Abstract
Object
Craniocervical decompression for Chiari malformation Type I (CM-I) and syringomyelia has been reported to fail in 10%–40% of patients. The present prospective clinical study was designed to test the hypothesis that in cases in which syringomyelia persists after surgery, craniocervical decompression relieves neither the physiological block at the foramen magnum nor the mechanism of syringomyelia progression.
Methods
The authors prospectively evaluated and treated 16 patients with CM-I who had persistent syringomyelia despite previous craniocervical decompression. Testing before surgery included the following: 1) clinical examination; 2) evaluation of the anatomy using T1-weighted MR imaging; 3) assessment of the syrinx and CSF velocity and flow using cine phase-contrast MR imaging; and 4) appraisal of the lumbar and cervical subarachnoid pressures at rest, during a Valsalva maneuver, during jugular compression, and following the removal of CSF (CSF compliance measurement). During surgery, ultrasonography was performed to observe the motion of the cerebellar tonsils and syrinx walls; pressure measurements were obtained from the intracranial and lumbar intrathecal spaces. The surgical procedure involved enlarging the previous craniectomy and performing an expansile duraplasty with autologous pericranium. Three to 6 months after surgery, clinical examination, MR imaging, and CSF pressure recordings were repeated. Clinical examination and MR imaging studies were then repeated annually.
Results
Before reexploration, patients had a decreased size of the CSF pathways and a partial blockage in CSF transmission at the foramen magnum. Cervical subarachnoid pressure and pulse pressure were abnormally elevated. During surgery, ultrasonographic imaging demonstrated active pulsation of the cerebellar tonsils, with the tonsils descending during cardiac systole and concomitant narrowing of the upper pole of the syrinx. Three months after reoperation, patency of the CSF pathways was restored and pressure transmission was improved. The flow of syrinx fluid and the diameter of the syrinx decreased after surgery in 15 of 16 patients.
Conclusions
Persistent blockage of the CSF pathways at the foramen magnum resulted in increased pulsation of the cerebellar tonsils, which acted on a partially enclosed cervical subarachnoid space to create elevated cervical CSF pressure waves, which in turn affected the external surface of the spinal cord to force CSF into the spinal cord through the Virchow-Robin spaces and to propel the syrinx fluid caudally, leading to syrinx progression. A surgical procedure that reestablished the CSF pathways at the foramen magnum reversed this pathophysiological mechanism and resolved syringomyelia. Elucidating the pathophysiology of persistent syringomyelia has implications for its primary and secondary treatment.
Keywords: syringomyelia; therapeutics; radiography; physiology; Arnold-Chiari malformation, Type I
Syringomyelia is more often associated with CM-I than any other etiology.17,18,37 The objective of surgical treatment of syringomyelia associated with the CM-I is to decompress the cerebellar tonsils, eliminate the syrinx, and prevent the progression of myelopathy. A reduced syrinx size, which is associated with disease stabilization, occurs after craniocervical decompression or syrinx fluid shunting. Unlike craniocervical decompression, syrinx shunting does not address the underlying cause of the syringomyelia, requires invasion of the spinal cord, and is prone to delayed side effects and malfunction. However, craniocervical decompression for CM-I and syringomyelia is not universally successful and has been reported to fail in 10%–40% of patients.1,12,34,39,50,51 When decompression fails to reverse syringomyelia, syrinx shunting is often performed as a secondary procedure despite its low long-term success rate.27,45,46
The pathophysiology of persistent syringomyelia following craniocervical decompression has not been examined. One potential benefit of defining this pathophysiology would be to improve the primary treatment of CM-I and syringomyelia in future patients. Another benefit would be to guide the surgeon in his or her selection of additional treatment if primary treatment fails.35 For example, if persistent or recurrent syringomyelia was attributed to the failure of a previous surgery to correct the pathophysiological abnormalities arising from a partial blockage of the CSF pathways at the foramen magnum, it would be reasonable to consider additional surgery at the foramen magnum with the goal of opening the CSF pathways.43,44,50 Alternatively, syrinx shunting might be a logical choice if the syrinx persisted despite eliminating the partial blockage.35 To determine whether the previous surgery effectively opened the CSF pathways at the foramen magnum, the patency of these pathways could be evaluated by 1) measuring their size using anatomical MR imaging; 2) determining the flow rate of CSF using cine phase-contrast MR imaging;13,32 3) measuring pressure transmission using the Queckenstedt test; and 4) comparing various physiological measurements with those made using the same techniques in patients with CM-I malformation and syringomyelia who had not undergone surgery.49 If these studies indicated that the previous surgery did not eliminate the anatomical and physiological block to CSF transmission at the foramen magnum, one might consider treating persistent syringomyelia by performing additional surgery at the foramen magnum to open the CSF pathways and reverse the pathophysiology of syringomyelia. This approach might have lower morbidity and long-term effectiveness than placing a drainage tube in the syrinx via myelotomy. Furthermore, the resolution of syringomyelia after additional surgery that successfully opened the CSF pathways at the foramen magnum would suggest that the previous surgery had not relieved the mechanism of syrinx progression.
Our purpose in the present prospective study was to define the pathophysiology of persistent syringomyelia after failed craniocervical decompression. In particular, we examined the theory that syrinx progression persists after craniocervical decompression because continued obstruction of the CSF pathways at the foramen magnum blocks the rapid efflux and influx of CSF between the head and spine, which normally compensates for brain expansion and contraction during the cardiac cycle. Specifically, we studied whether the basis of persistent or recurrent syringomyelia associated with CM-I is the same mechanism that had been established in patients at the primary presentation of CM-I and syringomyelia.22,42 Thus, the blockage would cause the cerebellar tonsils to be displaced in lieu of CSF, creating a piston effect during the cardiac cycle of the tonsils on the partially enclosed spinal subarachnoid space, which would lead to accentuated cervical subarachnoid pressure waves that would compress the spinal cord from without, direct CSF into the spinal cord,26,31 and produce pulsatile syrinx flow leading to syrinx progression.22,42 Determining the pathophysiology of persistent syringomyelia has considerable implications for its primary and secondary treatment.4,47
Methods
Study Protocol
Sixteen patients with a mean age of 35 ± 14 years (mean ± SD, range 12–58 years) and CM-I and syringomyelia that had not resolved or had recurred following craniocervical decompression were enrolled in the clinical research protocol entitled, “Establishing the Physiology of Syringomyelia” (NINDS 92-N-0226). All patients had progressive myelopathy caused by syringomyelia.
Craniocervical decompression had been performed 1–5 years previously in all patients except 1 who underwent surgery 20 years prior. Patients with ventricular or lumbar shunts were excluded from enrollment. Participants were evaluated before surgery, 1 week after surgery, 3–6 months after surgery, and then annually. The median follow-up interval after surgery was 6.2 years (range 0.7–13.2 years). Test results for the patient group were compared with data from 18 healthy volunteers, which were obtained in the protocol entitled, “Establishing Normal CSF Physiology to Allow Comparison with Syringomyelic CSF Physiology” (NINDS 94-N-0160).22 The Institutional Review Board of the NINDS approved the study. The risks in this protocol were deemed justifiable because the research was directed toward understanding the disease process in the patients and because the research may lead to knowledge that may improve the treatment of future patients with this condition. Informed consent was obtained from each adult patient, the parents of the only minor patient, and each healthy volunteer. All patients were evaluated and treated at the Clinical Center of the National Institutes of Health, Bethesda, Maryland.
Preoperative Evaluation
Clinical Evaluation
Signs and symptoms were graded as absent, mild, moderate, or severe. Grades for symptoms of headache, dysesthetic pain, extremity weakness, sensory loss, and impaired ambulation and for signs of weakness, atrophy, and ataxia were recorded and later compared with data collected after surgery. Ambulation and weakness were graded on standard scales, and the grades were converted to our scale for purposes of this analysis (Table 1). The ambulation scale was as follows: unaffected (normal ambulation), mild (with cane), moderate (with walker), and severe (nonambulatory). The muscle weakness scale set up as unaffected (5/5), mild (4/5), moderate (3/5, antigravity strength), and severe (1–2/5, less than antigravity).7
TABLE 1. Symptoms and signs in 16 patients with persistent syringomyelia*.
| No. of Patients |
||||||||
|---|---|---|---|---|---|---|---|---|
| Before Op |
After Op |
|||||||
| Symptom or Sign | Absent | Mild | Moderate | Severe | Absent | Mild | Moderate | Severe |
| headache | 6 | 2 | 6 | 2 | 13 | 2 | 0 | 1 |
| dysesthetic pain | 4 | 4 | 8 | 0 | 7 | 6 | 3 | 0 |
| subjective weakness | 5 | 6 | 0 | 5 | 11 | 1 | 2 | 2 |
| sensory loss reported | 2 | 0 | 9 | 5 | 1 | 6 | 7 | 2 |
| impaired ambulation | 5 | 6 | 2 | 3 | 9 | 4 | 1 | 2 |
| weakness per examination† | 7 | 4 | 3 | 2 | 11 | 2 | 2 | 1 |
| atrophy | 12 | 1 | 1 | 2 | 12 | 1 | 1 | 2 |
| spasticity | 10 | 1 | 2 | 3 | 12 | 1 | 1 | 2 |
| ataxia | 6 | 7 | 0 | 3 | 10 | 3 | 2 | 1 |
| sensory loss per examination | 2 | 3 | 5 | 6 | 3 | 6 | 3 | 4 |
Weakness: absent, 5/5; mild, 4/5; moderate, 3/5 (antigravity strength); severe, 1–2/5 (less than antigravity strength).
Operative reports from the prior surgery indicated that the dura mater was expanded by duraplasty in 13 cases and neither opened nor expanded in the other 3 cases. Materials reportedly used for duraplasty included allograft fascia lata (3 cases), collagen matrix (3 cases), xenograft (bovine) pericardium (2 cases), synthetic dural substitute (2 cases), autograft pericranium (1 case), autograft fascia lata (1 case), and “artificial dura” (1 case).
