Abstract
The effects of angiotensin-converting enzyme (ACE) inhibition and angiotensin II type 1 receptor blockade (ARB) on fibrinolysis and inflammation following cardiopulmonary bypass (CPB) are uncertain. This study tested the hypothesis that ACE inhibition enhances fibrinolysis and inflammation to greater extent than ARB in patients undergoing CPB.One week to five days prior to surgery, patients were randomized to ramipril 5mg/day,candesartan 16mg/day or placebo.ACE inhibition increased intraoperative bradykinin and tissue-type plasminogen activator (t-PA) concentrations compared to ARB. Both ACE inhibition and ARB decreased plasma transfusion compared to placebo, but only ACE inhibition decreased length of stay. Neither ACE inhibition nor ARB significantly affectedplasminogen activator inhibitor-1 (PAI-1), interleukin (IL)-6, IL-8, or IL-10 concentrations. ACE inhibition enhanced intraoperative fibrinolysis without increasing red cell transfusion risk. In contrast, neither ACE inhibition nor ARB affected the inflammatory response. ACE inhibitors and ARB may be safely continued until the day of surgery.
Keywords: Angiotensin-converting enzyme inhibition, angiotensin receptor blocker, renin-angiotensin system, cardiac surgery, cardiopulmonary bypass, plasminogen activator, interleukin, bradykinin
The fibrinolytic response to cardiopulmonary bypass (CPB) is biphasic. An initial hyperfibrinolytic phase is characterized by a rapid increase in plasma tissue-type plasminogen activator (t-PA) concentrations and bleeding. This is followed by a postoperative hypofibrinolytic phase associated with increased plasminogen activator inhibitor-1 (PAI-1) expression and decreased circulating t-PA.(1, 2) Disruption of fibrinolytic homeostasis results in hemorrhage during excessive fibrinolysis and thrombosis and inflammation during inappropriate fibrinolytic inhibition. Simultaneous with the fibrinolytic response, CPB induces a systemic inflammatory response characterized by interleukin (IL) production.(3, 4)Because increased PAI-1 and IL-6 concentrations are associated with an increased risk of postoperative atrial fibrillation, infection, and acute kidney injury,(5-7)drugs that alter the acute-phase response to CPB might decrease postoperative morbidity.
Inhibition of the renin-angiotensin system by angiotensin-converting enzyme (ACE) inhibitorsor angiotensin II type 1 (AT1) receptor blockers (ARBs) has been shown to decrease inflammation in patients with hypertension and rheumatoid arthritis.(8, 9)We previously demonstrated that continued ACE inhibition increases intraoperative t-PA concentrations and attenuates the rise in postoperative PAI-1 concentrations in two cardiac surgery studies in which patients were randomized to either continue or stop ACE inhibitor therapy prior to surgery.(2, 10)ACE inhibition decreasesthe formation of angiotensin II (a potent stimulus for PAI-1 production)(11), and decreases the degradation of bradykinin (a potent stimulus of t-PA(12) and IL-6 secretion).(13)ARBs block the effect of angiotensin II on AT1 receptors but do not significantly affect bradykinin degradation.(14, 15)Thus, to the extent that bradykinin contributes to either the fibrinolytic or the inflammatory response following CPB, ARB would be expected to potentiate the generation of bradykinin (and subsequent inflammation and fibrinolysis) during CPB to a lesser extentthan ACE inhibition. The effect of ACE inhibition and ARB on post-CPBinflammationremainsunclear. Some studies suggest that preoperative ACE inhibition blunts the postoperative inflammatory response,(16-18) whereas other studies found no effect(19) or an enhancement of the inflammatory response.(10)A study of perioperative ARB therapy failed to show a significant effect of ARB on IL-6 concentrations.(18)
This study tested the hypothesis that perioperative ACE inhibition enhances fibrinolysis and inflammation to greater extent thanARB in patients undergoing cardiac surgery requiring CPB.
Results
Patient Demographics
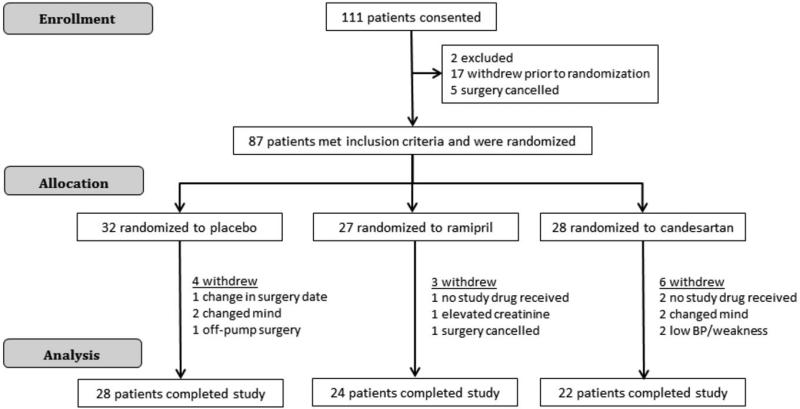
One hundred and eleven patients were consented to participate (Figure 1). Two patients did not meet inclusion/exclusion criteria. Seventeen subjects withdrew prior to randomization, and surgery was cancelled in five subjects. Eighty-seven subjects were randomized, and thirteen of those were excluded for the following reasons. Four subjects changed their mind and withdrew after randomization but prior to taking study medication, three subjects did not receive study drug in time, one subject's surgery date was changed, one subject's surgery was cancelled, two subjects experienced low blood pressure and weakness and stopped study drug, one subject's creatinine rose >1.6 mg/dl prior to surgery, and one subject had surgery without CPB. Seventy-four subjects completed the study protocol and were included in the final analysis.
Figure 1.
Study enrollment.
