysis (upper panel). The ratio of ABCA1 to GAPDH is shown as the percentage of control. Each data point shows the mean ± S.E. (n= 3) of separate experiments. The asterisk denotes a significant difference (P < 0.05). N.S.; no significant difference.
Role of CaMKIV in exendin-4-induced ABCA1 gene expression
Earlier studies have identified the components of the CaMKK cascade in exendin-4-induced ABCA1 promoter activity. The lack of information on whether this signal transduction cascade mediates the action of exendin-4 in INS-1 cells led us to examine whether CaMKIV has a role in exendin-4-induced transcription of the ABCA1 genes (Fig. 2B). To further characterize the role of CaMKIV in exendin-4 induced signalling to enhance ABCA1 expression, we have used siRNA to block CaMKIV-expression. INS-1 cells were exposed to CaMKIV specific or scramble siRNA and then treated with exendin-4. As shown in Fig. 2C, ABCA1 protein expression was increased in cells exposed to scrambled siRNA following stimulation with10 nM exendin-4. In contrast, exendin-4 induction of ABCA1 protein expression was significantly suppressed in cells treated with CaMKIV-siRNA. The above-mentioned findings support the idea that CaMKIV has a role in the exendin-4-induced ABCA1 expression.
Discussion
In this study, we found that the GLP-1 analogue, exendin-4 stimulated ABCA1 expression in the pancreatic β cell line – INS-1. The insulin secretagogue hormone GLP-1 and its structurally related peptide analogue, namely, exendin-4 are potent stimulators of the pancreatic β cell GLP-1 receptor [1, 3]. When administered to type 2 diabetic subjects, exendin-4 exerts multiple anti-diabetogenic effects: it stimulates insulin secretion, lowers fasting blood glucose levels and attenuates the elevation in blood glucose levels after ingestion of a meal. Such beneficial effects indicate its usefulness as a new treatment agent for diabetes [1].
Elevation of cholesterol levels in pancreatic islet cell, either in ob/ob mice that lacking ApoE and have diabetogenic obesity or in transformed β cell lines directly overloaded with cholesterol, reduces glucose-stimulated insulin secretion. This correlation is consistent with that between the reduction in insulin secretion and the elevation of pancreatic islet cell cholesterol levels in mice lacking β cell ABCA1 [4], suggesting that cholesterol has a direct effect on reduction of β cell function. The important role of β cell ABCA1 in glucose homeostasis was further underscored by the finding that rosiglitazone increases β cell ABCA1 expression [5]. In this study, we found that exendin-4 also has a stimulatory effect on ABCA-1 expression at the transcriptional level.
We have identified the role of the CaMKK/CaMKIV cascade in ABCA1 expression in response to exendin-4. We previously reported that both pancreatic β cells and the insulin-secreting cell line, INS-1, have this CaMKK/CaMKIV cascade and that this signal cascade plays an important role in glucose up-regulated transcriptional activation of the insulin gene [10]. Exendin-4 activated the phosphorylation of CaMKIV, this strongly suggests the possibility that activated CaMKIV might mediate the stimulatory effect of glucose-dependent insulin secretion through exendin-4.
In summary, we examined the role of the CaMKK/CaMKIV cascade in exendin-4-induced ABCA1 gene expression in the insulin-secreting pancreatic β cell line – INS-1. The results indicate that the activation of the CaMKK/CaMKIV cascade by exendin-4 stimulated ABCA1 gene transcription, suggesting that exendin-4 plays an important role in insulin secretion and cholesterol ester content in pancreatic β cells.
Supporting Information
Fig. S1 Effects of exendin-4 on protein and mRNAexpressions of ABCA1 in INS-1 cells. (A)Total cell lysatewas purified from the INS-1 cells treated with differentconcentrations of exendin-4 for 24 hrs. Western blot analysis wasperformed to examine ABCA1 expression. Expression of GAPDH wasstudied as the control, and the results are shown in the bottomlanes. The plot shows the ratio of ABCA1/GAPDH. Results arerepresented as mean ± S.E.M. of three experiments for eachtreatment group. The asterisk denotes a significant difference(P < 0.01). (B) Total RNA was extracted from theINS-1 cells treated with 10 nM of exendin-4 for 24 hrs. Real-timePCR was performed to analyse the ABCA1 mRNA expression. The plotshows the ratio of ABCA1/GAPDH mRNA. Results are represented asmean ± S.E.M. of three experiments for each treatment group.The asterisk denotes a significant difference (P < 0.01).
Fig. S2 Effect of exendin-4 on cholesterol ester contentand insulin secretion in INS-1 cells. Scramble siRNA (scramble) orsiRNA of ABCA1 was transfected into INS-1 cells and then INS-1cells were exposed to 10 nM exendin-4 for 24 hrs and cholesterolester content (A) and insulin section (B) weremeasured in total cell extract and media, respectively. Results arerepresented as mean ± S.E.M. of three experiments for eachtreatment group. The asterisk denotes a significant difference(*P < 0.01, **P < 0.05). N.S.; no significant difference.
Fig. S3 Time course of exendin-4-induced ABCA1 mRNA(A) and promoter activity (B). (A), INS-1cells were incubated in the medium with 5.6 mM glucose for theindicated periods of time in the presence of exendin-4. Real-timePCR was performed to analyse the ABCA1 mRNA expression. The plotshows the ratio of ABCA1/GAPDH mRNA. Results are represented asmean ± S.E.M. of three experiments for each treatment group.The asterisk denotes a significant difference (P < 0.01).(B), Cells were transfected with pABCA1-LUC and incubatedwith exendin-4 for indicated time after transfection. Each datapoint shows the mean ± S.E. of three separate transfectionsthat were performed on separate days. The asterisk denotes asignificant difference (P < 0.01).