Radiological Imaging of Anatomy
Three independent examiners evaluated midsagittal T1-weighted MR images of the posterior fossa and the cervical and thoracic spine. Low-signal areas were measured to determine the maximal anteroposterior diameter of the syrinx, length of the syrinx, anteroposterior diameter of the CSF pathways ventral and dorsal to the neural elements at the foramen magnum, size of the lateral ventricles, and evidence of a connection between the syrinx and the fourth ventricle. A line was drawn on the MR images between the basion and the posteroinferior aspect of the C-2 vertebral body; the distance between this line at the tip of the odontoid and the ventral dura was measured to determine the degree of retroflexion of the dens.19 Since the opisthion had been removed in the previous surgery, the level of the foramen magnum was established by extending a line posteriorly from the basion on the computer workstation (Carestream PACS, Carestream Health, Inc.). The distance between the most caudal extent of the cerebellar tonsils and the level of the foramen magnum was measured. The shape of the cerebellar tonsils (pointed or round) was also recorded.6
Radiological Imaging of Physiology
Cine phase-contrast MR imaging was performed at the foramen magnum and at C5–6. The velocity and flow rates of CSF and syrinx fluid were obtained in the superoinferior direction, with a positive sign denoting a velocity vector in the inferior direction and a negative sign indicating a vector in the superior direction (Signa Advantage Flow Analysis Software, Release 5.4, GE Medical Systems). Regions of interest included the 1) CSF pathway in the ventral half of the foramen magnum or spinal canal (at the C5–6 level), 2) CSF pathway in the dorsal half of the foramen magnum or spinal canal, 3) CSF pathway within the entire foramen magnum or spinal canal, and 4) syrinx. The region of interest for the ventral CSF pathway at the foramen magnum was carefully drawn to exclude the vertebral arteries that traverse that space.
Spinal Subarachnoid Pressure Recording and Physiological Testing
Preoperative testing of lumbar intrathecal and cervical subarachnoid pressures was performed after inserting 22-gauge spinal needles at the C1–2 and L4–5 levels using fluoroscopic guidance. Pressure recordings were performed in 15 of 16 patients (not in the minor patient) and were obtained while the patient was at rest, during Valsalva maneuver, and during jugular compression. The Valsalva maneuver consisted of the patient blowing into a plastic tube at a pressure of 40 mm Hg for 12 seconds.55 Jugular compression was performed by rapidly inflating a blood pressure cuff around the neck to a pressure of 60 mm Hg for 10 seconds; the rate of the rise in pressure (mm Hg/seconds) on compression and the fall in pressure on the release of compression was measured.29,49 Compliance (ml CSF/mm Hg) was measured by dividing the amount of CSF removed (10 ml) by the reduction in mean lumbar intrathecal pressure (mm Hg) that had resulted from CSF removal. Pressures were recorded on the hard disk drive of a Macintosh computer (Apple Computer, Inc.) using a system of Sorenson pressure transducers, a physiological monitor (Spacelabs Medical, Inc.), an analog-to-digital converter board (National Instruments Corp.), and data acquisition software (LabView, National Instruments Corp.). Recordings were displayed using graphical computer software (Kaleidagraph, Synergy Software). Pressure transducers were calibrated with a static water column.
Surgical Procedure
All patients underwent suboccipital craniectomy and upper cervical (C-1 and at least partial C-2) laminectomy. Patients were positioned prone on silicone gel pads that supported the chest and pelvis and allowed the abdomen to hang freely. The neck was slightly flexed. The head was held by a 3-pin Mayfield head holder (OMI, Inc.). Bony decompression at the foramen magnum was taken wide enough to decompress the entire posterior surface of the cerebellar tonsils, as determined by preoperative axial MR imaging measurements and as confirmed by intraoperative ultrasonography, which was performed before opening the dura. Since all patients had previously undergone C-1 laminectomy, identification of normal dura required removing at least part of the superior surface of the lamina of C-2. Subsequent opening of normal dura inferior to the surgical scar tissue provided a corridor into a relatively normal dorsal subarachnoid space and an opportunity to establish normal anatomical relationships before proceeding superiorly into a subarachnoid space that was narrowed by surgically induced adhesion of the dorsal dura, dural graft, and dural suture lines with the underlying spinal cord and cerebellar tonsils. Duraplasty was performed using autologous occipital pericranium.
Based on retrospective studies, craniocervical decompression and duraplasty are regarded as a nonexperimental treatment for persistent syringomyelia after craniocervical decompression for CM-I and syringomyelia.35,43,44,50 Despite support for syrinx shunting at least in some cases10,35 in the literature, it was not considered to be superior to reexploration craniocervical decompression and duraplasty and was not performed in this study.
Testing During Surgery
Physiological Testing
The extent of pressure monitoring during surgery was dictated by patient consent and surgeon discretion. All pressure measurements during surgery were performed under general inhalational anesthesia. Controlled ventilation maintained end-tidal CO2 levels around 35 torr. Positive end-expiratory pressure was not used. During surgery, catheters (NMT Corporation) were placed into the right lateral ventricle in 10 patients and the thecal sac at L4–5 in 13 patients. Baseline recordings were obtained. The rate of elevations in intracranial and lumbar intrathecal pressures in response to manually administered jugular compression and Valsalva (positive pressure ventilation to 40 mm Hg for 12 seconds) was measured.55
Intraoperative Imaging
After suboccipital craniectomy and laminectomy, but before opening the dura, ultrasonographic imaging was performed. Simultaneous CSF pressure (intracranial and lumbar intrathecal), hemodynamic pressure (central venous and radial artery), and electrocardiographic traces were plotted, which allowed us to establish the simultaneous relationship of pressures, syrinx size, and cerebellar tonsillar pulsation during the cardiac cycle. The response of cerebellar and syrinx pulsation to jugular compression, Valsalva, and dural opening was recorded. Ultrasonography was repeated after opening the dura and placing the dural graft.
Postoperative Evaluation
Six days after surgery, while the syrinx was still present, patients underwent cine phase-contrast MR imaging of the cervical spine. Three to 6 months after surgery, patients returned to the National Institutes of Health for anatomical midsagittal T1-weighted MR imaging of the posterior fossa and the cervical and thoracic spine, cine phase-contrast MR imaging, and cervical subarachnoid and lumbar intrathecal pressure testing. Patients were examined, and signs and symptoms were graded as absent, mild, moderate, or severe (Table 1). The grades for each sign or symptom from before and after surgery were compared, and the difference in scores was used to establish if the patient’s condition was improved, unchanged, or worse after surgery as compared with before surgery.7
Statistical Analysis
Data are expressed as the means ± SDs. Values from patients before and after surgery were compared with those from healthy volunteers by using the unpaired Student t-test. Test values from patients before surgery were compared with those after surgery by using the paired Student t-test. A p value ≤ 0.05 was considered significant.
Results
Preoperative Evaluation
Clinical Evaluation
Patients in whom previous craniocervical decompression and duraplasty had failed—that is, all patients in this study—most frequently reported symptoms of sensory loss, dysesthetic pain, weakness, ambulation difficulties, and headache. Signs of central cervical myelopathy and cerebellar dysfunction were often found on examination (Table 1).
Radiological Imaging of Anatomy
The mean anteroposterior diameter of the syringes measured 7.5 ± 3.3 mm, and their length was 17.5 ± 13.5 cm (Fig. 1A and Table 2). The anteroposterior width of the CSF pathways measured 1.2 ± 1.0 mm ventral and 0.8 ± 1.4 mm dorsal to the neural elements at the foramen magnum. These measurements were significantly smaller than the normal ventral value of 6.2 ± 1.8 mm (Fig. 2A) and dorsal value of 5.2 ± 2.6 mm (Fig. 2B; p < 0.0001 in both cases). Three patients had mild ventricular enlargement, and 2 patients had the suggestion of a communication between the fourth ventricle and the syrinx. The distance from the odontoid tip to the ventral dura was 4.7 ± 1.1 mm (range 2.3–6.5 mm) in all patients. The caudal extent of the cerebellar tonsils was 11.8 ± 7.9 mm inferior to the level of the foramen magnum. The cerebellar tonsils had a pointed shape in 11 cases and a rounded shape in the remaining 5. The length of the clivus (3.7 ± 0.4 mm) was shorter than normal (4.3 ± 0.4 mm, p < 0.0001). None of the patients in this study had instability or hypermobility at the craniocervical junction, basilar invagination, or cerebellar ptosis from excessive removal of the suboccipital bone.
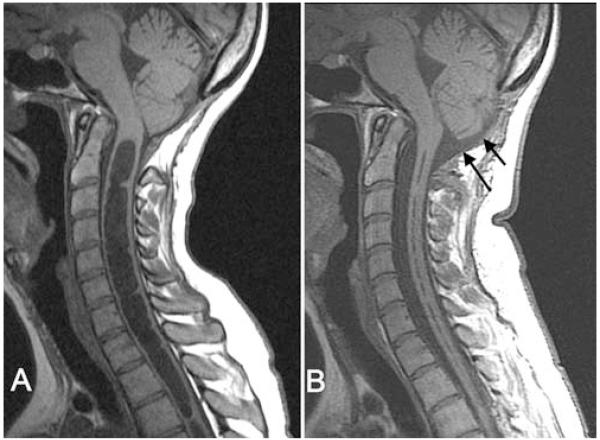
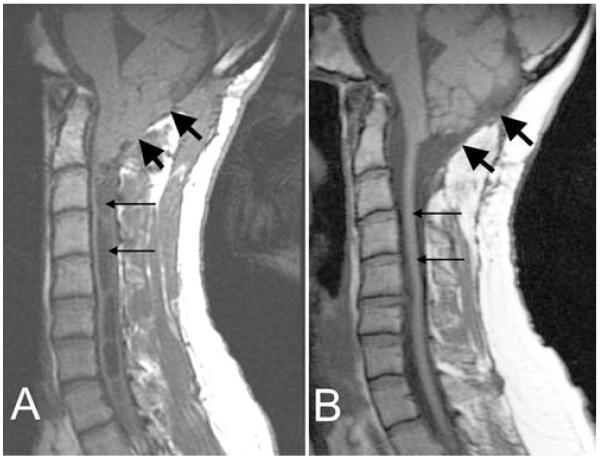
Fig. 1.