There were no significant differences among the three treatment groups in baseline subject characteristics (Table 1). PreoperativeACE activity (measured prior to CPB) was significantly lower in the ramipril group (8.1±1.0U/L) compared to the placebo (32.4±5.1 U/L, P<0.001 versus ramipril) and candesartan (29.0±2.9U/L; P<0.001 versus ramipril) groups, indicating effective inhibition of ACE activity by ramipril study drug. We achieved 75% inhibition of ACE activity with the ramipril 5mg/day dose. This is consistent with a previous study in which ramipril 2.5, 5, or 10 mg significantly decreased ACE activity compared to baseline by an average of 71% among all dose groups.(20)
Table 1.
Baseline Subject Characteristics
| Characteristics | Placebo (N=28) | Ramipril (N=24) | Candesartan (N=22) | P-value |
|---|---|---|---|---|
| Age (years) | 66.1±2.1 | 64.4±2.1 | 67.0±1.7 | 0.66 |
| Gender (Male), N (%) | 9 (32.1) | 12 (50.0) | 10 (45.5) | 0.40 |
| Race (White), N (%) | 26 (96.3) | 24 (100) | 22 (100) | 0.43 |
| BMI (kg/m2) | 29.6±1.6 | 29.1±1.2 | 30.7±1.7 | 0.68 |
| s-BP (mmHg) | 125.9±3.4 | 133.6±3.3 | 129.2±3.3 | 0.27 |
| d-BP (mmHg) | 74.0±2.3 | 72.6±1.5 | 69.7±2.2 | 0.34 |
| MAP (mmHg) | 88.3±2.5 | 89.8±3.5 | 91.5±3.3 | 0.76 |
| Hematocrit (%) | 40.1±0.8 | 41.7±0.8 | 40.7±1.0 | 0.42 |
| Potassium (meq/L) | 4.01±0.08 | 4.03±0.11 | 4.16±0.10 | 0.57 |
| Creatinine (mg/dL) | 1.00±0.05 | 1.02±0.04 | 1.01±0.05 | 0.77 |
| LVEF (%) | 53.6±2.1 | 57.1±2.3 | 58.2±2.7 | 0.37 |
| Medical History, N (%) | ||||
| Hypertension | 21 (75.0) | 17 (70.8) | 18 (81.8) | 0.68 |
| Current atrial fibrillation | 11 (39.3) | 8 (33.3) | 9 (40.9) | 0.85 |
| Diabetes | 9 (32.1) | 4 (16.7) | 9 (40.9) | 0.19 |
| Past smoking | 11 (39.3) | 10 (41.7) | 9 (40.9) | 0.98 |
| COPD | 3 (10.7) | 4 (16.7) | 3 (13.6) | 0.82 |
| Medications Prior to Randomization, N (%) | ||||
| ACEi | 8 (28.6) | 11 (45.8) | 8 (36.4) | 0.44 |
| ARB | 6 (21.4) | 2 (8.3) | 3 (13.6) | 0.41 |
| β-blocker | 19 (67.9) | 13 (54.2) | 9 (40.9) | 0.16 |
| Statin | 17 (60.7) | 14 (58.3) | 9 (40.9) | 0.33 |
| Diuretic | 17 (60.7) | 10 (41.7) | 14 (63.6) | 0.25 |
| Calcium channel blocker | 5 (19.2) | 7 (29.2) | 7 (31.8) | 0.57 |
BMI, body mass index; MAP, mean arterial pressure; LVEF, left ventricular ejection fraction; COPD, chronic obstructive pulmonary disease; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Intraoperative Subject Characteristics and Postoperative Outcomes
There were no significant differences among study groups in type of surgery, CPB time, cross-clamp time, use of aortic cross-clamp, hemoconcentration use during CPB, or use of steroids in the CPB pump prime (Table 2). In addition, inotropic support and use of vasopressors were similar among treatment groups.Blood loss, measured by chest tube output and need for surgical re-exploration, total fluids in and out, postoperative atrial fibrillation, AKI, and mortality were not significantly different among treatment groups (Table 3). The blood product transfusion exposure was similar among treatment groups except fresh frozen plasma was more frequently transfused in the placebo group. Hospital length of stay was significantly shorter in subjects randomized to receive ACE inhibitor therapy.
Table 2.
Intraoperative Characteristics
| Placebo (N=28) | Ramipril (N=24) | Candesartan (N=22) | P-value | |
|---|---|---|---|---|
| Surgery Type, N (%) | 0.45 | |||
| CABG surgery | 0 | 3 (12.5) | 1 (4.5) | |
| Valve surgery | 22 (78.6) | 17 (70.8) | 18 (81.8) | |
| CABG plus valve surgery | 5 (17.9) | 4 (16.7) | 3 (13.6) | |
| Other | 1 (3.6) | 0 | 0 | |
| Number of grafts | 1.4±0.5 | 2.9±0.5 | 1.75±0.5 | 0.11 |
| CPB time (min) | 138.5±10.4 | 131.4±10.6 | 142.3±10.3 | 0.77 |
| Aortic cross-clamp use, N (%) | 19 (67.9) | 13 (54.2) | 11 (50.0) | 0.40 |
| Aortic cross-clamp time (min) | 91.9±10.2 | 96.2±12.6 | 109.1±10.8 | 0.57 |
| Hemoconcentrator use, N (%) | 3 (10.7) | 1 (4.2) | 3 (13.6) | 0.53 |
| Lowest hematocrit during CPB (%) | 26.1±1.0 | 25.9±0.8 | 25.1±1.1 | 0.79 |
| Steroids in CPB pump prime, N (%) | 20 (71.4) | 17 (70.8) | 14 (63.6) | 0.82 |
| Inotropic drug support post CPB, N (%) | ||||
| Dobutamine | 10 (35.7) | 7 (29.2) | 6 (27.3) | 0.77 |
| Milrinone | 7 (25.0) | 6 (25.0) | 5 (22.7) | 0.98 |
| Norepinephrine | 24 (85.7) | 19 (79.2) | 21 (95.5) | 0.27 |
| Epinephrine | 2 (7.1) | 1 (4.3) | 3 (13.6) | 0.51 |
CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass.
