Abstract
Streptococcus gallolyticus subsp. gallolyticus (previously called Streptococcus bovis biotype I) infections have long been associated with colorectal cancer (CRC). This work aimed to investigate the CRC-associated humoral immune response to four pilus proteins of this bacterium by newly developed ELISAs. Pilus proteins are interesting diagnostic targets as they are the building blocks of pilin-like structures that mediate bacterial virulence and are readily exposed to the host immune system upon infection. The presence of serum antibodies against these pilus proteins was evaluated in Dutch and American populations. These analyses showed that an immune response to these antigens was specific for clinical S. gallolyticus subsp. gallolyticus infections, but that increased serum antibody titers to multiple pilus proteins in single individuals were rarely observed. However, a multiplex approach based on antibody titers against any of these four antigens resulted in assay sensitivities between 16% and 43% for the detection of early-stage CRC. Together these findings underscore the potential of a multi-antigen approach to complement diagnosis of S. gallolyticus subsp. gallolyticus–associated CRC.
Introduction
The human gastrointestinal tract is the habitat for a large and dynamic bacterial community, which is essential for digestion of food and the control of intestinal epithelial homeostasis (1, 2). Remarkably, although hundreds of bacterial species reside in the human intestinal tract, only the opportunistic pathogen Streptococcus gallolyticus subsp. gallolyticus (S. bovis biotype I), seems to benefit from the presence of premalignant colonic lesions to invade the human body (2–7). In this respect, S. gallolyticus subsp. gallolyticus can be regarded as a whistle-blower for colorectal cancer (CRC) as multiple studies showed that a (precursor of) CRC is detected in 33% to 100% of the individuals that undergo full bowel examination following diagnosis of this infection. Notably, our recent meta-analysis showed that this percentage is far above the prevalence of this disease in the asymptomatic age-matched population (8). S. gallolyticus subsp. gallolyticus is a known causative agent for infective endocarditis (IE), however, due to its mild virulence characteristics this bacterium can only establish a clinical infection in patients with preexisting heart valve abnormalities. Molecular studies suggested that S. gallolyticus subsp. gallolyticus is relatively invisible for the innate immune system due to its inert surface structure (9, 10; our unpublished observations), which implies that S. gallolyticus subsp. gallolyticus may cause subclinical infections in a substantial part of CRC patients (11). The latter idea was supported by our previous finding that the humoral immune response against the ribosomal protein L7/L12 from S. gallolyticus subsp. gallolyticus was significantly increased in early-stage CRC patients (12–14), which is indicative for an increased exposure to this bacterium. However, a drawback of this approach was the fact that the high conservation of this antigen in the bacterial kingdom was associated with a considerable amount of cross-reactivity in the immunoassay (14).
This study aimed at the development of new ELISAs exploiting antigens that are specific for S. gallolyticus subsp. gallolyticus strains. These candidate antigens concerned 4 cell wall peptidoglycan-anchored proteins that form pilin-like structures on the S. gallolyticus subsp. gallolyticus cell surface (15). Two of these proteins, annotated Gallo2178 (major pilin) and Gallo2179 (collagen-binding adhesin) are encoded by the pil1 locus that also encodes a sortase (Gallo2177) which is specifically responsible for the polymerization of these 2 LP×TG into a pilus structure. The pil1 locus is present in the majority of clinical S. gallolyticus subsp. gallolyticus IE isolates and involved in binding to collagen type I, biofilm formation, and virulence in a rat model of experimental endocarditis (to be published elsewhere). Interestingly, collagen I-binding capacity has also been proposed as a distinguished virulence feature of S. gallolyticus subsp. gallolyticus strains to facilitate its adherence to premalignant colonic sites (9). Collagen binding is likely to be mediated by Gallo2179, which contains a collagen-binding domain. The other 2 candidate antigens, Gallo1569 and Gallo2039, are major pilins related to Gallo2178, but encoded by the pil2 and pil3 operons, respectively (16). The pil2 operon has a low conservation among S. gallolyticus subsp. gallolyticus strains, whereas homologous pil3 operons can also be found in Streptococcus infantarius subsp. infantarius strains.