Midsagittal T1-weighted MR images demonstrating the posterior fossa and cervical spine before (A) and after (B) reexploration craniocervical decompression. After reexploration (B), the subarachnoid space at the foramen magnum expanded (arrows), the tips of the cerebellar tonsils rounded, and the syrinx diameter decreased dramatically.
TABLE 2. Comparison of MR imaging features in 16 patients with CM-I and persistent syringomyelia and 18 healthy volunteers*.
| Patients |
Healthy Volunteers |
||
|---|---|---|---|
| Feature | Measurement Before Revision | Measurement 3–6 Mos After Op | Measurement |
| syrinx | |||
| diameter (mm) | 7.5 ± 3.3 | 2.6 ± 2.4† | NA |
| length (no. of levels) | 9.8 ± 6.5 | 6.1 ± 5.7† | NA |
| length (cm) | 17.5 ± 13.5 | 10.7 ± 11.8† | NA |
| tonsillar herniation below FM (mm) | 11.8 ± 7.9‡ | 7.5 ± 5.0†‡ | 0.3 ± 1.0 |
| pointed tonsils (no. of cases) | 11 | 1 | |
| rounded tonsils (no. of cases) | 5 | 15 | |
| FM CSF pathway (mm) | |||
| ventral AP diameter | 1.2 ± 1.0‡ | 2.1 ± 1.5†‡ | 6.2 ± 1.8 |
| dorsal AP diameter | 0.8 ± 1.4‡ | 6.8 ± 6.0† | 5.2 ± 0.6 |
| clivus length (cm) | 3.7 ± 0.4‡ | 3.7 ± 0.3‡ | 4.3 ± 0.4 |
Values expressed as the means ± SDs. Abbreviations: AP = anteroposterior; FM = foramen magnum; NA = not applicable.
Significant (p < 0.05) when compared with patient’s parameter before revision surgery (paired t-test).
Significant (p < 0.05) when compared with healthy volunteers (unpaired t-test).
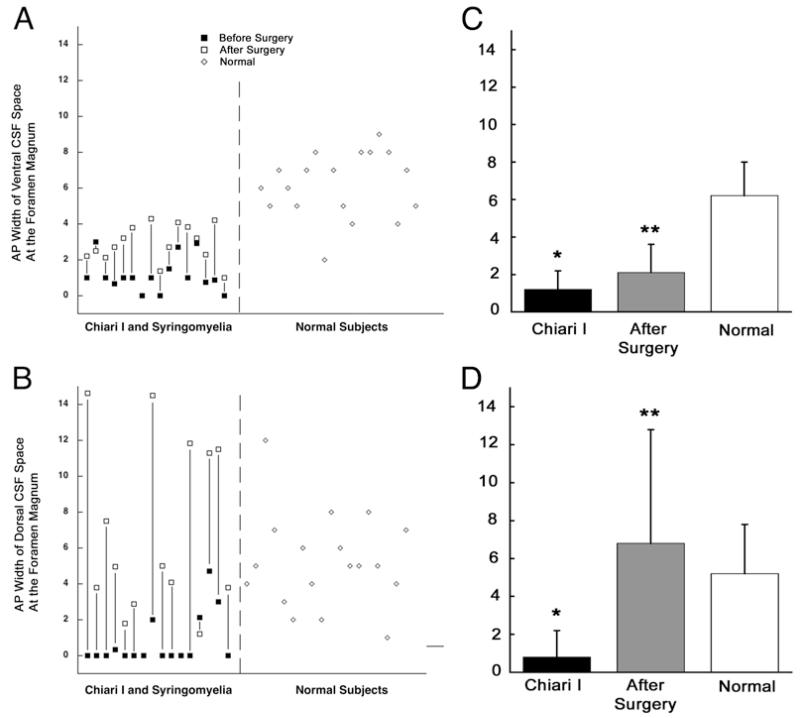
Fig. 2.
Graphs showing the anteroposterior (AP) diameter of the subarachnoid space ventral to the medulla (A) and dorsal to the cerebellar tonsils (B) at the foramen magnum both in patients with CM-I and syringomyelia (before and after surgery) and in healthy volunteers. Bar graphs demonstrating mean values for the widths of ventral (C) and dorsal (D) CSF spaces. Before surgery the ventral CSF space in patients with CM-I was significantly decreased as compared with that in healthy volunteers. After surgery, the CSF space increased significantly as compared with before surgery. Before surgery the dorsal CSF space in patients with CM-I was significantly decreased compared with that in healthy volunteers. After surgery the CSF space increased significantly as compared with before surgery. Error bars on the bar graphs indicate 1 SD above the mean value. *p < 0.001. **p < 0.0014.
Radiological Imaging of Physiology
The velocity (cm/second) of CSF at the foramen magnum was abnormally high ventrally (p < 0.006) but normal dorsally (Table 3). Its velocity across the entire foramen magnum was significantly increased (p < 0.01) compared with normal. The velocity of syrinx fluid was comparable with CSF velocity at the foramen magnum and in the surrounding spinal canal. Peak flow rates of CSF (ml/second) at the foramen magnum were normal ventrally and much less than normal dorsally (p < 0.0001; Table 4). The flow rate of CSF across the entire foramen magnum was normal. Syrinx fluid moved inferiorly during systole and superiorly during diastole (Fig. 3A and B).
TABLE 3. Cerebrospinal fluid velocity measurements in the subarachnoid space and syrinx before and after surgery in 14 patients and in 18 volunteers*.
| Patients |
Healthy Volunteers |
|||
|---|---|---|---|---|
| Feature | Measurement Before Revision |
Measurement 1 Wk Postop |
Measurement 3–6 Mos Postop |
Measurement |
| FM ventral SAS | ||||
|
| ||||
| peak velocity inf direction (systole) | 1.60 ± 1.06† | 1.07 ± 0.53 | 1.40 ± 0.62† | 0.80 ± 0.42 |
| peak velocity sup direction (diastole) | −1.52 ± 0.93† | −1.18 ± 0.54 | −1.43 ± 0.60† | −0.84 ± 0.50 |
| sup-inf velocity change | 3.12 ± 1.86† | 2.26 ± 0.94 | 2.83 ± 0.94† | 1.64 ± 0.85 |
|
| ||||
| FM dorsal SAS | ||||
|
| ||||
| peak velocity inf direction (systole) | 0.22 ± 0.24 | 0.43 ± 0.18 | 0.32 ± 0.45 | 0.33 ± 0.26 |
| peak velocity sup direction (diastole) | −0.40 ± 0.35 | −0.32 ± 0.10 | −0.45 ± 0.56 | −0.51 ± 0.44 |
| sup-inf velocity change | 0.63 ± 0.41 | 0.76 ± 0.23 | 0.77 ± 0.54 | 0.84 ± 0.50 |
|
| ||||
| FM entire SAS | ||||
|
| ||||
| peak velocity inf direction (systole) | 1.04 ± 0.83† | 0.70 ± 0.33 | 0.70 ± 0.52 | 0.49 ± 0.28 |
| peak velocity sup direction (diastole) | −1.06 ± 0.72† | −0.71 ± 0.32 | −0.64 ± 0.36 | −0.59 ± 0.43 |
| sup-inf velocity change | 2.10 ± 1.47† | 1.41 ± 0.61 | 1.33 ± 0.71 | 1.09 ± 0.61 |
|
| ||||
| syrinx | ||||
|
| ||||
| peak velocity inf direction (systole) | 1.69 ± 1.37 | 1.06 ± 0.91 | 0.56 ± 0.71‡ | NA |
| peak velocity sup direction (diastole) | −0.95 ± 0.63 | −0.89 ± 1.09 | −0.35 ± 0.49‡ | NA |
| sup-inf velocity change | 2.63 ± 1.82 | 1.95 ± 1.60 | 0.90 ± 1.17‡ | NA |
|
| ||||
| C5–6 ventral SAS | ||||
|
| ||||
| peak velocity inf direction (systole) | 1.94 ± 1.19 | 1.62 ± 0.78 | 2.26 ± 1.40 | 2.41 ± 1.04 |
| peak velocity sup direction (diastole) | −1.52 ± 1.04 | −1.32 ± 0.78 | −1.61 ± 0.73 | −1.37 ± 0.67 |
| sup-inf velocity change | 3.45 ± 2.12 | 2.94 ± 1.42 | 3.87 ± 1.27 | 3.78 ± 1.53 |
|
| ||||
| C5–6 dorsal SAS | ||||
|
| ||||
| peak velocity inf direction (systole) | 0.74 ± 0.68 | 0.63 ± 0.68 | 1.24 ± 1.27 | 1.26 ± 1.20 |
| peak velocity sup direction (diastole) | −0.46 ± 0.33† | −0.48 ± 0.43 | −0.86 ± 0.68 | −0.93 ± 0.67 |
| sup-inf velocity change | 1.19 ± 0.92 | 1.12 ± 1.05 | 2.10 ± 1.91 | 2.19 ± 1.79 |
|
| ||||
| C5–6 entire SAS | ||||
|
| ||||
| peak velocity inf direction (systole) | 1.37 ± 0.88 | 1.20 ± 0.67† | 2.02 ± 1.09 | 1.90 ± 0.90 |
| peak velocity sup direction (diastole) | −1.05 ± 0.76 | −0.92 ± 0.52 | −1.44 ± 0.60 | −1.10 ± 0.69 |
| sup-inf velocity change | 2.42 ± 1.58 | 2.11 ± 1.09 | 3.46 ± 1.59 | 2.99 ± 1.50 |
Values expressed as the means ± SDs in centimeter/second. Abbreviations: inf = inferior; SAS = subarachnoid space; sup = superior.