Table 3.
Postoperative Characteristics
| Placebo (N=28) | Ramipril (N=24) | Candesartan (N=22) | P-value | |
|---|---|---|---|---|
| Chest tube output 24hrs (ml) | 437±35 | 470±59 | 511±119 | 0.67 |
| Total fluid in 24hrs (ml) | 3002±190 | 2675±207 | 3107±245 | 0.28 |
| Total fluid out 24hrs (ml) | 2123±210 | 1997±133 | 1937±163 | 0.77 |
| Time to extubation (hours) | 17.4±4.1 | 13.5±4.3 | 10.0±2.2 | 0.15 |
| Transfusion, N (%) | ||||
| PRBC | 19 (67.9) | 15 (62.5) | 16 (72.7) | 0.76 |
| FFP | 17 (60.7) | 7 (29.2)* | 7 (31.8)* | 0.04 |
| Platelets | 13 (46.4) | 7 (29.2) | 5 (22.7) | 0.18 |
| Cryoprecipitate | 2 (7.1) | 1 (4.2) | 1 (4.5) | 0.87 |
| Morbidity, N (%) | ||||
| Acute renal failure (STS criteria)† | 0 | 1 (4.2) | 1 (4.5) | 0.53 |
| Acute kidney injury (stage I)‡ | 8 (28.6) | 5 (23.8) | 8 (36.4) | 0.51 |
| Re-exploration | 1 (3.6) | 2 (8.3) | 1 (4.5) | 0.73 |
| Stroke | 2 (7.1) | 1 (4.2) | 1 (4.5) | 0.87 |
| New onset atrial fibrillation | 5 (17.9) | 4 (16.7) | 6 (27.3) | 0.62 |
| Pacemaker placement | 6 (21.4) | 1 (4.2) | 2 (9.1) | 0.14 |
| Hospital length of stay (days) | 7.7±0.5 | 6.3±0.6* | 8.1±1.0 | 0.04 |
| In-hospital mortality, N (%) | 0 | 0 | 1 (4.5) | 0.30 |
PRBC, packed red blood cells; FFP, fresh frozen plasma.
P<0.05 versus placebo.
200% increase and at least 2 mg/dl serum creatinine postoperatively.
50% or 0.3 mg/dL increase in serum creatinine within 72 hours of surgery (AKIN criteria).
Effect of CPB on the fibrinolytic and inflammatory response
CPB was associated with a significant increase in t-PA antigen (13.5±0.9 ng/ml preoperatively to 35.6±1.9 ng/ml post-bypass, P<0.001) and PAI-1 antigen (16.4±1.3 ng/ml preoperatively to 43.1±3.2 ng/ml post-bypass, P<0.001). The PAI-1:t-PA molar ratio decreased significantly (1.9±0.1 preoperativelyto 1.3±0.1 60min of CPB, P<0.001), reflective of the initial pro-fibrinolytic phase of CPB. CPB was also associated with increasedexpression of the pro-inflammatory markers IL-6 (7.3±1.2 pg/ml preoperatively to a peak concentration of 206.0±38.1pg/ml on POD1, P<0.001) and IL-8 (14.0±1.6 pg/ml preoperatively to a peak concentration of 73.9±10.2 pg/ml post-bypass, P<0.001) and the anti-inflammatory marker IL-10 (4.7±0.4 pg/ml preoperatively to a peak concentration of 662.2± 111.2 pg/ml post-bypass, P<0.001).
Effect of study drug on the fibrinolytic and inflammatory response
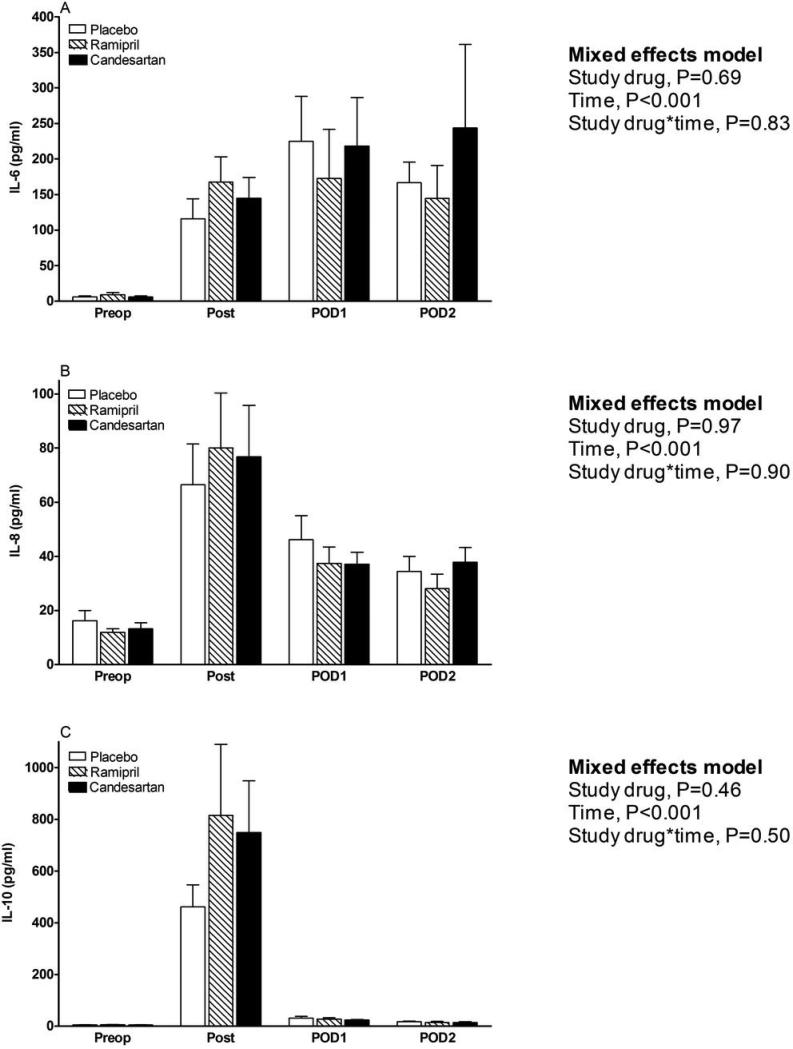
Preoperative t-PA antigen, PAI-1 antigen, and the PAI-1:t-PA molar ratio were not significantly different among treatment groups (all P-values > 0.44).Although study drug did not significantly affect t-PA antigen concentrations over time (P=0.28 for effect of study drug over time, Figure 2A)we observed a trend for the interaction between study drug and t-PA over time. Because of this interaction trend and the fact that the fibrinolytic response following CPB surgery is characterized by an initial hyperfibrinolytic intraoperative phase and a subsequent hypofibrinolytic postoperative phase, we investigated the effect of study drug on intraoperative and postoperative t-PA antigen concentrations separately, using the Wilcoxon rank-sum test. Ramipril significantly increased the t-PA antigen concentrations and decreased the PAI-1:t-PA antigen molar ratio compared to candesartan measured at the conclusion of surgery (post-bypass). Study drug did not affectPAI-1 antigen concentrations (P=0.84 for effect of study drug, Figure 2B).Preoperative IL-6, IL-8, and IL-10 concentrations were similar among treatment groups (all P-values >0.57). There was no effect of study drug on IL-6, IL-8 or IL-10 concentrations over time (P=0.69, P=0.97, P=0.46 respectively for effect of study drug, Figure 3A, B and C).
Figure 2.