Our current data showed that ELISAs with these 4 antigens were indeed specific for S. gallolyticus subsp. gallolyticus infections. Furthermore, our data showed a highly selective humoral immune response to these antigens in CRC patients. However, a multimarker approach could identify a substantial number of these patients. This finding argues in favor of developing extended multiplex assays based on specific antigens from CRC-associated bacteria as screening tool for CRC.
Materials and Methods
Patient material
Blood samples were derived from the same collections as used before in our studies (14). However, here, we primarily focused on the early stages of CRC (i.e., colorectal adenomas and local stage of colorectal cancer). Serum samples from 37 CRCs, 12 polyp patients (6 adenomas, 2 villous adenomas, and 4 undefined polyps), and 15 patients with a clinical bacterial infection [Escherichia coli. (3), Klebsiella pneumoniae (3), Salmonella typhimurium (3), Streptococcus pneumonia (3), or S. gallolyticus subsp. gallolyticus (3; CRC diagnosed in 1 patient] who had been admitted to the Radboud University Nijmegen Medical Centre (Nijmegen, the Netherlands) were used. Patients suffering from bacterial infections were recognized as such by a positive blood culture and routine microbial typing. As control, serum samples from 27 healthy blood donors (>50 years), who did not undergo colonic evaluation, were used. In addition, plasma samples from 33 CRC, 11 polyp patients, and 47 healthy controls who participated in a population-based case–control study in Metropolitan Detroit, were included as a second independent study population. CRC samples concerned localized disease (stage I or II), with the exception of 7 Detroit cases with unknown stage. All cases in both the Nijmegen and Detroit population underwent colonic evaluation. The use of the samples was approved by the Medical Ethical Committee of Nijmegen/Arnhem (#2006/078) and Wayne State University Human Investigation Committee (#0409000504); informed consent was obtained when required. Serum and plasma samples were stored at −80°C until use.
Antigen overproduction and purification
DNA fragments internal to gallo2179, gallo2178, gallo2039and gallo1569 were produced by PCR using genomic DNA of UCN34 strain as template and the primers described in Supplementary Table S1. These DNA fragments were digested with the appropriate enzymes (NheI/NdeI and BamH1) and cloned into pET28-a(+) (Novagen). The resulting plasmids were introduced into E. coli strain DH5α for sequence analysis or BL21(lDE3) for protein expression. For overproduction, cells were grown aerobically at 37°C until OD600nm = 0.4 after which recombinant gene expression was induced by 1 mmol/L isopropyl β-D-1-thiogalac-topyranoside. Recombinant His-tagged antigens were purified (>95%) under native conditions by affinity chromatography using the Nickel-NTA columns according to the manufacturers’ recommendations (Novagen). Cell lysates and isolated protein fractions were analyzed by SDS-PAGE, and accurate protein concentrations were determined with the BCA system (Pierce) prior to use in ELISAs.
ELISA measurements
Antigens ELISAs were built and conducted as described before (14). A few improvements were made as we observed that background levels due to nonspecific binding of serum immunoglobulins to the ELISA plate could vary significantly between samples. First, after antigen (AG) coating for at least 18 hours at 4°C, the well was extensively blocked by 1% bovine serum albumin (BSA) in PBS-Tween20 (0.1%) for 2 hours at 37°C. In addition, for each antigen-coated well, a duplicate well on the same plate was incubated in coating buffer without antigens and subsequently blocked with 1% BSA (blank). Finally, 1% BSA was added to all incubation buffers that were used during the ELISA measurements. The optical density of horseradish peroxidase–converted 3,3′,5,5′-tetramethylbenzidine substrate was quantified at a wavelength of 450 nm in a spectrophotometer. Samples were measured in duplicate and titers of a specific sample were calculated as the mean OD450AG−OD450blank and expressed as arbitrary S. gallolyticus units (SGU) based on a reference sample from a S. gallolyticus-infected patient that was measured in every plate. Titers were set to zero in case of negative values.