Significant (p < 0.05) when compared with health volunteers (unpaired t-test).
Significant (p < 0.05) when compared with measurement obtained before revision surgery (paired t-test).
TABLE 4. Cerebrospinal fluid flow measurements in the subarachnoid space and syrinx before and after surgery in 14 patients and in 18 volunteersw*.
| Patients |
Healthy Volunteers |
|||
|---|---|---|---|---|
| Feature | Measurement Before Revision |
Measurement 1 Wk After Op |
Measurement 3–6 Mos After Op |
Measurement |
| FM ventral SAS | ||||
|
| ||||
| peak flow inf direction (systole) | 0.98 ± 0.74 | 1.08 ± 0.56 | 1.64 ± 0.76†‡ | 1.08 ± 0.52 |
| peak flow sup direction (diastole) | −1.10 ± 0.77 | −1.12 ± 0.46 | −1.73 ± 0.86†‡ | −1.12 ± 0.45 |
| sup-inf flow change | 2.08 ± 1.25 | 2.20 ± 0.88 | 3.37 ± 1.34†‡ | 2.20 ± 0.80 |
|
| ||||
| FM dorsal SAS | ||||
|
| ||||
| peak flow inf direction (systole) | 0.11 ± 0.12† | 0.66 ± 0.61‡ | 0.86 ± 0.94‡ | 0.65 ± 0.56 |
| peak flow sup direction (diastole) | −0.12 ± 0.12† | −0.50 ± 0.52 | −1.02 ± 2.47 | −0.77 ± 0.47 |
| sup-inf flow change | 0.22 ± 0.23† | 1.16 ± 1.12‡ | 1.89 ± 3.13 | 1.42 ± 0.76 |
|
| ||||
| FM entire SAS | ||||
|
| ||||
| peak flow inf direction (systole) | 1.09 ± 0.95 | 1.76 ± 0.86 | 2.12 ± 1.27‡ | 1.57 ± 0.88 |
| peak flow superior direction (diastole) | −1.48 ± 1.09 | −1.85 ± 1.00 | −2.23 ± 2.12 | −1.71 ± 0.64 |
| sup-inf flow change | 2.58 ± 1.59 | 3.62 ± 1.73 | 4.35 ± 2.97 | 3.28 ± 1.09 |
|
| ||||
| syrinx | ||||
|
| ||||
| peak flow inf direction (systole) | 0.58 ± 0.56 | 0.17 ± 0.27‡ | 0.04 ± 0.07‡ | NA |
| peak flow sup direction (diastole) | −0.36 ± 2.72 | −0.12 ± 0.17‡ | −0.03 ± 0.05‡ | NA |
| sup-inf flow change | 0.94 ± 0.76 | 0.29 ± 0.42‡ | 0.07 ± 0.11‡ | NA |
|
| ||||
| C5–6 ventral SAS | ||||
|
| ||||
| peak flow inf direction (systole) | 1.37 ± 1.04 | 1.33 ± 0.80 | 2.25 ± 1.10 | 1.8 ± 0.59 |
| peak flow sup direction (diastole) | −1.15 ± 1.05 | −1.06 ± 0.73 | −1.65 ± 0.74† | −1.02 ± 0.43 |
| sup-inf flow change | 2.52 ± 2.03 | 2.38 ± 1.46 | 3.90 ± 1.70† | 2.82 ± 0.86 |
|
| ||||
| C5–6 dorsal SAS | ||||
|
| ||||
| peak flow inf direction (systole) | 0.30 ± 0.29 | 0.35 ± 0.42 | 0.62 ± 0.90 | 0.67 ± 0.67 |
| peak flow superior direction (diastole) | −0.31 ± 0.50 | −0.26 ± 0.26 | −0.42 ± 0.49 | −0.45 ± 0.29 |
| sup-inf flow change | 0.60 ± 0.72 | 0.61 ± 0.66 | 1.04 ± 1.38 | 1.12 ± 0.95 |
|
| ||||
| C5–6 entire SAS | ||||
|
| ||||
| peak flow inf direction (systole) | 1.51 ± 1.18† | 1.60 ± 1.05† | 2.73 ± 1.13 | 2.46 ± 0.99 |
| peak flow superior direction (diastole) | −1.23 ± 1.14 | −1.21 ± 0.80 | −1.95 ± 0.76 | −1.40 ± 0.70 |
| sup-inf flow change | 2.74 ± 2.27 | 2.82 ± 1.76 | 4.70 ± 1.74 | 3.87 ± 1.57 |
Values expressed as the means ± SD in milliliters/second.
Significant (p < 0.05) when compared with healthy volunteers (unpaired t-test).
Significant (p < 0.05) when compared with value before revision surgery (paired t-test).
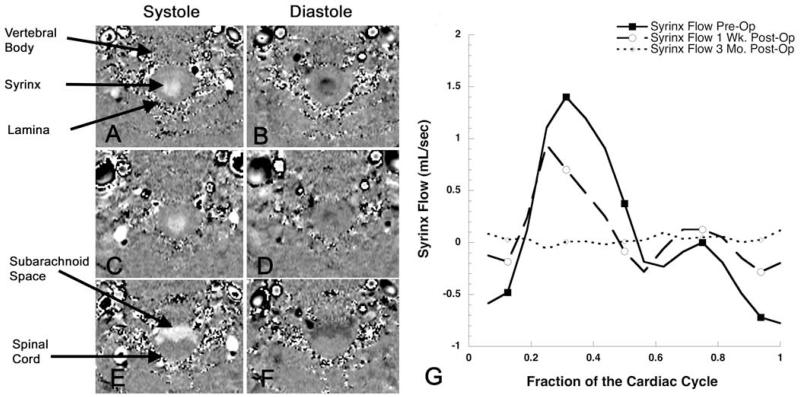
Fig. 3.
Cine phase-contrast MR images obtained in the axial plane at the C5–6 level during 16 segments of the cardiac cycle before (A and B), 1 week after (C and D), and 3 months after (E and F) reexploration surgery. Fluid flow in the inferior direction (during systole) is white and in the superior direction (during diastole) is black. Following surgery that restored the CSF pathways at the foramen magnum, the flow of syrinx fluid (G) during the cardiac cycle steadily decreased, and flow in the spinal subarachnoid space increased as the spinal cord resumed a normal morphology (E).
Spinal Subarachnoid Pressure Recording and Physiological Testing
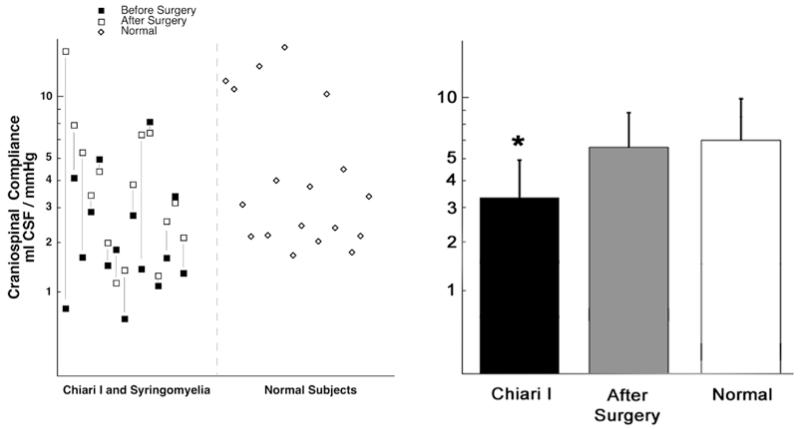
Measuring cervical subarachnoid pressure before surgery was possible in 6 patients and impossible in the other patients because severe narrowing of the dorsal subarachnoid space, as documented on cervical MR imaging, increased the risk of C1–2 puncture. The mean pressure in the cervical subarachnoid space of evaluable patients was elevated (16.7 ± 2.7 mm Hg, normal 11.2 ± 2.5 mm Hg; p < 0.0003; Table 5). Cervical pulse pressure was 2.8 ± 1.0 mm Hg, which was significantly greater than normal (1.6 ± 0.6 mm Hg; p < 0.002). The mean lumbar pressure was also elevated (p < 0.05), but lumbar pulse pressure was not. Cerebrospinal fluid pressure transmission across the foramen magnum was compromised, as shown by the rate of increase in lumbar intrathecal pressure (patients 3.5 ± 2.6 mm Hg/second, volunteers 6.3 ± 2.0 mm Hg/second, p < 0.006) in response to jugular compression (Queckenstedt test) and the rate of decrease in lumbar intrathecal pressure (patients −8.2 ± 5.6 mm Hg/second, volunteers −17.6 ± −8.5 mm Hg/second, p < 0.0002; Fig. 4) following the release of jugular compression. Craniospinal compliance in the patients (3.4 ± 1.7 ml CSF/mm Hg) was significantly decreased when compared with that in healthy volunteers (6.3 ± 2.0 ml CSF/mm Hg; p < 0.02; Fig. 5).