Effects of study drug on markers of fibrinolysis. A) tissue-type plasminogen activator (t-PA) antigen concentrations. B) plasminogen activator inhibitor-1 (PAI-1) antigen concentrations. C) PAI-1:t-PA molar ratios. Preop indicates preoperative, 60min indicates 60min of cardiopulmonary bypass, post indicates post-bypass, and POD indicates postoperative day.
Figure 3.
Effects of study drug on markers of inflammation. A) Interleukin (IL)-6. B) IL-8. C) IL-10. Preop indicates preoperative, post indicates post-bypass, and POD indicates postoperative day.
Clinical correlates
Post-bypass PAI-1 antigen (all subjects) correlated with duration of CPB (r2=0.31, P<0.001; Figure 4A) and was higher in subjectsthat underwent aorta cross-clamping (49.5±4.9 ng/ml versus 34.3±3.0 ng/ml, P=0.03). Mixed effects modelsthat included type of surgery (valve, CABG, or combined surgery) as a covariate indicatedan effect between surgery type and post-bypass PAI-1 antigen (P=0.002), but no effect between surgery type and t-PA antigen or PAI-1 to t-PA molar ratio.Including type of surgery in the model did not impact the effect of study drug on these biomarkers.
Figure 4.
A) Correlation between duration of cardiopulmonary bypass (CPB) and post-bypass plasminogen activator inhibitor-1 (PAI-1) antigen. B) Correlation between duration of CPBand post-bypass interleukin (IL)-6.
Similar to PAI-1 antigen, post-bypass IL-6 concentrations correlated with the duration of CPB (r2=0.50, P<0.001; Figure 4B). Surgery type was also associated with differences in post-bypass IL-6 concentrations, asvalve only surgery subjects had significantly lower IL-6 concentrations compared to subjects who had coronary artery bypass graft (CABG)only surgery (88.9±10.6 versus 323.8±53.6 pg/ml, P<0.001). In addition, post-bypass IL-6 concentrations were higher in subjects who underwentaorta cross-clamping (186.2±27.8 versus 79.0±12.1 pg/ml, P=0.006). Treatment with steroids, added to the CPB pump prime, was associated with lower post-bypass IL-6 concentrations (116.7±17.8 versus 195.7±40.7 pg/ml, P=0.046). The duration of CPB, type of surgery, aortic cross-clamp use, and pump prime steroids accounted for 66% of the variability observed in post-bypass IL-6 concentrations. The inclusion of these variables as potential confounders in the mixed effects model, however, did not change the effect of study drug on IL-6 concentrations.
Kinin Response
Bradykinin concentrations increased 2.7 fold in the entire cohort, from 51.5±4.7 fmol/ml before surgery to 139.6±23.7 fmol/ml post-bypass (P<0.001).Post-bypass bradykinin concentrationswere higher in the ramipril group compared to the candesartan group (175.4±61.0 versus 113.3±28.7 fmol/ml, P=0.03), and bradykinin concentrations during CPB correlated with t-PA antigen concentrations(r2=0.23, P<0.001). Peak bradykinin concentrations did not correlate with post-bypass IL-6, IL-8 or IL-10 concentrations (all P-values >0.43).
Discussion
This prospective, placebo-controlled, randomized clinical trial revealed four findings about the role of the renin-angiotensin systemduring CPB in adult patients without severe chronic kidney disease or severe systolic heart failure. 1) ACE inhibition enhances intraoperative fibrinolysis (increases t-PA antigen and decreases PAI-1:t-PA molar ratio) without increasing blood loss or transfusion exposure risk. 2) Neither ACE inhibition nor ARB affects PAI-1 concentrations.3) Perioperative ACE inhibition or ARB does not significantly affect the CPB-induced interleukin response. 4) While is it common practice to stop ACE inhibitors and ARBsseveral days prior to surgery that involves CPB, this study provides additional data suggesting that continuation of these drugs is safe.