Results
Specific humoral immune response to S. gallolyticus pilus proteins
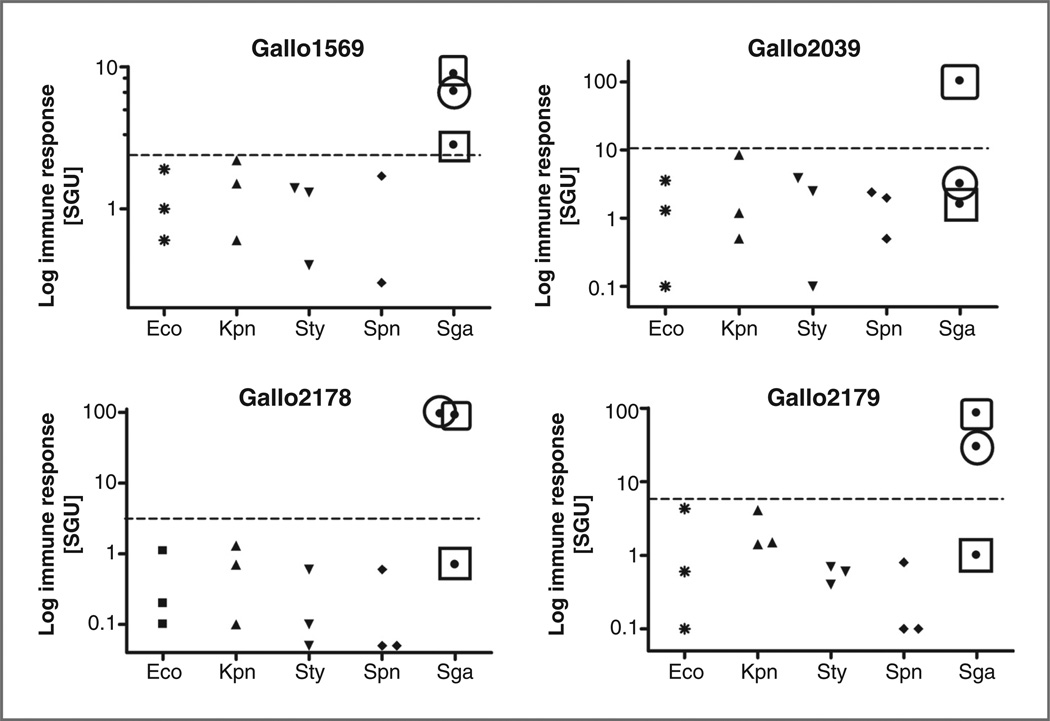
The current study aimed at the development of new ELISAs exploiting antigens that are specific for S. gallolyticus subsp. gallolyticus strains (further designated as S. gallolyticus; ref. 8). To this purpose, purified pilus proteins Gallo1569, -2039, -2178, and -2179 from S. gallolyticus were used as immunoglobulin G (IgG)-capturing antigens in an ELISA setup. To evaluate whether an antibody response to these antigens was specific for S. gallolyticus infections, the corresponding serum IgG levels were determined in serum from patients exhibiting various clinical bacterial infections. As shown in Fig. 1, the anti-Gallo2178 and -2179 IgG levels in serum from 2 out of 3 S. gallolyticus-infected patients were several order of magnitude higher than those in control patients diagnosed with E. coli, K. pneumoniae, S. typhimuriumor S. pneumonia infections. For the Gallo2039 antigen, this was only observed in 1 patient. The anti-Gallo1569 IgG levels were mildly increased in serum from all 3 S. gallolyticus-infected individuals, but they displayed superior discrimination between S. gallolyticus and the other clinical bacterial infections. These data clearly illustrate that an antibody response to these antigens could be a useful clinical marker of S. gallolyticus infection. However, it also points out that this humoral immune response has a high interindividual variation.