TABLE 5. Pressure recordings in 15 patients with persistent syringomyelia and in 18 healthy volunteers*.
| Measurement | Patients Before Revision Op | Patients After Revision Op (p value) |
Healthy Volunteers (p value) |
|---|---|---|---|
| cervical pressure (mm Hg) | |||
| mean pressure | 16.7 ± 2.7† | 10.9 ± 3.1 (0.11)‡ | 11.2 ± 2.5 (0.0003) |
| pulse pressure | 2.8 ± 1.0† | 2.1 ± 1.4 (0.27)‡ | 1.6 ± 0.6 (0.002) |
| lumbar pressure (mm Hg) | |||
| mean pressure | 13.7 ± 4.4 | 11.0 ± 2.7 (0.03)§ | 11.2 ± 2.3 (0.97) |
| pulse pressure | 1.0 ± 0.7 | 1.0 ± 0.6 (0.78) | 1.0 ± 0.5 (0.97) |
| craniospinal compliance (ml CSF/mm Hg) | 3.4 ± 1.7† | 5.3 ± 3.5 (0.10) | 6.0 ± 4.0 (0.02) |
| jugular compression | |||
| rate of increase (mm Hg/second) | 3.5 ± 2.6† | 5.6 ± 3.3 (0.004)§ | 6.3 ± 2.0 (0.006) |
| peak intrathecal pressure | 32.5 ± 11.6 | 37.3 ± 8.6 (0.10) | 35.4 ± 8.9 (0.42) |
Values expressed as the means ± SDs. One patient (a minor) did not undergo pressure recording.
Significant difference compared with healthy volunteers (unpaired t-test).
Only 6 patients underwent cervical pressure recording after surgery.
Significant difference compared with before revision surgery (paired t-test).
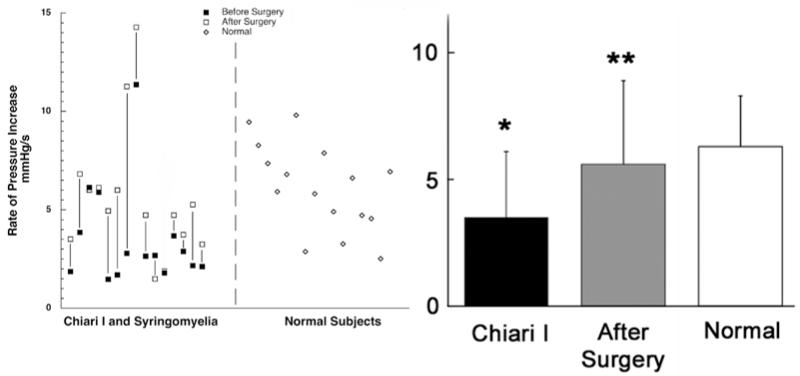
Fig. 4.
Graphs demonstrating the rate of rise in lumbar intrathecal pressure during jugular compression in patients with CM-I and syringomyelia (before and after surgery) and in healthy volunteers. Before surgery the rate of pressure increase in patients with CM-I was significantly decreased compared with that in healthy volunteers. After surgery the rate of elevation in pressure increased significantly as compared with before surgery. *p < 0.006. **p < 0.004.
Fig. 5.
Graphs showing compromised craniospinal compliance before surgery in patients with CM-I and normal compliance after surgery. Compliance (ml CSF/mm Hg) was measured by dividing the amount of CSF removed (10 ml) by the resultant reduction in lumbar intrathecal pressure (mm Hg). Before surgery compliance was significantly decreased in the CM-I group as compared with healthy volunteers. *p < 0.02.
Testing During Surgery
Physiological Testing
The mean intracranial pressure and pulse pressure were 14.4 ± 3.6 mm Hg and 4.5 ± 1.9 mm Hg, respectively, compared with a mean lumbar pressure of 13.7 ± 3.3 mm Hg and pulse pressure of 2.1 ± 1.5 mm Hg. The mean intracranial pressure at baseline was 14.4 ± 3.6 mm Hg and increased to a maximum 23.5 ± 4.1 mm Hg during the Valsalva maneuver. The mean lumbar pressure at baseline was 13.7 ± 3.3 mm Hg and increased to a maximum of 24.1 ± 4.0 mm Hg during the Valsalva maneuver. Note that the Valsalva maneuver failed to produce significant pressure differentials between intracranial and spinal subarachnoid spaces (Table 6). In contrast, during jugular compression there was a more rapid and greater increase in intracranial pressure than in lumbar intrathecal pressure, creating a craniospinal pressure differential of 12.6 mm Hg.
TABLE 6. Intraoperative pressure recordings in 11 patients before and during Valsalva maneuver and jugular compression*.
| Valsalva Maneuver |
Jugular Compression |
||||
|---|---|---|---|---|---|
| Pressure | Baseline Pressure (mm Hg) |
Max Pressure (mm Hg) |
Rate of Rise in Pressure (mm Hg/sec) |
Max Pressure (mm Hg) |
Rate of Rise in Pressure (mm Hg/sec) |
| intracranial | 14.4 ± 3.6 | 23.5 ± 4.1 | 2.8 ± 0.8 | 27.3 ± 8.3† | 4.0 ± 2.3† |
| lumbar | 13.7 ± 3.3 | 24.1 ± 4.0 | 2.9 ± 1.2 | 14.7 ± 4.7 | 2.2 ± 1.1 |
| craniospinal differential^ | −0.7 ± 1.3 | −0.6 ± 1.1 | −0.1 ± 0.9 | 12.6 ± 9.6 | 1.8 ± 2.3 |
Values expressed as the means ± SDs.
Significant (p < 0.05) when compared with lumbar values for maximal pressure and for the rate of rise in pressure.
‡Craniospinal pressure differential is the intracranial pressure minus lumbar pressure.
Intraoperative Imaging and Operative Findings
Ultrasonographic evaluation was performed after making the posterior midline incision, dissecting soft tissue off the posterior fossa and upper cervical lamina, and exposing the dura. On imaging, an intervening CSF space was not present between the dorsal dural membrane and the cerebellar tonsils. The cerebellar tonsils pulsated actively, descending during cardiac systole and ascending during diastole. Syringes that extended to the level of exposure contracted 1–2 mm during cardiac systole. Valsalva did not change syrinx diameter. Jugular compression produced reversible inferior movement of the tonsils and variable compression of the upper pole of the syrinx. On visually inspecting the surgical field, the reason for previous treatment failure was thought to be due to inadequate bone removal at the foramen magnum (6 cases), pseudomeningocele and intradural adhesions (4 cases), extradural bands and intradural adhesions (2 cases), intradural adhesions alone (2 cases), intradural adhesions accompanied by a thin membrane covering the foramen of Magendie (1 case), and intradural adhesions accompanied by 2 arachnoid veils bridging the dorsal subarachnoid space (1 case). Surgical intervention included removing enough bone at the foramen magnum to completely decompress the entire posterior surface aspect of the cerebellar tonsils. Previous surgery resulted in adherence of the arachnoid of the cisterna magna to the dura so that opening the dura resulted in some degree of opening the arachnoid membrane. In cases in which a dural graft was present, adhesions of the graft to underlying arachnoid and cerebellum were densest along the suture line between the graft and surrounding dura. Pseudomeningoceles were associated with an increased reaction between the dorsal dura, arachnoid, and underlying neural elements, regardless of the type of dural substitute that had been placed. Adhesions between the dura or dural graft and the cerebellum, medulla, and spinal cord were cut sharply. In the case with a membrane covering the foramen of Magendie, CSF issued from the foramen after removing this membrane. In places where the graft adhered especially tenaciously to neural structures, the dural graft was cut around points of attachment, leaving small islands of graft on the neural structures, which released the previous graft and allowed it to be removed without traumatizing the neural elements. After removing the preexisting graft, the dural opening was extended superiorly and inferiorly in a Y-shape. The superior portion of the previous scalp incision was extended several centimeters superiorly to obtain a triangular pericranial graft from the occipital area measuring 4–5 cm in length and width. This larger dural graft was placed to recreate a CSF cistern dorsal to the cerebellar tonsils.
Postoperative Evaluation
Clinical Symptoms and Signs
Patients tolerated surgery without complication except for 1 patient who had decreased proprioception in the right upper extremity persisting for a few weeks after surgery. Most patients noted some improvement in ataxia, sensory function, dysesthetic pain, and headache. Neurological function as evaluated by neurological examination remained stable or improved after reoperation in all patients at the 3-month follow-up (Table 1), and in all but 1 patient at a later follow-up. In this patient, dense arachnoiditis was found at surgery, which prevented the dorsal dura from being released from the dorsal surface of the spinal cord and medulla; after surgery the syrinx did not collapse, but the patient remained neurologically stable for more than 4 years. Chronic headaches and gait ataxia related to hydrocephalus developed 4.5 years after surgery; a ventriculoperitoneal shunt was placed, which relieved these symptoms. Eighteen months later, the patient underwent another craniocervical decompression and duraplasty that neither relieved his symptoms nor resolved his syringomyelia.
Radiological Imaging of Anatomy
When evaluated 3–12 months postsurgery, syrinx diameter (presurgery 7.5 ± 3.3 mm, postsurgery 2.6 ± 2.4 mm; p < 0.0001) and length (presurgery 17.5 ± 13.5 mm, postsurgery 10.7 ± 11.8 mm; p < 0.006) had significantly decreased in 15 of 16 patients (Fig. 1B and Table 2). Tonsillar herniation (presurgery 11.8 ± 7.9, postsurgery 7.5 ± 5.0, p < 0.01) significantly improved in 15 of 16 patients, and the cerebellar tonsils assumed a rounded shape in all but 1 patient. The anteroposterior width of the CSF pathways after surgery measured 2.1 ± 1.5 mm ventral and 6.8 ± 6.0 mm dorsal to the neural elements at the foramen magnum (Fig. 2). These measurements were significantly larger than the preoperative values of 1.2 ± 1.0 mm ventral (p < 0.0001) and 0.8 ± 1.4 mm dorsal (p < 0.002).
Radiological Imaging of Physiology
After surgery the total velocity of the CSF at the foramen magnum in both inferior and superior directions became normal (Table 3). Postoperative syrinx velocity was significantly decreased in both inferior (p < 0.003) and superior (p < 0.01) directions when compared with preoperative values.
