Abstract
Small leucine-rich proteoglycans (SLRPs), such as decorin and biglycan, regulate the assembly and turnover of collagenous matrix. The aim of the study was to analyse the effect of chronic rosuvastatin treatment on decorin, biglycan and the collagen matrix in ApoE-deficient mice. Twenty-week-old male ApoE-deficient mice received normal chow or 20 mg rosuvastatin/kg × day for 32 weeks. Subsequently, matrix composition was analysed by histochemistry and immunostaining at the aortic root and in innominate arteries of ApoE deficient mice as well as in human carotid endarterectomy specimens. Immunoblotting of proteoglycans was performed from aortic extracts of ApoE-deficient mice. Immunohistochemistry and immunoblotting revealed strongly increased decorin and biglycan deposition in atherosclerotic plaques at the aortic root and in innominate arteries. In contrast, versican and perlecan expression was not changed by rosu-vastatin. Furthermore, matrix metalloproteinase 2 and gelatinolytic activity were decreased in response to rosuvastatin and a condensed collagen-rich matrix was formed. In carotid endarterectomy specimens of statin-treated patients increased decorin and biglycan accumulation was detected as well. Drug treatment did not change low-density lipoprotein (LDL) plasma levels in ApoE-deficient mice and did not significantly affect lipid retention at the aortic root level as demonstrated by oil-red O staining and immunohistochemistry of LDL. Long-term treatment with rosuvastatin caused pronounced remodelling of atherosclerotic plaque matrix characterized specifically by enrichment with SLRPs and formation of a condensed collagen matrix. Therefore, decorin and biglycan might represent novel targets of statin treatment that contribute to a stable plaque phenotype.
Keywords: decorin, biglycan, remodelling, atherosclerotic plaque stability, statins
Introduction
Statins have been shown to reduce the cardiovascular mortality in both patients with elevated serum cholesterol [1] and in patients with low to normal serum cholesterol [2]. The beneficial effect of statins on cardiovascular events is correlated to the strong low-density lipoprotein (LDL) lowering effect [3]. However, statins are believed also to reduce cardiovascular risk independently of their cholesterol-lowering activity [4]. So called pleiotropic effects of statins, such as inhibition of inflammation or increased availability of nitric oxide have been identified in vivo and in vitro[5]. Rosuvastatin, a hydrophilic, hepatoselective statin with strong lipid-lowering activity [6], has been shown to reduce inflammation [7], increase nitric oxide synthase function and reduce atherosclerotic lesion formation in animal models of atherosclerosis [8, 9].
The composition and the turnover of extracellular matrix (ECM) within the vessel wall and atherosclerotic plaques contribute to plaque progression, the risk of plaque rupture, erosion and in turn stenosis and thromboembolic complications [10–12]. The collagen content of the fibrous cap is thought to be a key parameter of plaque stability [13]. It has been convincingly shown that statins increase the collagen content of vascular lesions likely through inhibition of matrix metalloproteinase (MMP) expression and activation leading to decreased collagen proteolysis [14].
However, in addition to synthesis and degradation of collagen, the three-dimensional arrangement of collagen fibrils and the embedded collagen binding proteins contribute to the biological functions of collagen-rich matrices [15]. Decorin and biglycan are secreted, small leucine-rich proteoglycans (SLRPs) that bind to collagen type I and type III thereby modifying collagen fibrillogen-esis and fibril packing [16]. Studies performed in decorin deficient mice have shown that in the absence of decorin, collagen fibril formation is perturbed resulting in irregular thickness of collagen fib-rils and weakened mechanical strength as seen in the dermis of decorin deficient mice [17]. Bigylcan deficient mice also display a collagen related phenotype in bone development and present a disturbance of ossification [18]. From in vitro studies we know that statins cause vascular smooth muscle cells (SMCs) to synthesize proteoglycans with modified glycosaminoglycan chains that display reduced capacity to bind LDL [19]. Therefore it is likely that the degree of proteoglycan accumulation in the plaque and the specific composition of proteoglycans regulate both collagen matrix assembly and lipid retention and thus play an important role in the progression of atherosclerosis. However, the interrelationships between collagen matrix assembly and SLRPs in response to statin treatment or any other drug treatment are still unknown.
Therefore, the present study characterizes the effects of chronic treatment of ApoE-deficient mice with rosuvastatin with respect to proteoglycan composition and its relation to the collagen matrix of atherosclerotic plaques. To mimic the common clinical setting characterized by initiation of long-term statin treatment in individuals already affected by atherosclerosis, ApoE-deficient mice were treated for 32 weeks beginning at the age of 20 weeks.