Fibrinolysis
In non-surgical populations, most studies suggest that chronic ACE inhibition decreases PAI-1 concentrations, whereas ARB has no effect.(21)In contrast to our prior studiesin surgical populations,(2, 10)perioperativeACE inhibition did not attenuatethe increase in postoperative PAI-1 concentrations. This finding may be explained by a difference in study methodologies. The current clinical trial included mostly valve surgery patients randomized to a single fixed dose ACE inhibitor, whereas in our previous studies(2, 10) patients were on chronic diverse ACE inhibitor therapies at different doses before they were randomized to either continue or discontinue their current ACE inhibitor therapy.Even though the dose of ACE inhibitor may affect the PAI-1 response (22) the HEART study investigators(23)reported that both low-dose and full-dose ramipril decreases PAI-1 to a similar extent.The dose of ramipril in this study, although not the maximum dose, was sufficient to suppress ACE activity compared toplacebo and ARB groups. We cannot exclude the possibility that our results may have been different if we studied only patients on chronic ACE inhibitor therapy.Taken together with prior studies, the current study suggests that short-term ACE inhibition does not affect the PAI-1 response, whereas withdrawal of chronic ACE inhibitionmay lead to an increased PAI-1 response following CPB.The effect of ARB on the fibrinolytic response following CPB has not been studied. ARB treatment did not affect PAI-1 concentrations. The lack of effect of ARB on the fibrinolytic response may be attributable to non-AT1 receptor subtypes mediating the effect of angiotensinII on endothelial PAI-1 expression.(24)Consistent with our previous studiesperioperative ACE inhibition increases t-PA during cardiac surgery.(2)
Inflammatory Response
CPB was associated with a significant increase in postoperative inflammatory markers. Neither ACE inhibition nor ARB significantly affected the increase in postoperative pro- or anti-inflammatory markers. In prior studies preoperative ACE inhibition hasbeen associated a decreased,(16-18)an increased,(10) or an unchanged(19)cardiac surgery-induced inflammatory response. In another study ARB tended to decrease IL-6 concentrations following CPB surgery.(18)Comparison of our findings to these studies isdifficult because of study methodology differences. For example, the study by Radaelli et al(17)included predominantly male CABG surgery patients and only demonstrated inflammation attenuation with high-dose ACE inhibitorplus high-dose statintherapy, and this effect did not persistsix hours after cross-clamp removal. Our study population on the other hand underwent more diverse surgeryand statin use was not randomized. Despite our use of a lower dose of ramipril, ACE activity was significantly suppressed with this dose. The majority of the postoperative IL-6 variability observed in our study was secondary to duration of CPB, aorticcross-clamping, type of surgery, and administration of steroids. Including these variables in the mixed effects model still did not reveal any effect of renin-angiotensin blockade on the inflammatory response.
Bradykinin concentrations were measured to investigate the contributions of bradykinin to the inflammatory response because bradykinin has been shown to increase circulating IL-6,(13, 25)and ACE inhibition and to a lesser extent ARB increase bradykinin concentrations by decreasing bradykinin metabolism.(14, 26)As expected, ACE inhibition increased bradykinin concentrations(27)and although increased bradykinin correlated with increased t-PA concentrations in our study, there were no significant associationsamong bradykinin and inflammatory marker concentrations. Ongoing studies of perioperative bradykinin receptor antagonist (NCT00223704) usewill help elucidate the role of bradykinin in postoperative inflammation.
Clinical Outcomes
In the current studywe made the additional observation that increased markers of intraoperative fibrinolysis are not necessarily associated with increased postoperative blood loss or blood product exposure risk. In fact, ACE inhibition was associated with less exposure to fresh frozen plasma compared to placebo. Study group differences in other predictors of coagulopathy, hemorrhage, and transfusion, such as surgical hemostasis, platelet dysfunction, or hemodilution may explain this finding.ACE inhibition was not associated with any safety concerns but was associated with a decreased length of hospital stay, suggesting additional benefits of perioperative ACE inhibition. Prior retrospective studies either demonstrated no difference(28) or an increase(29) in adverse clinical outcomes associated with preoperative ACE inhibition. This study was not powered to assess the impact of ACE inhibition on clinical outcomes, and therefore clinical trials are needed to determine whether preoperative ACE inhibition impacts clinical outcomes.
Limitations
Contrary to prior studies that focused on CABG only surgical populations,(2, 16-19)we included a diverse study population that reflects standard cardiac practice but may have led to little observed effect of renin-angiotensin system blockade on clinical fibrinolytic and inflammatory markers. We cannot exclude the possibility that our results may have been different if we studied a homogenous surgery population.Nonetheless, controlling for type of surgery did not change the effect of study drug on clinical fibrinolytic and inflammatory markers.On the other hand, our diverse study population allows for generalizability of our study results to diverse cardiac surgical populations.
In conclusion, this study demonstrates that perioperative ACE inhibition increases intraoperative t-PA and bradykinin concentrations but does not increase blood loss or transfusion exposure risk, perioperative ARB does not affect plasma markers or clinical indicators of fibrinolysis, and neither ACE inhibition nor ARB alter the inflammatory response to CPB. The modest effect of ACE inhibition on the fibrinolytic balance (with no increase in detrimental effects such as blood loss or blood product transfusion) and absence of a significant effect on the inflammatory response would suggest that renin-angiotensin system inhibition plays a minor role in the postoperative fibrinolytic and inflammatory response and that ACE inhibitors and ARBsmay be safely continued until the day of surgery. Additional prospective trials are needed to assess the impact of preoperative ACE inhibition and ARB on clinical outcomes.