Ventral flow in the inferior and superior directions at the foramen magnum significantly increased after surgery compared with before surgery (p < 0.03 and p < 0.05, respectively; Table 4). Dorsal flow was significantly higher in the inferior direction when compared with preoperative values. Syrinx fluid flow decreased dramatically in both the inferior and superior directions after surgery (Fig. 3).
Spinal Subarachnoid Pressure Recording and Physiological Testing
Lumbar intrathecal pressure decreased significantly after surgery. The mean and pulse pressure in the cervical subarachnoid space decreased and entered the normal range. Pressure transmission on jugular compression increased to 5.6 ± 3.3 mm Hg/second, which was greater than before surgery and similar to the normal value of 6.3 ± 2.0 mm Hg/second (Table 5 and Fig. 4). Compliance increased after surgery and was similar to normal values (Fig. 5).
Discussion
The basis of persistent syringomyelia in patients with CM-I after craniocervical decompression suggests 1) syringomyelia in these patients might not originate from an obstruction of the CSF pathways at the foramen magnum; 2) the introduction of a mechanism not previously present due to the surgery itself; or 3) an obstruction of the CSF pathways at the foramen magnum that was not effectively relieved by the previous surgery.44,50 The latter possibility offers the simplicity of a single mechanism underlying the syringomyelia in these patients. Establishing the basis for syrinx persistence after craniocervical decompression would shed light on the mechanism of syringomyelia and guide decisions for selecting the treatment in patients with CM-I-related syringomyelia.
Patients in our study manifested central cervical myelopathy that had progressed after a previous surgical procedure. Our investigations using conventional MR imaging, cine phase-contrast MR imaging, and the Queckenstedt test demonstrated that the earlier surgery had failed to open the CSF pathways at the foramen magnum. Normally, CSF displacement from the intracranial subarachnoid cisterns into the cervical subarachnoid space permits the brain to expand within the rigid cranium during cardiac systole. In contrast, patients with CM-I have obstructed CSF transmission across the foramen magnum so that normal brain expansion results in inferior displacement of the cerebellar tonsils during cardiac systole.22,42 Tonsillar descent was documented by ultrasonographic imaging during surgery. Descent of the cerebellar tonsils into a partially enclosed spinal subarachnoid space with low compliance created enlarged cervical subarachnoid pulse pressure waves, which were evident on CSF pressure recordings obtained before surgery. Pulsatile caudal flow of the syrinx fluid, which leads to syrinx progression, was seen on cine phase-contrast MR imaging studies performed before surgery.
Obstructed CSF transmission across the foramen magnum was remedied by removing enough bone to completely decompress the posterior surface of the cerebellar tonsils, separating the previous dural graft and adjacent dura from the posterior surface of the cerebellum and placing a new dural graft of autologous pericranium. The expansile duraplasty created a CSF space dorsal to the cerebellar tonsils and spinal cord and expanded the volume of the posterior fossa. Following surgery that effectively expanded the CSF pathways at the craniocervical junction, conventional MR imaging, cine phase-contrast MR imaging, and the Queckenstedt test demonstrated relief of the anatomical and physiological block at the foramen magnum (Figs. 1-7).3 Relief of the block reduced pulsation of the cerebellar tonsils, increased compliance, reduced cervical pulse pressure (but not lumbar intrathecal pulse pressure) (Table 5), and reduced CSF movement into the syrinx. The pulsatile movement of the syrinx fluid progressively abated after surgery, syrinx size decreased, and neurological signs and symptoms stabilized or improved. The surgical procedure corrected the pathophysiological consequences of the CM-I and reversed persistent syringomyelia without resorting to the placement of shunt tubing into the syrinx to create a conduit for syrinx drainage.27
Fig. 7.
Midsagittal T1-weighted MR images of the posterior fossa and cervical spine before (A) and 8 years after (B) reexploration craniocervical decompression and duraplasty. The CSF spaces at the foramen magnum were absent (A, thick arrows), and a large syrinx distended the cervical spinal cord (thin arrows). Reexploration and craniocervical decompression enlarged the subarachnoid space at the foramen magnum (B, thick arrows) and led to syrinx resolution (thin arrows). Note the reduced diameter of the spinal cord (B), which resulted from previous injury to the spinal cord by the syrinx.
Radiographic studies have documented that the CSF pathways are usually able to expand at the foramen magnum after reexploration decompression and duraplasty. Opening the CSF pathways did not require either resection or coagulation of the cerebellar tonsils.4,16,30,56 A comparison of the pre- and postoperative T1-weighted MR images showed that the expansion of the CSF pathways and posterior fossa resulted in normal cerebellar morphology, with ascent of the cerebellar tonsils and acquisition of a rounded, rather than a pointed, shape (Table 2). It has been reported that CM-I is associated with a small posterior fossa.5,40,41,48,52 The tendency of the cerebellum to return to its normal morphology after a surgery that enlarged the posterior fossa lends support to the contention that CM-I is a secondary deformation that arises from a small posterior fossa and not a primary abnormality of the cerebellum.22,54
Inferior protrusion of the cerebellar tonsils through the foramen magnum narrowed the CSF pathway in patients with CM-I. Previous craniocervical procedures were often ineffective in decompressing the cerebellar tonsils because bone was removed only near the midline or because fibrous bands within or on the external surface of the dura (in cases in which only bone removal had been performed) pressed the dura into the tonsils. These procedures had not adequately expanded the CSF pathways to permit free pulsatile movement of CSF in the subarachnoid space and lead to syrinx resolution. However, these limited procedures did not produce extensive scarring within the subarachnoid space, permitting subsequent surgery to encounter tissue planes that had not been invaded surgically. The presence of a clear CSF space between the spinal cord and dura immediately inferior to the tonsils on MR imaging studies predicted that arachnoiditis would not extend beyond the posterior fossa (Fig. 1A).
Our results indicate that patients with radiographic evidence of a surgically remediable problem, such as inadequate bony decompression at the foramen magnum or an uncomplicated pseudomeningocele, have a high likeli-hood of lasting resolution of syringomyelia and stabilization of neurological deficits with further surgery focused on the foramen magnum. In our study, successful expansion of the CSF pathways led to reduced lumbar intrathecal pressure and increased compliance, effects that accompanied improved CSF flow.22 Authors of other studies have reported that patients with hydrocephalus, CM-I, and syringomyelia may benefit from third ventriculostomy.9,11,21,36,38 On the other hand, shunting of CSF from the lumbar intrathecal space can increase tonsillar herniation in patients with CM-I and lead to cerebellar and medullary dysfunction.57 Because a direct comparison of craniocervical decompression with syrinx shunting was not performed in this study, the superiority of one treatment over the other remains speculative. In patients who have not previously undergone surgical treatment of CM-I and syringomyelia, most comparative studies28,58 demonstrate the superiority of craniocervical decompression over syrinx shunting, with some exceptions.23,24 Syrinx shunts may produce spinal cord dysfunction—acutely from placing the myelotomy or later from delayed tethering of the spinal cord.46,53 The functional half-life of syrinx shunts is only 4 years, which is a relatively short period for patients with long life expectancies.46 Shunts are foreign bodies that are susceptible to infection. A case can be made for selecting craniocervical decompression and duraplasty over syrinx shunting because of the potential risks and limited long-term effectiveness of syrinx shunts. However, these shunts may be an effective and necessary last resort in the treatment of patients in whom extensive arachnoiditis prevents craniocervical decompression from effectively opening the CSF pathways.10
None of the patients in this study had basilar invagination, instability, hypermobility at the craniocervical junction, or prominent (> 9 mm) retroflexion of the dens, which are other causes for the failure of decompressive craniectomy and should be excluded before performing a reexploration.2,15,19,20 In addition, none of the patients had signs of cerebellar ptosis.14,25 We attempted to remove enough bone laterally at the foramen magnum and at the C-1 level to fully decompress the posterior aspect of the cerebellar tonsils. Bone removal in the posterior fossa was carried 2–3 cm superior to the foramen magnum. The dura was opened in the standard Y-shape, starting in the midline inferior to the cerebellar tonsils and extending superiorly to expose the posteroinferior aspect of both cerebellar hemispheres. A triangular duraplasty was placed, tenting across the craniocervical junction, enlarging the subarachnoid space dorsal to the neural elements at the foramen magnum, and increasing the volume of the posterior fossa. Although methods of craniocervical decompression can vary, it is generally agreed that surgical procedures for CM-I and syringomyelia should relieve the impaction of cerebellar tonsils and the obstruction of free CSF circulation at the foramen magnum.22,28,42
Without treatment, central cervical myelopathy would have been expected to progress during the follow-up period.8,33,51 Despite their previously unsuccessful craniocervical decompression, patients accepted the rationale for reexploration surgery and were receptive to the performance of a surgical procedure that did not require fresh exploration of another anatomical region or placement of a syrinx shunt. Most patients noted some improvement in symptoms after surgery, but radiographically demonstrated resolution of the syrinx did not consistently erase all neurological deficits that were present before surgery. After syringes resolved, the diameter of the spinal cord in some patients was smaller than normal, reflecting the irreversible alteration of the spinal cord that resulted from syringomyelia (Fig. 7B). None of the patients with syringomyelia resolution after surgery have had a radiographically demonstrated recurrence of syringomyelia or neurological progression during the follow-up period that averaged more than 6 years. Therefore, this treatment compares favorably with other treatments for failed syringomyelia surgery, such as shunting of syrinx fluid to the subarachnoid space, pleura, or peritoneal cavity.35,58
Finally, the clinical, radiographic, and physiological findings in this study are similar to those reported in an earlier paper, which described patients with CM-I and syringomyelia who had not previously undergone surgical treatment,22 and are consistent with the theory previously proposed for the mechanism of syringomyelia.42 Syringomyelia that persists radiographically and progresses neurologically after craniocervical decompression surgery is evidence that the previous surgery did not relieve obstruction of the CSF pathways at the foramen magnum, which initiates the pathophysiological mechanism of syrinx progression. Thus, a single mechanism is sufficient to explain syringomyelia in both settings. Myelopathy does not progress in the occasional patient who has slow but eventual radiographic syrinx resolution after craniocervical decompression.54 In patients with progressive myelopathy from persistent syringomyelia, treatment should be administered without delay to prevent the development of additional irreversible neurological deficits.