Methods
Animals
ApoE−/− mice were obtained from Taconic M&B (Bomholt, Denmark). Male mice were kept under routine conditions. Rosuvastatin, provided by AstraZeneca, was pelleted into the chow by ssniff GmbH (Soest, Germany). At the age of 20 weeks mice received either normal chow (control) or 20 mg rosuvastatin/kg × day for 32 additional weeks. All experiments were performed according to the guidelines for the use of experimental animals as given by ‘Deutsches Tierschutzgesetz’ and according to the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health.
Morphometric analysis and image analysis
Plaque area, cell density and SMC content of the fibrous cap were determined in a blinded manner at the aortic root lesions using analysis software (Soft Imaging System, Münster, Germany). If not stated otherwise the aortic root lesions were chosen for analysis because they represent a well defined area with homogenous atherosclerotic plaques. However, key parameters were also investigated in innominate arteries. Analysis of plaque rupture was not attempted. Intima-media ratio was used to compare the extent of neointimal hyperplasia between control and rosuvastatin treated animals. Brightfield images were taken using ColorViewII and AnalySIS 3.2 software (Soft Imaging System) and analysed by ImageJ 1.37v software (National Institute of Health, Bethesda, MD, USA) using the colour deconvolution technique as described earlier [20]. Threshold values were chosen as described before and positive staining was expressed as percentage of total intimal area [20].
Immunohistochemical analysis
The hearts and innominate arteries (brachiocephalic arteries) were paraffin embedded for immunohistochemical staining. SLRPs were detected with polyclonal rabbit antisera against murine biglycan (1:1000, LF 106) and murine decorin (1:1000, LF 113), kindly provided by Larry Fisher (National Institute of Dental and Cranofacial Research, National Institutes of Health, Bethesda, MD, USA). Versican was detected with a polyclonal rabbit antibody (GAG-α domain) from Chemicon (Billerica, MA, USA; 1:50) and per-lecan with a monoclonal rat antibody (Seikagaku, Tokyo, Japan; 1:50). Type I and II collagen cleavage by collagenases was visualized by COL 2 3/4C short polyclonal rabbit antibody (IBEX Diagnistics, Montreal, Quebec, Canada; 1:100). MMP-2 (1:250) and MMP-9 (C-20, 1:50) were detected by polyclonal antibodies from Novus Biologicals (Littleton, CO, USA) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), respectively. All secondary antibodies were obtained from Santa Cruz. Detection was performed with diaminobenzidine (Zytomed, Berlin, Germany) as chromogen. ApoB-48 was detected using K23300R (BioDesign, Saco, ME, USA; 1:50) recognizing mouse apoB-48 and human apoB-100 [21]. In situ zymogra-phy was performed on cryosections as described previously [22].
Histochemical stainings
Collagen accumulation was detected on 3 μm paraffin sections by picro-sirius red staining. Qualitative analysis of collagen deposition was performed with polarized light microscopy [23].
Lipid accumulation in the plaques
Hearts were fixed in 4% neutral buffered paraformaldehyde for 2 hrs and subsequently transferred into 20% sucrose in PBS solution. Hearts were frozen in OCT tissue freezing medium (Jung, Leica Instruments, Nussloch, Germany) in liquid isopentan at −40°C and 14 μm sections of the aortic root were stained with Oil Red O for lipid accumulation.
In situ Zymography
DQ-Gelatin (Invitrogen, Carlsbad, CA, USA) was diluted in 1% UGT-agarose (Sigma-Aldrich, Munich, Germany) in phosphate buffered saline pH 7.45 at 60°C and cooled to 37°C. Subsequently, DQ-Gelatin was applied to the unfixed frozen sections (10 μm) of aortic roots. The gel was allowed to solidify at 4°C in the dark before the sections were incubated in a humid chamber for 72 hrs at 37°C. As controls sections were incubated with the MMP inhibitors TIMP-1 (10 μmol/l, Sigma-Aldrich) or 1.10-phenanthroline (10 mmol/l, Sigma-Aldrich) which inhibited the cleavage of DQ-gelatin (data not shown). Nuclei were stained with DAPI (1 μg/ml).