Fig. S4 Effects of various inhibitors on ABCA1 expressionin INS-1 cells with 10 nM exendin-4. Western blot analysis of totalcell protein extracted from INS-1 cells with various inhibitorswith or without 10 nM exendin-4 (Ex-4) is shown. Abundance of GAPDHserved as control and is shown on the bottom of each lane,and the ratio of ABCA1 to GAPDH is shown as percent of control inthe figure. A graph showing the mean ± S.E.M. of threeexperiments for each treatment group is shown. The asterisk denotesa significant difference (P < 0.01). N.S.; no significant difference. Cont; no inhibitor, STO; CaMK inhibitor (STO-609), H-89; PKA inhibitor, LY: phosphatidylinositol 3-kinase inhibitor (LY-294002), Bis: PKC inhibitor (bisindolylmaleimide I).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 2.Holz GG. A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 2000;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 4.Tall AR, Wang N. Tangier disease as a test of the reverse cholesterol transport hypothesis. J Clin Invest. 2000;106:1205–7. doi: 10.1172/JCI11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunham LR, Kruit JK, Pape TD, et al. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13:340–7. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- 6.Langmann T, Porsch-Ozcürümez M, Heimerl S, et al. Identification of sterol-independent regulatory elements in the human ATP-binding cassette transporter A1 promoter: role of Sp1/3, E-box binding factors, and an oncostatin M-responsive element. J Biol Chem. 2002;277:14443–50. doi: 10.1074/jbc.M110270200. [DOI] [PubMed] [Google Scholar]
- 7.Tokumitsu H, Brickey DA, Glod J, et al. Activation mechanisms for Ca2+/calmodulin-dependent protein kinase IV. J Biol Chem. 1994;269:28640–7. [PubMed] [Google Scholar]
- 8.Tokumitsu H, Soderling TR. Requirements for calcium and calmodulin in the calmodulin kinase activation cascade. J Biol Chem. 1996;271:5617–22. doi: 10.1074/jbc.271.10.5617. [DOI] [PubMed] [Google Scholar]
- 9.Tokumitsu H, Hatano N, Inuzuka H, et al. Mechanism of the generation of autonomous activity of Ca2+/calmodulin-dependent protein kinase IV. J Biol Chem. 2004;279:40296–302. doi: 10.1074/jbc.M406534200. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Murao K, Sayo Y, et al. The role of calcium/calmodulin-dependent protein kinase cascade in glucose upregulation of insulin gene expression. Diabetes. 2004;53:1475–81. doi: 10.2337/diabetes.53.6.1475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Effects of exendin-4 on protein and mRNAexpressions of ABCA1 in INS-1 cells. (A)Total cell lysatewas purified from the INS-1 cells treated with differentconcentrations of exendin-4 for 24 hrs. Western blot analysis wasperformed to examine ABCA1 expression. Expression of GAPDH wasstudied as the control, and the results are shown in the bottomlanes. The plot shows the ratio of ABCA1/GAPDH. Results arerepresented as mean ± S.E.M. of three experiments for eachtreatment group. The asterisk denotes a significant difference(P < 0.01). (B) Total RNA was extracted from theINS-1 cells treated with 10 nM of exendin-4 for 24 hrs. Real-timePCR was performed to analyse the ABCA1 mRNA expression. The plotshows the ratio of ABCA1/GAPDH mRNA. Results are represented asmean ± S.E.M. of three experiments for each treatment group.The asterisk denotes a significant difference (P < 0.01).
Fig. S2 Effect of exendin-4 on cholesterol ester contentand insulin secretion in INS-1 cells. Scramble siRNA (scramble) orsiRNA of ABCA1 was transfected into INS-1 cells and then INS-1cells were exposed to 10 nM exendin-4 for 24 hrs and cholesterolester content (A) and insulin section (B) weremeasured in total cell extract and media, respectively. Results arerepresented as mean ± S.E.M. of three experiments for eachtreatment group. The asterisk denotes a significant difference(*P < 0.01, **P < 0.05). N.S.; no significant difference.
Fig. S3 Time course of exendin-4-induced ABCA1 mRNA(A) and promoter activity (B). (A), INS-1cells were incubated in the medium with 5.6 mM glucose for theindicated periods of time in the presence of exendin-4. Real-timePCR was performed to analyse the ABCA1 mRNA expression. The plotshows the ratio of ABCA1/GAPDH mRNA. Results are represented asmean ± S.E.M. of three experiments for each treatment group.The asterisk denotes a significant difference (P < 0.01).(B), Cells were transfected with pABCA1-LUC and incubatedwith exendin-4 for indicated time after transfection. Each datapoint shows the mean ± S.E. of three separate transfectionsthat were performed on separate days. The asterisk denotes asignificant difference (P < 0.01).
Fig. S4 Effects of various inhibitors on ABCA1 expressionin INS-1 cells with 10 nM exendin-4. Western blot analysis of totalcell protein extracted from INS-1 cells with various inhibitorswith or without 10 nM exendin-4 (Ex-4) is shown. Abundance of GAPDHserved as control and is shown on the bottom of each lane,and the ratio of ABCA1 to GAPDH is shown as percent of control inthe figure. A graph showing the mean ± S.E.M. of threeexperiments for each treatment group is shown. The asterisk denotesa significant difference (P < 0.01). N.S.; no significant difference. Cont; no inhibitor, STO; CaMK inhibitor (STO-609), H-89; PKA inhibitor, LY: phosphatidylinositol 3-kinase inhibitor (LY-294002), Bis: PKC inhibitor (bisindolylmaleimide I).