Conclusions
The findings of the physiological and anatomical studies performed before, during, and after craniocervical decompression for persistent CM-I and syringomyelia demonstrated that the previous surgical intervention had not relieved the obstruction of the CSF pathways at the foramen magnum. Patients had active pulsation of the cerebellar tonsils, increased cervical subarachnoid CSF pulse pressure, and longitudinal movement of syrinx fluid during the cardiac cycle. Reoperation enlarged the CSF pathways at the foramen magnum and improved pressure transmission there. Pathophysiological consequences of the blockage of CSF pathways, including increased cervical subarachnoid CSF pulse pressure and syrinx fluid motion, were reduced after surgery. These findings are consistent with a mechanism of syrinx origin that arises outside, rather than inside, the spinal cord. Reoperation in most cases effectively opened the CSF pathways at the foramen magnum, reduced the size of the syrinx, produced symptomatic improvement, and arrested neurological deterioration.
Supplementary Material
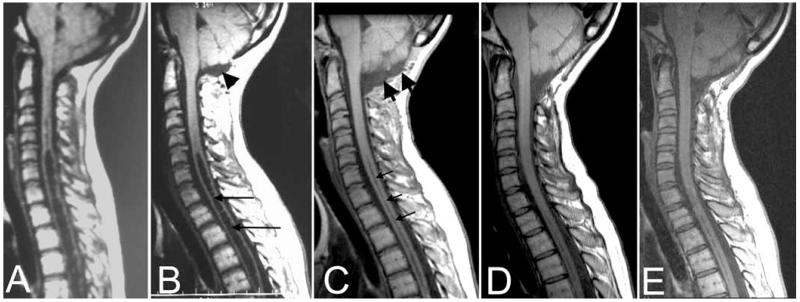
Fig. 6.
Midsagittal T1-weighted MR images of the posterior fossa and cervical spine before (A) and after (B) initial craniocervical decompression and then 3 months (C), 5 years (D), and 10 years (E) after reexploration surgery. After the initial surgery (B), myelopathy progressed and the syrinx (long arrows) did not resolve despite the creation of a CSF space dorsal and inferior to the cerebellar tonsils (arrowhead). At reexploration surgery, a pseudomeningocele was found dorsal to the dura. Reexploration craniocervical decompression removed the pseudomeningocele, enlarged the subarachnoid space at the foramen magnum (C, thick arrows), and led to syrinx resolution (C, thin arrows) that appears to be permanent (D and E).
Acknowledgment
The authors thank Ms. Devera Schoenberg, M.Sc., for her professional editorial review of the manuscript.
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health.
Abbreviations used in this paper
- CM-I
Chiari malformation Type I
- NINDS
National Institute of Neurological Disorders and Stroke
Footnotes
Disclosure
Author contributions to the study and manuscript preparation include the following. Conception and design: Heiss, Oldfield. Acquisition of data: Heiss, Smith, DeVroom, Patronas, Butman, Thomas. Analysis and interpretation of data: Heiss, Suffredini, Oldfield. Drafting the article: Heiss. Critically revising the article: Heiss, Butman, Oldfield. Reviewed final version of the manuscript and approved it for submission: Heiss. Statistical analysis: Heiss, Suffredini. Administrative/technical/material support: Heiss, Suffredini, Smith, DeVroom, Butman, Thomas, Oldfield. Study supervision: Heiss, Oldfield.
References
- 1.Alleyne CH, Jr, Barrow DL. Immune response in hosts with cadaveric dural grafts. Report of two cases. J Neurosurg. 1994;81:610–613. doi: 10.3171/jns.1994.81.4.0610. [DOI] [PubMed] [Google Scholar]
- 2.Aronson DD, Kahn RH, Canady A, Bollinger RO, Towbin R. Instability of the cervical spine after decompression in patients who have Arnold-Chiari malformation. J Bone Joint Surg Am. 1991;73:898–906. [PubMed] [Google Scholar]
- 3.Arora P, Pradhan PK, Behari S, Banerji D, Das BK, Chhabra DK, et al. Chiari I malformation related syringomyelia: radio-nuclide cisternography as a predictor of outcome. Acta Neurochir (Wien) 2004;146:119–130. doi: 10.1007/s00701-003-0180-5. [DOI] [PubMed] [Google Scholar]
- 4.Asgari S, Engelhorn T, Bschor M, Sandalcioglu IE, Stolke D. Surgical prognosis in hindbrain related syringomyelia. Acta Neurol Scand. 2003;107:12–21. doi: 10.1034/j.1600-0404.2003.01357.x. [DOI] [PubMed] [Google Scholar]
- 5.Badie B, Mendoza D, Batzdorf U. Posterior fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery. 1995;37:214–218. doi: 10.1227/00006123-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Barkovich AJ, Wippold FJ, Sherman JL, Citrin CM. Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol. 1986;7:795–799. [PMC free article] [PubMed] [Google Scholar]
- 7.Batzdorf U. Syringomyelia related to abnormalities at the level of the craniovertebral junction. In: Batzdorf U, editor. Syringomyelia: Current Concepts in Diagnosis and Treatment. Williams & Wilkins; Baltimore: 1991. pp. 163–182. [Google Scholar]
- 8.Boiardi A, Munari L, Silvani A, Porta E, Scuratti A, Lodrini S. Natural history and postsurgical outcome of syringomyelia. Ital J Neurol Sci. 1991;12:575–579. doi: 10.1007/BF02336954. [DOI] [PubMed] [Google Scholar]
- 9.Buxton N, Jaspan T, Punt J. Treatment of Chiari malformation, syringomyelia and hydrocephalus by neuroendoscopic third ventriculostomy. Minim Invasive Neurosurg. 2002;45:231–234. doi: 10.1055/s-2002-36195. [DOI] [PubMed] [Google Scholar]
- 10.Cacciola F, Capozza M, Perrini P, Benedetto N, Di Lorenzo N. Syringopleural shunt as a rescue procedure in patients with syringomyelia refractory to restoration of cerebrospinal fluid flow. Neurosurgery. 2009;65:471–476. doi: 10.1227/01.NEU.0000350871.47574.DE. [DOI] [PubMed] [Google Scholar]
- 11.Decq P, Le Guérinel C, Sol JC, Brugières P, Djindjian M, Nguyen JP. Chiari I malformation: a rare cause of noncommunicating hydrocephalus treated by third ventriculostomy. J Neurosurg. 2001;95:783–790. doi: 10.3171/jns.2001.95.5.0783. [DOI] [PubMed] [Google Scholar]
- 12.Depreitere B, Van Calenbergh F, van Loon J, Goffin J, Plets C. Posterior fossa decompression in syringomyelia associated with a Chiari malformation: a retrospective analysis of 22 patients. Clin Neurol Neurosurg. 2000;102:91–96. doi: 10.1016/s0303-8467(00)00073-1. [DOI] [PubMed] [Google Scholar]
- 13.Dolar MT, Haughton VM, Iskandar BJ, Quigley M. Effect of craniocervical decompression on peak CSF velocities in symptomatic patients with Chiari I malformation. AJNR Am J Neuroradiol. 2004;25:142–145. [PMC free article] [PubMed] [Google Scholar]
- 14.Duddy MJ, Williams B. Hindbrain migration after decompression for hindbrain hernia: a quantitative assessment using MRI. Br J Neurosurg. 1991;5:141–152. doi: 10.3109/02688699108998460. [DOI] [PubMed] [Google Scholar]
- 15.Fenoy AJ, Menezes AH, Fenoy KA. Craniocervical junction fusions in patients with hindbrain herniation and syringohydromyelia. J Neurosurg Spine. 2008;9:1–9. doi: 10.3171/SPI/2008/9/7/001. [DOI] [PubMed] [Google Scholar]
- 16.Fischer EG. Posterior fossa decompression for Chiari I deformity, including resection of the cerebellar tonsils. Childs Nerv Syst. 1995;11:625–629. doi: 10.1007/BF00300718. [DOI] [PubMed] [Google Scholar]
- 17.Foster JB, Hudgson P. The pathology of communicating syringomyelia. In: Barnett HJM, Foster JB, Hudgson P, editors. Syringomyelia. Vol. 1. WB Saunders; Philadelphia: 1973. pp. 79–103. [Google Scholar]
- 18.Gardner WJ, Angel J. The mechanism of syringomyelia and its surgical correction. Clin Neurosurg. 1958;6:131–140. doi: 10.1093/neurosurgery/6.cn_suppl_1.131. [DOI] [PubMed] [Google Scholar]
- 19.Grabb PA, Mapstone TB, Oakes WJ. Ventral brain stem compression in pediatric and young adult patients with Chiari I malformations. Neurosurgery. 1999;44:520–528. doi: 10.1097/00006123-199903000-00050. [DOI] [PubMed] [Google Scholar]
- 20.Grahame R, Bird HA, Child A. The revised (Brighton 1998) criteria for the diagnosis of benign joint hypermobility syndrome (BJHS) J Rheumatol. 2000;27:1777–1779. [PubMed] [Google Scholar]
- 21.Hayhurst C, Osman-Farah J, Das K, Mallucci C. Initial management of hydrocephalus associated with Chiari malformation Type I-syringomyelia complex via endoscopic third ventriculostomy: an outcome analysis. J Neurosurg. 2008;108:1211–1214. doi: 10.3171/JNS/2008/108/6/1211. [DOI] [PubMed] [Google Scholar]
- 22.Heiss JD, Patronas N, DeVroom HL, Shawker T, Ennis R, Kammerer W, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg. 1999;91:553–562. doi: 10.3171/jns.1999.91.4.0553. [DOI] [PubMed] [Google Scholar]
- 23.Hida K, Iwasaki Y. Syringosubarachnoid shunt for syringomyelia associated with Chiari I malformation. Neurosurg Focus. 2001;11(1):E7. doi: 10.3171/foc.2001.11.1.8. [DOI] [PubMed] [Google Scholar]