Western blot
The thoracic and abdominal aorta was dissected between the aortic sinus and the renal arteries and stripped from all adherent tissue including adven-titia. Subsequently the tissue was freeze dried and aliquots representing 10 mg dry weight of total aorta per mouse were used for immunoblotting to allow quantitative comparisons. For this purpose the freeze dried tissue was homogenized with 1 ml extraction buffer containing 4 mol/l guanidine hydrochloride (Sigma), 0.05 mol/l sodium acetate (Sigma) and 2% Triton X100 (Merck, Darmstadt, Germany), 1 mmol/l phenylmethylsulphonylfluo-ride (Sigma), 10 mmol/l N-ethylmaleimide (Sigma), 10 mmol/l EDTA (Sigma). Protein was extracted for 12 hrs at 4°C. The guanidine extract was applied to 4 ml of a Sephadex G50 column (Sigma) which was equilibrated with urea buffer (8 mol/l urea, 0.05 mol/l sodium acetate, 0.5% Triton X100, 0.2 mol/l NaCl) and eluted with 1.5 ml of urea buffer. Subsequently, the samples were purified using a DEAE-anion exchange column, precipitated and processed for Western analysis as described previously [24]. Western blots were performed with a rabbit anti-rat decorin antibody (LF113, 1:1000) and a rabbit anti-rat biglycan antibody (LF106, 1:500) as described [24]. Detection was performed with HRP-conjugated anti-rabbit IgG (1:10.000, Santa Cruz) and an enzyme-linked chemiluminescence procedure (Luminol, Santa Cruz).
Cell culture
Human coronary smooth muscle cells were from Promocell (Heidelberg, Germany). For experiments the SMC were grown in Dulbecco's modified Eagle's medium containing 10% FCS (foetal calf serum), 100 units/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere with 5% CO2 at 37°C. Cells were growth-arrested by serum withdrawal for 24 hrs and were subsequently treated with the compounds to be studied.
Collagen gels were prepared as described by Vernon and Gooden [25]. Briefly, adhesion free gels with final concentration of 1.25 mg PureCol collagen/400 μl gel (Collagen type 1, Nutacon, Leimuiden, Netherlands) populated with 20 × 103 vascular smooth muscle cells (VSMC) per gel, with or without 1 μM of Rosuvastatin were polymerized for 2 hrs at 37°C and harvested for RNA isolation after additional 24 hrs.
Plasma lipid analysis
Blood was collected by heart puncture and anticoagulated with 100 mM EDTA in isotonic sodium chloride solution. Plasma was prepared via cen-trifugation at 850 ×g for 15 min. at 4°C and stored at − 20°C for later analysis. Cholesterol and triglycerides were quantified using enzymatic kits from Randox (Antrim, UK).
Human endarterectomy specimens
For analysis of plaques derived from patients with symptomatic carotid artery stenosis frozen sections of carotid endarterectomy specimens of untreated (n= 10) and statin (atorvastatin, n= 3; pravastatin, n= 1; sim-vastatin; n= 6) treated patients were used. Statin-treated patients were patients with mild hypercholesterolemia and taking statin therapy for at least 1 month before surgery. Patients were part of an observational cohort. The study was approved by the institutional review board of University of Chieti-Pescara, Italy, and the patients gave written consent. The accumulation of human decorin and biglycan was determined by co-immunostaining of decorin (LF 136, 1:200) and biglycan (LF 51, 1:800) detected simultaneously by diaminobenzidine (DAB, Zymed, Germany) as a chromogene. All secondary antibodies were obtained from Santa Cruz.
Statistical analysis
Data are presented as the mean ± S.E.M. Statistical analysis was performed by unpaired t-test or one-way anova followed by comparison of selected pairs (Bonferroni). A value of P < 0.05 was considered significant.
Results
Accumulation of collagen
The experiments were initiated using 20-week-old mice being fed a normal chow diet. At that time the ApoE-deficient mice had already developed atherosclerotic lesions at the aortic root. Treatment of 20-week old ApoE-deficient mice with rosuvastatin (20 mg/kg × day) for 32 weeks did not affect body weight and mortality. Furthermore rosuvastatin had no significant effects on plasma cholesterol levels (control, 562.2 ± 28.8 mg/dl; rosuvastatin, 493. 8 ± 23. 3 mg/dl, n= 11–17). Plasma triglycerides were decreased by rosuvastatin (control, 223.7 ± 32.2 mg/dl; rosuvastatin, 109.3 ± 10.8 mg/dl, P < 0.05, n= 11–17) and free fatty acids remained unchanged (control: 0.64 ± 0.075 mmol/l, rosuvastatin, 0.604 ± 0.051 mmol/l). The cell density in the intima was significantly reduced by rosuvastatin (control, 2052 ± 161.7 cells/mm2; rosuvastatin, 1543 ± 108.1 cells/mm2, n= 10, P < 0.05) which suggested that over time the amount of ECM within the plaque intima increased. Furthermore, intimal areas and inti-mal/medial ratios slightly increased after 32 weeks of treatment with rosuvastatin (data not shown).