Figure 1.
Pilin antibody levels in patients with systemic bacterial infections. Anti-Gallo1569, -Gallo2039, -Gallo2178, and -Gallo2179 IgG levels were measured in serum samples from patients with clinical infections with E. coli (Eco; n = 3), K. pneumoniae (Kpn; n = 3), S. typhimurium (Sty; n = 3) S. pneumonia (Spn; n= 3), or S. gallolyticus (Sga; n= 3). Anti-pilin IgG levels were expressed as arbitrary SGU. The data from S. gallolyticus-infected patients are differentially marked to allow assessment of the intraindividual differences in the responses to these 4 antigens.
Selective humoral immune response to S. gallolyticus pilus proteins in CRC patients
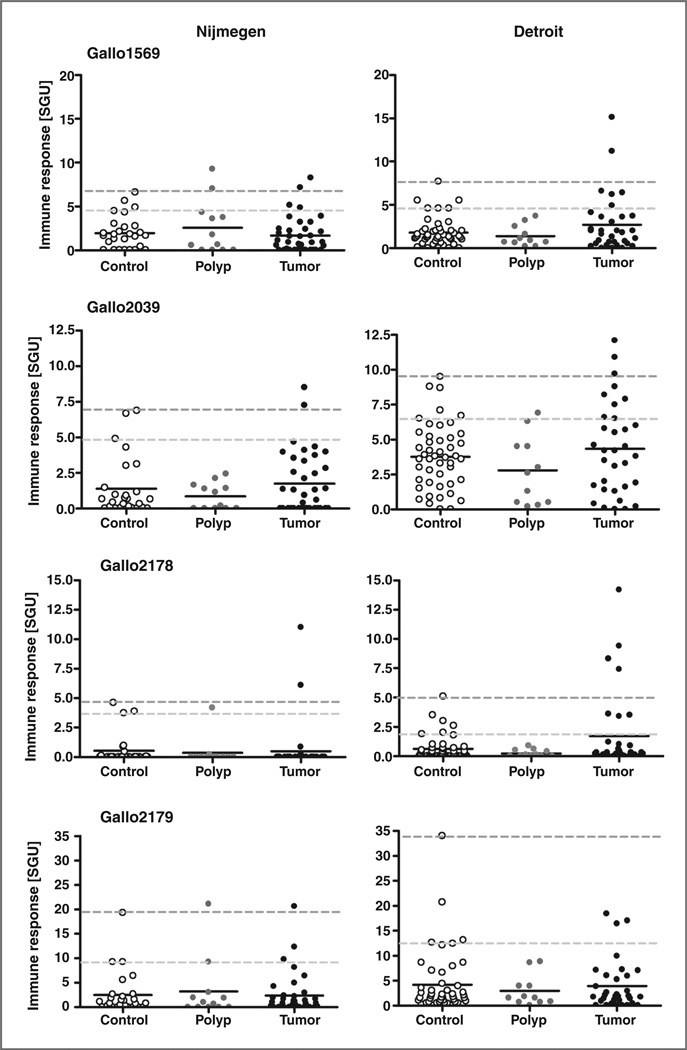
To investigate whether subclinical infections with S. gallolyticus can be monitored in CRC patients by the developed pilus ELISAs, serum/plasma from polyp, early-stage CRC patients, and asymptomatic age-matched individuals from the Nijmegen (serum) and Detroit (plasma) populations (14) were measured. As shown in Fig. 2, the IgG titers in serum/plasma of CRC patients never reached those observed in patients with a clinical S. gallolyticus infection. In addition, no significant differences were observed between the polyp, CRC, and control groups. However, with a few exceptions, the highest IgG titers were observed in serum/plasma from CRC patients.