- 24.Hida K, Iwasaki Y, Koyanagi I, Sawamura Y, Abe H. Surgical indication and results of foramen magnum decompression versus syringosubarachnoid shunting for syringomyelia associated with Chiari I malformation. Neurosurgery. 1995;37:673–679. doi: 10.1227/00006123-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Holly LT, Batzdorf U. Management of cerebellar ptosis following craniovertebral decompression for Chiari I malformation. J Neurosurg. 2001;94:21–26. doi: 10.3171/jns.2001.94.1.0021. [DOI] [PubMed] [Google Scholar]
- 26.Itoh Y, Kuwahara N, Sasajima T, Mizoi K, Hatazawa J. Spinal cord edema preceding syringomyelia associated with Chiari I malformation—case report. Neurol Med Chir (Tokyo) 2002;42:410–413. doi: 10.2176/nmc.42.410. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki Y, Hida K, Koyanagi I, Abe H. Reevaluation of syringosubarachnoid shunt for syringomyelia with Chiari malformation. Neurosurgery. 2000;46:407–413. doi: 10.1097/00006123-200002000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Klekamp J, Batzdorf U, Samii M, Bothe HW. The surgical treatment of Chiari I malformation. Acta Neurochir (Wien) 1996;138:788–801. doi: 10.1007/BF01411256. [DOI] [PubMed] [Google Scholar]
- 29.Lakke JPWF. Queckenstedt’s Test. Electromanometric Examination of CSF Pressure on Jugular Compression and Its Clinical Value. Excerpta Medica Foundation; Amsterdam: 1969. [Google Scholar]
- 30.Lazareff JA, Galarza M, Gravori T, Spinks TJ. Tonsillectomy without craniectomy for the management of infantile Chiari I malformation. J Neurosurg. 2002;97:1018–1022. doi: 10.3171/jns.2002.97.5.1018. [DOI] [PubMed] [Google Scholar]
- 31.Levy EI, Heiss JD, Kent MS, Riedel CJ, Oldfield EH. Spinal cord swelling preceding syrinx development. Case report. J Neurosurg. 2000;92(1 Suppl):93–97. doi: 10.3171/spi.2000.92.1.0093. [DOI] [PubMed] [Google Scholar]
- 32.Levy LM. MR identification of Chiari pathophysiology by using spatial and temporal CSF flow indices and implications for syringomyelia. AJNR Am J Neuroradiol. 2003;24:165–166. [PMC free article] [PubMed] [Google Scholar]
- 33.Mariani C, Cislaghi MG, Barbieri S, Filizzolo F, Di Palma F, Farina E, et al. The natural history and results of surgery in 50 cases of syringomyelia. J Neurol. 1991;238:433–438. doi: 10.1007/BF00314649. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Symon L. Surgical management of syringomyelia—current results. Surg Neurol. 1989;32:258–265. doi: 10.1016/0090-3019(89)90227-9. [DOI] [PubMed] [Google Scholar]
- 35.Mazzola CA, Fried AH. Revision surgery for Chiari malformation decompression. Neurosurg Focus. 2003;15(3):E3. doi: 10.3171/foc.2003.15.3.3. [DOI] [PubMed] [Google Scholar]
- 36.Métellus P, Dufour H, Levrier O, Grisoli F. Endoscopic third ventriculostomy for treatment of noncommunicating syringomyelia associated with a Chiari I malformation and hydrocephalus: case report and pathophysiological considerations. Neurosurgery. 2002;51:500–504. [PubMed] [Google Scholar]
- 37.Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, Wolpert C, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery. 1999;44:1005–1017. doi: 10.1097/00006123-199905000-00042. [DOI] [PubMed] [Google Scholar]
- 38.Mohanty A, Suman R, Shankar SR, Satish S, Praharaj SS. Endoscopic third ventriculostomy in the management of Chiari I malformation and syringomyelia associated with hydrocephalus. Clin Neurol Neurosurg. 2005;108:87–92. doi: 10.1016/j.clineuro.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 39.Navarro R, Olavarria G, Seshadri R, Gonzales-Portillo G, McLone DG, Tomita T. Surgical results of posterior fossa decompression for patients with Chiari I malformation. Childs Nerv Syst. 2004;20:349–356. doi: 10.1007/s00381-003-0883-1. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa M, Sakamoto H, Hakuba A, Nakanishi N, Inoue Y. Pathogenesis of Chiari malformation: a morphometric study of the posterior cranial fossa. J Neurosurg. 1997;86:40–47. doi: 10.3171/jns.1997.86.1.0040. [DOI] [PubMed] [Google Scholar]
- 41.Nyland H, Krogness KG. Size of posterior fossa in Chiari type 1 malformation in adults. Acta Neurochir (Wien) 1978;40:233–242. doi: 10.1007/BF01774749. [DOI] [PubMed] [Google Scholar]
- 42.Oldfield EH, Muraszko K, Shawker TH, Patronas NJ. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3–15. doi: 10.3171/jns.1994.80.1.0003. [DOI] [PubMed] [Google Scholar]
- 43.Paré LS, Batzdorf U. Syringomyelia persistence after Chiari decompression as a result of pseudomeningocele formation: implications for syrinx pathogenesis: report of three cases. Neurosurgery. 1998;43:945–948. doi: 10.1097/00006123-199810000-00125. [DOI] [PubMed] [Google Scholar]
- 44.Sacco D, Scott RM. Reoperation for Chiari malformations. Pediatr Neurosurg. 2003;39:171–178. doi: 10.1159/000072467. [DOI] [PubMed] [Google Scholar]
- 45.Schijman E, Steinbok P. International survey on the management of Chiari I malformation and syringomyelia. Childs Nerv Syst. 2004;20:341–348. doi: 10.1007/s00381-003-0882-2. [DOI] [PubMed] [Google Scholar]
- 46.Sgouros S, Williams B. A critical appraisal of drainage in syringomyelia. J Neurosurg. 1995;82:1–10. doi: 10.3171/jns.1995.82.1.0001. [DOI] [PubMed] [Google Scholar]
- 47.Sindou M, Chávez-Machuca J, Hashish H. Cranio-cervical decompression for Chiari type I-malformation, adding extreme lateral foramen magnum opening and expansile duroplasty with arachnoid preservation. Technique and long-term functional results in 44 consecutive adult cases—comparison with literature data. Acta Neurochir (Wien) 2002;144:1005–1019. doi: 10.1007/s00701-002-1004-8. [DOI] [PubMed] [Google Scholar]
- 48.Stovner LJ, Bergan U, Nilsen G, Sjaastad O. Posterior cranial fossa dimensions in the Chiari I malformation: relation to pathogenesis and clinical presentation. Neuroradiology. 1993;35:113–118. doi: 10.1007/BF00593966. [DOI] [PubMed] [Google Scholar]
- 49.Tachibana S, Iida H, Yada K. Significance of positive Queckenstedt test in patients with syringomyelia associated with Arnold-Chiari malformations. J Neurosurg. 1992;76:67–71. doi: 10.3171/jns.1992.76.1.0067. [DOI] [PubMed] [Google Scholar]
- 50.Tubbs RS, Webb DB, Oakes WJ. Persistent syringomyelia following pediatric Chiari I decompression: radiological and surgical findings. J Neurosurg. 2004;100(5 Suppl Pediatr):460–464. doi: 10.3171/ped.2004.100.5.0460. [DOI] [PubMed] [Google Scholar]
- 51.Van Calenbergh F, Hoorens G, Van den Bergh R. Syringomyelia: a retrospective study Part II: diagnostic and therapeutic approach. Acta Neurol Belg. 1990;90:100–110. [PubMed] [Google Scholar]
- 52.Vega A, Quintana F, Berciano J. Basichondrocranium anomalies in adult Chiari type I malformation: a morphometric study. J Neurol Sci. 1990;99:137–145. doi: 10.1016/0022-510x(90)90150-l. [DOI] [PubMed] [Google Scholar]
- 53.Wester K, Pedersen PH, Kråkenes J. Spinal cord damage caused by rotation of a T-drain in a patient with syringoperitoneal shunt. Surg Neurol. 1989;31:224–227. doi: 10.1016/0090-3019(89)90122-5. [DOI] [PubMed] [Google Scholar]
- 54.Wetjen NM, Heiss JD, Oldfield EH. Time course of syringomyelia resolution following decompression of Chiari malformation Type I. J Neurosurg Pediatr. 2008;1:118–123. doi: 10.3171/PED/2008/1/2/118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams B. Simultaneous cerebral and spinal fluid pressure recordings. 2. Cerebrospinal dissociation with lesions at the foramen magnum. Acta Neurochir (Wien) 1981;59:123–142. doi: 10.1007/BF01411198. [DOI] [PubMed] [Google Scholar]
- 56.Williams B. Surgery for hindbrain related syringomyelia. Adv Tech Stand Neurosurg. 1993;20:107–164. doi: 10.1007/978-3-7091-6912-4_4. [DOI] [PubMed] [Google Scholar]
- 57.Williams B. Syringomyelia. Neurosurg Clin N Am. 1990;1:653–685. [PubMed] [Google Scholar]
- 58.Williams B, Sgouros S, Nenji E. Cerebrospinal fluid drainage for syringomyelia. Eur J Pediatr Surg. 1995;5(Suppl 1):27–30. doi: 10.1055/s-2008-1066259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.