The assumption of increased ECM accumulation was confirmed by collagen staining. Collagen as detected by sirius red staining was increased within the atherosclerotic plaques of rosuvastatin treated mice (Fig. 1A–C). To further characterize the changes of collagen matrix, Sirius red staining was analysed under polarized light. In control mice, red polarizing collagen was clearly detectable in the media and also within the plaque intima and fibrous cap. However, the amount of red polarizing collagen was strongly increased in the plaques of ApoE−/− mice treated with rosuvastatin (Fig. 1D–F) indicating a tight arrangement of collagen fibrils.
Fig 1.
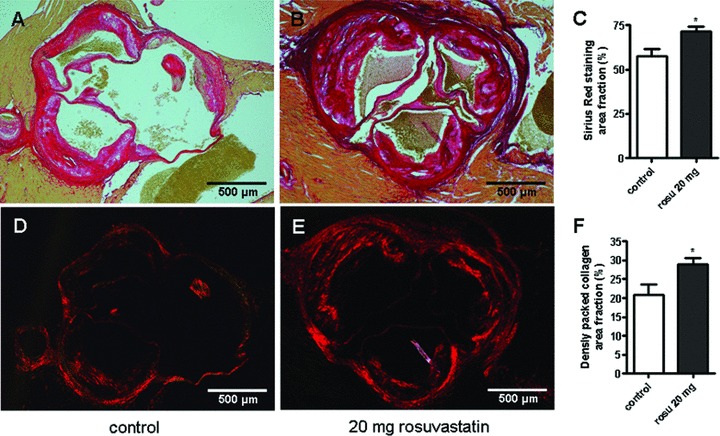
Increased accumulation of collagen in aortic root lesions of ApoE−/− mice in response to rosuvastatin. ApoE−/− mice were chronically treated with rosuvastatin (20 mg/kg × day) from 20 to 52 weeks of age. Sirius red staining of collagen and plaque morphology at the aortic root. (A) control; (B) rosuvastatin treated ApoE−/−; (C) quantitative image analysis. Tightly packed collagen matrix was visualized by birefringence analysis of picrosirus red staining under polarized light of (D) control; (E) rosuvastatin treated ApoE−/−; (F) quantitative image analysis, *P < 0.05; control, n= 10, rosu-vastatin, n= 18.
Rosuvastatin increases accumulation of collagen binding proteoglycans
A mechanism that might increase the packing of collagen fibrils as detected in Fig. 1 is the accumulation of the collagen binding SLRPs, decorin and biglycan. As shown in Fig. 2A–C, decorin accumulation analysed by immunostaining was increased throughout the plaque intima including the entire fibrous cap in rosuvastatin-treated animals. Furthermore, decorin was increased also in the medial layer and in the circumference of the aortic root. The immunohistochemical results were supported by Western blot analysis using aortic extracts (Fig. 2G, H). Decorin presented as a typical double band [26] and was significantly increased in aortic extracts from rosuvastatin treated mice as determined by densitometric scanning of the upper band. In a similar manner to decorin, the accumulation of another collagen binding SLRP, biglycan, was also dramatically increased in the neointima of aortic root lesions (Fig. 2D–F) and in aortic extracts (Fig. 2I, J) as detected by immunohistochemistry and Western blotting, respectively. In contrast to decorin and biglycan, versican and perlecan were not affected by the pharmacological treatment (not shown). In addition, atherosclerotic lesions of innominate arteries that represent vascular lesions were examined. The results were very similar to those achieved from plaques at the aortic root (Fig. 3). Specifically, the amount of tightly arranged collagen was strongly increased as demonstrated by birefringence analysis (Fig. 3D–F) whereas total collagen remained unchanged. Image analysis of biglycan (Fig. 3G–I) and decorin (Fig. 3J–L) showed increased signals of both SLRPs that were even more pronounced as at the aor-tic root (compared to Fig. 2).
Fig 2.