Figure 2.
Pilin antibody levels in patients with CRC and asymptomatic controls. Anti-Gallo1569, -Gallo2039, -Gallo2178, and -Gallo2179 IgG levels were measured in serum (Nijmegen population) and plasma samples (Detroit population) from polyp and CRC patients and in samples from asymptomatic age-matched controls. Anti-pilin IgG levels were expressed as arbitrary SGU; median levels are indicated. Nijmegen population: controls (n = 27), polyp patients (n = 12), and CRC patients (n = 37). Detroit population: controls (n = 47), polyp patients (n = 11), and CRC patients (n = 33). Cutoff values used to calculate assay specificity as indicated in Table 1 are indicated as dashed lines; upper line reflects 100% specificity (no positive controls) and lower line reflects 78% specificity (i.e., the 95 percentile in each control group) which adjusts for a 22% incidence of polyps in the control group.
The potential of S. gallolyticus pilus antigens for CRC diagnostics
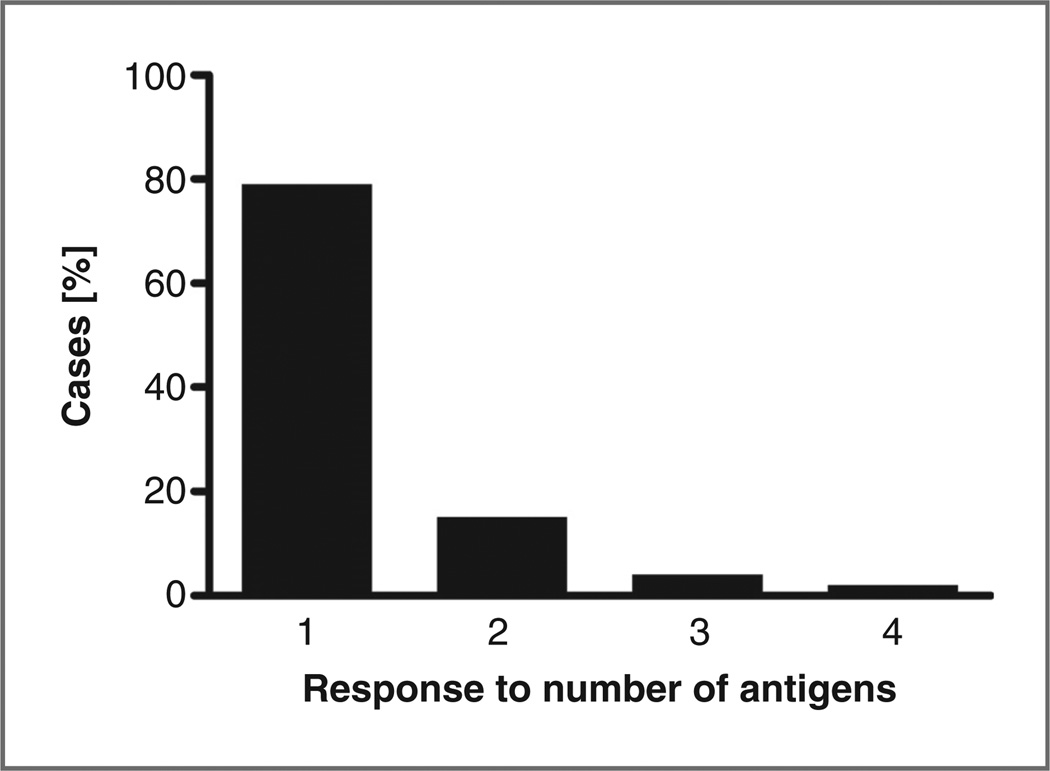
To assess to which extent an antibody response to S. gallolyticus pilus proteins can be instrumental in the diagnosis of CRC, a simple strategy was employed in which the highest IgG level in the control group was used as cutoff value for the diseased groups. This implicates that the assay has a 100% specificity as all controls are classified as not diseased. Table 1A shows that no single antigen has clear superior diagnostic value over another, and the sensitivity for polyps or CRC is only marginal when assessed for single pilin antigens. The highest sensitivity for CRC (4 positive identifications; 9%) was obtained for IgG titers against Gallo2178 in the Detroit population. When a combined approach was used in which antibody titers against any one of the 4 pilus antigens was considered to be diagnostic for polyps or tumors, the sensitivity for both the Nijmegen and Detroit populations was increased to 16%. As about 22% of the asymptomatic elderly population carries polyps (17), which may form a portal of entry for S. gallolyticusthe sensitivity of the ELISAs for CRC was calculated using lower cutoff levels (i.e., 95 percentile in each control group), that correspond to an overall specificity of about 78%. As