Methods
Adult patients undergoing cardiac surgery with the use of CPB were eligible for study. Exclusion criteria included left ventricular ejection fraction less than 30%, serum potassium greater than 5.0 mEq/L, serum creatinine greater than 1.6 mg/dL, and inability to discontinue current ACE inhibitor or ARB.
One week to five days prior to surgery, patients were randomized to treatment with placebo, ramipril (2.5 mg the first three days followed by 5mg/day, with the dose reduced to 2.5mg/d on the first postoperative day only), or candesartan (16 mg/day).The ramipril 5mg daily dosing was chosen to achieve significant inhibition of ACE activity without compromising patient safety (a larger dose of ramipril may have increased perioperative hypotension and withholding of study drug protocol violations).Randomization was stratified by prior ACE inhibitor and ARB use. Preexisting ACE inhibitor and ARB therapy was stopped at randomization. All other preoperative medications were continued until the day of surgery. Safety criteria for stopping study medication were hypotension (defined as SBP less than 90 mmHg or prolonged need for vasopressors), serum potassium greater than 5.5 mEq/L if confirmed by a repeated measurement, and acute renal failure (ARF), defined using Society of Thoracic Surgery (STS) criteria. The STS defines post cardiac surgery ARF as any serum creatinine concentration greater or equal to 2.0 mg/dL and 2-fold (200%) greater than baseline.The study (ClinicalTrials.gov Identifier: NCT00607672) was approved by the Vanderbilt University Human Research Protection Program and the TN Valley Healthcare System Institutional Review Board and conducted according to the Declaration of Helsinki. All patients provided written informed consent.
Primary and Secondary Endpoints
The primary endpoint was the effects of study drug on the fibrinolytic response, quantified by measurement of plasma t-PA and PAI-1 antigen concentrations, and the effects of study drug on the inflammatory response, quantified by measurement of plasma IL-6, IL-8 and IL-10 concentrations. Because ACE inhibition has been shown to impact fibrinolysis, kidney injury, and atrial fibrillation,(5, 29, 30)we also assessed postoperative blood loss, transfusion requirements, re-exploration for bleeding, inotropic and vasopressor use, new onset atrial fibrillation, and changes in serum creatinine as secondary endpoints. Acute kidney injury (AKI) was defined according to Acute Kidney Injury Network (AKIN) criteria,(31) specifically any increase in subject serum creatinine concentration of 50% or 0.3 mg/dL (26.5 μmol/L) within 72 hours of surgery. The AKIN urine output criteria for AKI diagnosis were not used due to confounding by intravascular hypovolemia and diuretic use,(32) both of which are common among cardiac surgery patients.
Standardized Patient Treatment
Anesthesia management and CPB were conducted according to institutional protocol. Induction of anesthesia was achieved with either etomidate or propofol and maintained with isoflurane, fentanyl, air, and oxygen. Muscle relaxation was achieved and maintained with pancuronium or rocuronium. Hemodynamics were invasively monitored with an arterial line (Arrow International, Reading, PA) and a pulmonary artery catheter (Edwards Lifesciences, Irvine, CA). CPB was achieved with a roller pump (Medtronic, Minneapolis, MN); heparin coated circuit (Carmeda®) and a Trillium® hollow fiber oxygenator (Medtronic, Minneapolis, MN). Heparin was used for anticoagulation during CPB with an initial dose of 300U/kg and supplemented with additional heparin to achieve and maintain an activated clotting time (ACT) >400seconds. All patients received ε-aminocaproic acid (antifibrinolytic drug) as a bolus of 100mg/kg over 30 minutes prior to CPB and then 25mg/kg/hour throughout surgery. Temperature management included cooling to 28° to 30°C, temperature uncorrected blood gas management (alpha stat), and cold anterograde and retrograde cardioplegia techniques when an aortic cross-clamp was applied. At the conclusion of CPB, anticoagulation was reversed with 250 mg protamine, with additional 50 mg aliquots administered to achieve baseline ACT. Vasopressors were used at separation from CPB if the mean arterial blood pressure was less than 60 mmHg. Inotropes were used for separation from CPB for the following criteria: left ventricular ejection fraction less than 40%, CPB time longer than 120 minutes, cardiac index less than 2 l/min/m2,or evidence of new onset left ventricular dysfunction by transesophageal echocardiography. Use of inotropes and/or vasopressors in the postoperative period was at the discretion of the intensive care physicians. Patients were transfused according to the following guidelines: packed red blood cells (PRBC) were transfused for a hematocrit less than 20% during CPB and for a hematocrit less than 25% after CPB. Platelets were transfused in 5-U sets for ongoing microvascular bleeding despite a normalized ACT or CPB duration > 120 minutes. Fresh frozen plasma (FFP) was given for an INR>1.5 or for continued bleeding only after platelets were given. Transfusion requirements were recorded from the beginning of surgery until hospital discharge.