shown in Table 1B, this yielded an increased sensitivity of 20% in the Nijmegen population and 43% in the Detroit population. Figure 3 shows that most of these positive cases were diagnosed by increased IgG titers against only one of the four antigens. An immune response to 2, 3, or 4 antigens was only detected in 15%, 4%, and 2% of the cases, respectively. These data exemplify the added value of a multimarker approach for the detection of CRC based on bacterial antigens.
Table 1.
Sensitivity and specificity of S. gallolyticus pilin–based ELISAs for the detection of CRCa
| Nijmegen (49 cases and 27 noncases) |
Detroit (44 cases and 47 noncases) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Antigen | Cutoffs | Positive cases/ noncasesa |
Sensitivity (%) |
Specificity (%) |
Cutoffs | Positive cases/ noncasesa |
Sensitivity (%) |
Specificity (%) |
| A | ||||||||
| Gallo1569 | 6.6 | 3/0 | 6 | 100 | 7.7 | 2/0 | 5 | 100 |
| Gallo2039 | 6.9 | 2/0 | 4 | 100 | 9.5 | 3/0 | 7 | 100 |
| Gallo2178 | 4.6 | 2/0 | 4 | 100 | 5.1 | 4/0 | 9 | 100 |
| Gallo2179 | 19.2 | 2/0 | 4 | 100 | 34.0 | 0/0 | 0 | 100 |
| Any of the above | 8/0 | 16 | 100 | 7/0 | 16 | 100 | ||
| B | ||||||||
| Gallo1569 | 4.9 | 5/2 | 10 | 93 | 4.6 | 6/4 | 14 | 92 |
| Gallo2039 | 4.9 | 2/2 | 4 | 93 | 6.7 | 8/4 | 18 | 92 |
| Gallo2178 | 3.7 | 3/2 | 6 | 93 | 2.0 | 7/4 | 16 | 92 |
| Gallo2179 | 9.2 | 5/2 | 10 | 93 | 12.4 | 3/4 | 7 | 92 |
| Any of the above | 10/6 | 20 | 78 | 19/10 | 43 | 78 | ||
CRC includes both polyps and tumor cases; the lower cutoff value in B represents the 95 percentile in each control group.
Number of cases and noncases above the specified ELISA cutoff.
Figure 3.
Distribution of anti-pilin IgG levels. From the cases that were positively diagnosed at a specificity level of approximately 78% (Table 1B), the number of antigens to which IgGs were detected was plotted against percentage of cases.
Discussion
Inspired by the results of our previous studies that were indicative of an increased exposure to S. gallolyticus antigens in early-stage CRC patients (14), we here aimed at the development of ELISAs for the specific detection of human infections with this bacterium and the assessment of their utility for CRC diagnosis.
Four pilus proteins from S. gallolyticus were purified and used to develop ELISAs to measure the corresponding serum IgG levels. Pilus proteins are surface exposed and carry out key functions during infection and are quite specific for S. gallolyticus. This is for instance reflected by the unique collagen-binding capacity of this bacterium mediated by Gallo2179, which is absent in the closely related bacteria S. infantarius subsp. infantarius and S. gallolyticus subsp. macedonicus (9). Notably, homologues of Gallo2039 are also found in related species, which implies that an anti-body response against this antigen could be associated with a decreased specificity for S. gallolyticus subsp. gallolyticus infections. Interestingly, the ELISA based on Gallo2039 did indeed yield the highest average IgG levels, which was most prominently observed in the Detroit population (Fig. 2). For the other 3 antigens, the corresponding IgG levels were in most individuals not significantly increased compared to the background (blank) level, which underscores the specificity of the strong response that was observed in a minority of CRC cases.
The responses in patients with known clinical S. gallolyticus infections show a variable IgG response (Fig. 1), which could be due to the individual status if the immune system and/or strain-specific differences in pilus expression (to be published elsewhere). It should be realized that bacterial pilin expression and the corresponding immune response are likely to be different during local infiltration of the bowel wall (subclinical infection) in CRC patients. However, on the basis of 3 S. gallolyticus-infected patients, we may estimate that no more than 66% of infected patients can be distinguished as such based on increased IgG levels to any of the 4 pilus antigens tested. The maximum sensitivity for the diagnosis of CRC is further decreased by the fact that the presence of S. gallolyticus in the large bowel of CRC patients may not exceed 50% (18), while the presence of genomic DNA of this bacterium in tumor tissue samples was reported to be around 35% (19). On the basis of these findings, the estimated limit of the current 4 S. gallolyticus pilus antigen–based approach for CRC detection would lay between 35% and 50%. Promisingly, our current data are indicative of an assay sensitivity between 20% to 43%, assuming that it is also diagnostic for adenomatous polyps with a prevalence of about 22% in the general elderly population (see Table 1).
Evidently, the above assumptions need to be validated with a properly designed study using serum samples from colonoscopy-controlled samples from healthy individuals, CRC patients as well as from patients with inflammatory bowel disease (IBD), who have reported bowel colonization rates in between healthy controls and CRC patients (4). Furthermore, the reassessment of bowel colonization of S. gallolyticus and its association with IBD, CRC, and adenoma tissues are both subjects of our ongoing investigations. To further increase the detection of CRC with this antigen approach, additional S. gallolyticus-specific antigens should be added to the here presented panel of 4 antigens, together with antigens from other CRC-associated bacteria and/or CRC tumor antigens (20, 21). Finally, the specificity of this multi-antigen approach should be validated in serum from patients having clinical infections with related Gram-positive pathogens (e.g., enterococcal infections), which is also among the subjects of our ongoing investigations. Nevertheless, our current proof of concept study clearly shows that S. gallolyticus pilus proteins are promising antigens to aid in the immunodiagnosis of S. gallolyticus-associated CRC.
Supplementary Material
Acknowledgments
The authors thank Anneke Geurts-Moespot, Nicolai Grebenchtchikov, Guus Kortman, Erwin Wiegerinck, Dorine Swinkels, and our other colleagues from the Department of Laboratory Medicine for stimulating discussions, technical support, and/or collection/selection of serum samples from cases and controls.
Grant Support
A. Boleij was supported by the Dutch Cancer Society (KWF; project KUN 2006-3591), R. Roelofs was supported by the Dutch Digestive Diseases Foundation (MLDS; project WO 10-53), and I. Kato was supported in part by the US NIH (Research grant R01-CA93817).