Assays
Blood samples were obtained to measure ACE activity,PAI-1, t-PA antigen, and inflammatory markers at five time points: 1) after induction of anesthesia and prior to surgical incision (preoperative), 2) at 60 min of CPB, 3) after separation from CPB and administration of protamine (post-bypass), 4) on postoperative day (POD)1, and 5) on POD2. All blood samples were taken from the indwelling arterial line. Not all markers were assayed at each time point. Blood for measurement of PAI-1 and t-PA was collected in vacutainer tubes containing 0.5ml 0.5 M citrate buffer (Tcoag Ireland Ltd., Ireland). All blood samples were collected on ice and centrifuged immediately at 4°C for 20 minutes. Plasma samples were stored at –80°C until the time of assay. PAI-1 antigen (TriniLIZE PAI-1 Antigen, Tcoag Ireland Ltd., Ireland) and t-PA antigen (TriniLIZE t-PA Antigen, Tcoag Ireland Ltd., Ireland)levels were determined using a 2-site enzyme-linked immunosorbent assay as previously described.(33) The PAI-1:t-PA molar ratio (an indicator of fibrinolytic balance) was determined using a molecular weight of 70,000 g/mol for t-PA and 50,000 g/mol for PAI-1. Blood for measurement of bradykinin was drawn into cold anhydrous ethanol (4:1 blood to ethanol). After 1 hour at 4°C, the mixture was centrifuged (15 minutes, 3000 rpm, 10°C) and the supernatant decanted and frozen at –80°C until the time of assay. Bradykinin concentrations were determined using a commercially available enzyme immunoassay (Peninsula Laboratories, Inc. Division of Bachem, San Carlos, CA). A panel of human inflammatory cytokines consisting of IL-6, IL-8, and IL-10 were simultaneously measured using the Human Inflammation Cytokine Cytometric Bead array kit (BD Biosciences Pharmingen, San Diego, CA, USA) by the Vanderbilt University Immunology Core laboratory. Serum ACE activity was determined by a three-step colorimetric assay in which ACE hydrolyzes the substrate p-hydroxybenzoyl-glycyl-L-histidyl-L-leucine, and subsequent reactions lead to the formation of quinoneimine dye, which was measured spectrophotometrically (Fujirebio America Inc., Fairfield, NJ, U.S.A.).
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM) unless otherwise indicated. Based on preliminary data we powered the study to detect a 16 ng/ml difference in postoperative day 1 PAI-1 between either the ARB group or the ACE inhibitor group and the placebo group, assuming a standard deviation of 20 ng/ml. Twenty-six subjects per group provides 80% power with a 0.05 two-sided significance level. Discrete variables were compared among treatment groups using chi-square test or Fisher exact test depending on the number of events for three-group or pair-wise comparisons. Continuous data were compared among treatment groups using one-way analysis of variance (ANOVA) or Kruskal-Wallis test, depending on normality of the data. Correlations were evaluated using the Spearman's rank or Pearson correlation coefficient depending on normality of the data. Longitudinal measures of PAI-1, t-PA, PAI-1:t-PA ratio, and interleukins were analyzed using mixed effects models with fixed effects of drug treatment (placebo, ramipril, candesartan) and time since randomization. We included a random subject effect and a first-order autoregressive process [AR(1)] to adjust for any errors in the mixed effects model. In separate mixed effects models, CPB time, type of surgery, use of aortic cross-clamp, and/or use of steroids in the CPB pump prime were included to adjust for their potential effects on the inflammatory response.
Predictors of post-bypass IL-6 concentrations were additionally assessed using linear regression. CPB time, type of surgery, use of aortic cross-clamp, and use of steroids in the pump prime were included in this model as independent variables. A 2-tailed P value less than 0.05 was considered statistically significant. Statistical analyses were performed with the statistical package IBM SPSS for Windows (Version 19.0, IBM, New York, NY) and SAS for Windows (Version 9, Cary, NC).
Acknowledgements
We would like to thank Jeff Petro for his technical assistance.This research was funded by the NIH [HL085740, HL077389, and HL060609] and supported in part by Vanderbilt CTSA [Grant 1 UL1 RR024975] from the National Center for Research Resources, NIH.
Footnotes
Conflict of Interest/Disclosure
None of the authors has a conflict of interest or disclosure.
Frederic T. Billings Wrote Manuscript
Jorge M. Balaguer Wrote Manuscript
Chang Yu Wrote Manuscript Designed Research Analyzed Data
Patricia Wright Performed Research
Michael R. Petracek Wrote Manuscript
John G. Byrne Wrote Manuscript
Nancy J. Brown Wrote Manuscript Designed Research
Mias Pretorius Wrote Manuscript Designed Research Analyzed Data
References
- 1.Chandler WL, et al. Individual variations in the fibrinolytic response during and after cardiopulmonary bypass. Thromb Haemost. 1995;74:1293–1297. [PubMed] [Google Scholar]
- 2.Pretorius M, Murphey LJ, McFarlane JA, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition alters the fibrinolytic response to cardiopulmonary bypass. Circulation. 2003;108:3079–3083. doi: 10.1161/01.CIR.0000105765.54573.60. [DOI] [PubMed] [Google Scholar]
- 3.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamura T, Wakusawa R, Okada K, Inada S. Elevation of cytokines during open heart surgery with cardiopulmonary bypass: participation of interleukin 8 and 6 in reperfusion injury. Can J Anaesth. 1993;40:1016–1021. doi: 10.1007/BF03009470. [DOI] [PubMed] [Google Scholar]
- 5.Pretorius M, Donahue BS, Greelish JP, Yu C, Roden DM, Brown NJ. Plasminogen activator inhibitor-1 as predictor of postoperative atrial fibrillation following cardiopulmonary bypass. Circulation. 2007;116:I-1–I-7. doi: 10.1161/CIRCULATIONAHA.106.677906. [DOI] [PubMed] [Google Scholar]
- 6.Sander M, et al. Increased interleukin-6 after cardiac surgery predicts infection. Anesth Analg. 2006;102:1623–1629. doi: 10.1213/01.ane.0000215998.21739.48. [DOI] [PubMed] [Google Scholar]