Footnotes
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Streptococcus bovis: causal or incidental involvement in cancer of the colon? Int J Cancer. 2006;119:xi–xii. doi: 10.1002/ijc.22314. [DOI] [PubMed] [Google Scholar]
- 4.Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of the colon. N Eng J Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- 5.Klein RS, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Streptococcus bovis septicemia and carcinoma of the colon. Ann InternMed. 1979;91:560–562. doi: 10.7326/0003-4819-91-4-560. [DOI] [PubMed] [Google Scholar]
- 6.Corredoira J, Alonso MP, Coira A, Casariego E, Arias C, Alonso D, et al. Characteristics of Streptococcus bovis endocarditis and its differences with Streptococcus viridans endocarditis. Eur J Clin Microbiol Infect Dis. 2008;27:285–291. doi: 10.1007/s10096-007-0441-y. [DOI] [PubMed] [Google Scholar]
- 7.Burnett-Hartman AN, Newcomb PA, Potter JD. Infectious agents and colorectal cancer: a review of Helicobacter pylori, Streptococcus bovis, JC virus, and human papillomavirus. Cancer Epidemiol Biomarkers Prev. 2008;17:2970–2979. doi: 10.1158/1055-9965.EPI-08-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boleij A, van Gelder MMHJ, Swinkels DW, Tjalsma H. Clinical importance of S. gallolyticus infections among colorectal cancer patients: systematic review and meta-analysis. Clin Infect Dis. 2011;53:870–878. doi: 10.1093/cid/cir609. [DOI] [PubMed] [Google Scholar]
- 9.Boleij A, Muytjens CM, Bukhari SI, Cayet N, Glaser P, Hermans PW, et al. Novel clues on the specific association of Streptococcus gallolyticus subsp gallolyticus with colorectal cancer. J Infect Dis. 2011;203:1101–1109. doi: 10.1093/infdis/jiq169. [DOI] [PubMed] [Google Scholar]
- 10.Hirota K, Osawa R, Nemoto K, Ono T, Miyake Y. Highly expressed human sialyl Lewis antigen on cell surface of Streptococcus gallolyticus. Lancet. 1996;347:760. doi: 10.1016/s0140-6736(96)90109-9. [DOI] [PubMed] [Google Scholar]
- 11.Haimowitz MD, Hernandez LA, Herron RM. A blood donor with bacteraemia. Lancet. 2005;365:1596. doi: 10.1016/S0140-6736(05)66462-8. [DOI] [PubMed] [Google Scholar]
- 12.Tjalsma H, Scholler-Guinard M, Lasonder E, Ruers TJ, Willems HL, Swinkels DW. Profiling the humoral immune response in colon cancer patients: diagnostic antigens from Streptococcus bovis. Int J Cancer. 2006;119:2127–2135. doi: 10.1002/ijc.22116. [DOI] [PubMed] [Google Scholar]
- 13.Tjalsma H, Lasonder E, Scholler-Guinard M, Swinkels DW. Shotgun immunoproteomics to identify disease associated bacterial antigens: application to human colon cancer. Proteomics Clin Appl. 2007;1:429–434. [Google Scholar]
- 14.Boleij A, Roelofs R, Schaeps RM, Schüulin T, Glaser P, Swinkels DW, et al. Increased exposure to bacterial antigen RpL7/L12 in early stage colorectal cancer patients. Cancer. 2010;116:4014–4022. doi: 10.1002/cncr.25212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telford JL, Barocchi MA, Margarit I, Rappuoli R, Grandi G. Pili in grampositive pathogens. Nat Rev. 2006;4:509–519. doi: 10.1038/nrmicro1443. [DOI] [PubMed] [Google Scholar]
- 16.Rusniok C, Couvé E, Da Cunha V, El Gana R, Zidane N, Bouchier C, et al. Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J Bacteriol. 2010;192:2266–2276. doi: 10.1128/JB.01659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 18.Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of the colon. N Engl J Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- 19.Abdulamir AS, Hafidh RR, Bakar FA. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer. 2010;9:249. doi: 10.1186/1476-4598-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tjalsma H. Identification of biomarkers for colorectal cancer through proteomics-based approaches. Expert Rev Proteomics. 2010;7:879–895. doi: 10.1586/epr.10.81. [DOI] [PubMed] [Google Scholar]
- 21.Tjalsma H. Hybrid multiplex assays for the early detection of colorectal cancer: a perspective. 2011;35:10–12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.