- 7.Liu KD, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 9.Flammer AJ, et al. Angiotensin-converting enzyme inhibition improves vascular function in rheumatoid arthritis. Circulation. 2008;117:2262–2269. doi: 10.1161/CIRCULATIONAHA.107.734384. [DOI] [PubMed] [Google Scholar]
- 10.Fleming GA, Billings FT, Klein TM, Bichell DP, Christian KG, Pretorius M. Angiotensin-converting enzyme inhibition alters the inflammatory and fibrinolytic response to cardiopulmonary bypass in children. Pediatr Crit Care Med. 2011;12:532–538. doi: 10.1097/PCC.0b013e3181fe3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Gaboury CL, Conlin PR, Seely EW, Williams GH, Vaughan DE. Stimulation of plasminogen activator inhibitor in vivo by infusion of angiotensin II. Evidence of a potential interaction between the renin-angiotensin system and fibrinolytic function. Circulation. 1993;87:1969–1973. doi: 10.1161/01.cir.87.6.1969. [DOI] [PubMed] [Google Scholar]
- 12.Brown NJ, Nadeau JH, Vaughan DE. Selective stimulation of tissue-type plasminogen activator (t-PA) in vivo by infusion of bradykinin. Thromb Haemost. 1997;77:522–525. [PubMed] [Google Scholar]
- 13.Huang CD, Tliba O, Panettieri RA, Jr., Amrani Y. Bradykinin induces interleukin-6 production in human airway smooth muscle cells: modulation by Th2 cytokines and dexamethasone. Am J Respir Cell Mol Biol. 2003;28:330–338. doi: 10.1165/rcmb.2002-0040OC. [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ, Krum H, Esler MD. Losartan increases bradykinin levels in hypertensive humans. Circulation. 2005;111:315–320. doi: 10.1161/01.CIR.0000153269.07762.3B. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsumi Y, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brull DJ, Sanders J, Rumley A, Lowe GD, Humphries SE, Montgomery HE. Impact of angiotensin converting enzyme inhibition on post-coronary artery bypass interleukin 6 release. Heart. 2002;87:252–255. doi: 10.1136/heart.87.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radaelli A, et al. Inflammatory activation during coronary artery surgery and its dose-dependent modulation by statin/ACE-inhibitor combination. Arterioscler Thromb Vasc Biol. 2007;27:2750–2755. doi: 10.1161/ATVBAHA.107.149039. [DOI] [PubMed] [Google Scholar]
- 18.Trevelyan J, Brull DJ, Needham EWA, Montgomery HE, Morris A, Mattu RK. Effect of enalapril and losartan on cytokines in patients with stable angina pectoris awaiting coronary artery bypass grafting and their interaction with polymorphisms in the interleukin-6 gene. Am J Cardiol. 2004;94:564–569. doi: 10.1016/j.amjcard.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Kwapisz MM, et al. The effect of intravenous quinaprilat on plasma cytokines and hemodynamic variables during cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18:53–58. doi: 10.1053/j.jvca.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Ruddy MC, Mroczek WJ. Comparison of ramipril and enalapril in patients with essential hypertension. Pharmacotherapy. 1993;13:224–228. [PubMed] [Google Scholar]
- 21.Fogari R, Zoppi A. Antihypertensive drugs and fibrinolytic function. Am J Hypertens. 2006;19:1293–1299. doi: 10.1016/j.amjhyper.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Pahor M, et al. Fosinopril versus amlodipine comparative treatments study: a randomized trial to assess effects on plasminogen activator inhibitor-1. Circulation. 2002;105:457–461. doi: 10.1161/hc0402.102929. [DOI] [PubMed] [Google Scholar]
- 23.Vaughan DE, Rouleau JL, Ridker PM, Arnold JM, Menapace FJ, Pfeffer MA. Effects of ramipril on plasma fibrinolytic balance in patients with acute anterior myocardial infarction. HEART Study Investigators. Circulation. 1997;96:442–447. doi: 10.1161/01.cir.96.2.442. [DOI] [PubMed] [Google Scholar]
- 24.Kerins DM, Hao Q, Vaughan DE. Angiotensin induction of PAI-1 expression in endothelial cells is mediated by the hexapeptide angiotensin IV. J Clin Invest. 1995;96:2515–2520. doi: 10.1172/JCI118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi R, et al. Bradykinin stimulates IL-6 and IL-8 production by human lung fibroblasts through ERK- and p38 MAPK-dependent mechanisms. Eur Respir J. 2000;16:452–458. doi: 10.1034/j.1399-3003.2000.016003452.x. [DOI] [PubMed] [Google Scholar]
- 26.Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339:1285–1292. doi: 10.1056/NEJM199810293391804. [DOI] [PubMed] [Google Scholar]
- 27.Pretorius M, McFarlane JA, Vaughan DE, Brown NJ, Murphey LJ. Angiotensin-converting enzyme inhibition and smoking potentiate the kinin response to cardiopulmonary bypass. Clin Pharmacol Ther. 2004;76:379–387. doi: 10.1016/j.clpt.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Rady MY, Ryan T. The effects of preoperative therapy with angiotensin-converting enzyme inhibitors on clinical outcome after cardiovascular surgery. Chest. 1998;114:487–494. doi: 10.1378/chest.114.2.487. [DOI] [PubMed] [Google Scholar]
- 29.Miceli A, et al. Effects of angiotensin-converting enzyme inhibitor therapy on clinical outcome in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2009;54:1178–1184. doi: 10.1016/j.jacc.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Belluzzi F, Sernesi L, Preti P, Salinaro F, Fonte ML, Perlini S. Prevention of recurrent lone atrial fibrillation by the angiotensin-II converting enzyme inhibitor ramipril in normotensive patients. J Am Coll Cardiol. 2009;53:24–29. doi: 10.1016/j.jacc.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 31.Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2010;26:509–515. doi: 10.1093/ndt/gfq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker PM, et al. Baseline fibrinolytic state and the risk of future venous thrombosis. A prospective study of endogenous tissue-type plasminogen activator and plasminogen activator inhibitor. Circulation. 1992;85:1822–1827. doi: 10.1161/01.cir.85.5.1822. [DOI] [PubMed] [Google Scholar]