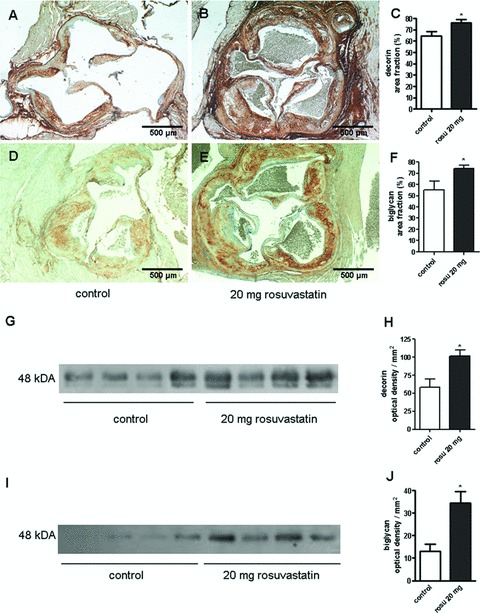
Accumulation of decorin and biglycan was increased in aortic root plaques and in aortic extracts in response to rosuvastatin. (A, D) control and (B, E) rosuvastatin. (A, B) decorin immonostaining; (D, E) biglycan immunostaining; (C, F) quantitative image analysis of intimal SLRP staining; *, P < 0.05; control, n= 8, rosuvastatin, n= 10. (G–J) to verify the immunostaining Western blotting of both SLRPs was performed from aortic extracts normalized to mg dry weight of total aorta. (G, I) Western blots, (G) decorin and I, biglycan; (H, J) quantitative densitometry, n= 4; *, P < 0.05.
Fig 3.
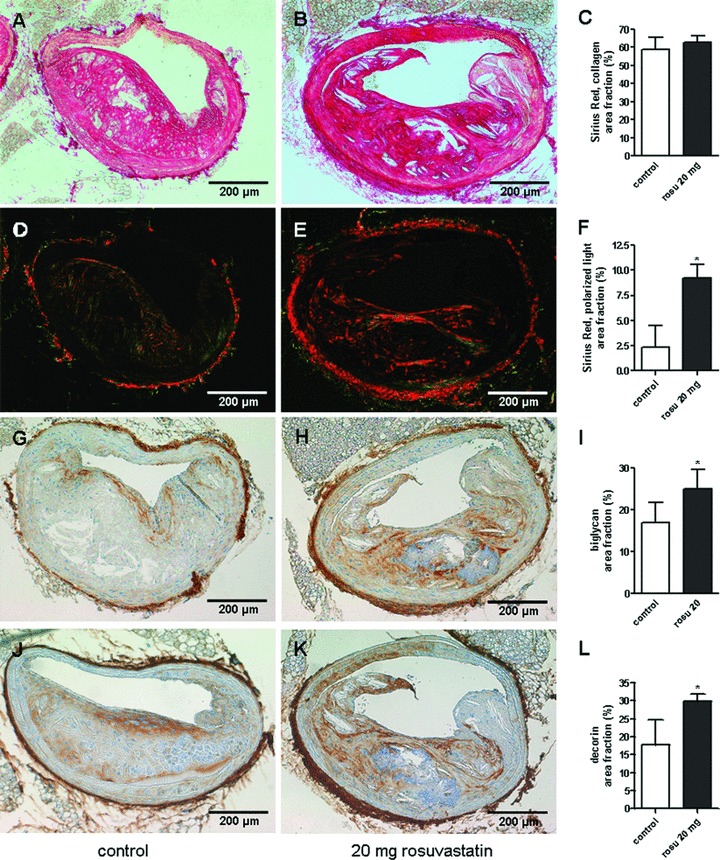
Condensed collagen matrix and increased accumulation of SLRPs in innominate arteries of rosuvastatin treated ApoE−/− mice. Collagen and collagen matrix arrangement were analysed by picrosirius red staining and birefringence analysis, respectively. In addition decorin and biglycan were detected by immunohistochemistry in plaques of innominate arteries. (A, D, G, J) control and (B, E, H, K) rosuvastatin. (A–F) picrosirius red staining and quantitative image analysis. (D, E) picrosirius red staining viewed under polarized light. (G–I) biglycan immunostaining and quantitative image analysis. (J–L) decorin immunostaining and quantitative image analysis; control, n= 4, rosuvastatin, n= 11, *, P < 0.05.
Decreased cleavage of collagen
MMPs cleave both collagen and SLRPs. Therefore to address a possible mechanism for increased collagen and SLRP accumulation we determined the expression and activity of MMPs. Collagen epitopes generated by cleavage of type I collagen, were visualized by immunohistochemistry using an antibody detecting collagen neoepitopes (COL 2 3/4C). As depicted in Fig. 4A–C the specific collagen neoepitope staining in the aortic root was markedly reduced after rosuvastatin treatment, indicating reduced turnover of collagen by MMPs. Furthermore the ratio of collagen neoepi-topes and total collagen was markedly reduced (Fig. 4D). In situ zymography was used to visualize the gelatinolytic activity within the plaques on frozen sections. As depicted in Fig. 4D–F the gelatinolytic activity was considerably reduced in rosuvastatin treated animals. In accordance with the reduced collagen cleavage immunohistochemistry revealed significantly reduced expression of MMP-2, whereas MMP-9 expression levels were not altered (Fig. 4H, I).
Fig 4.
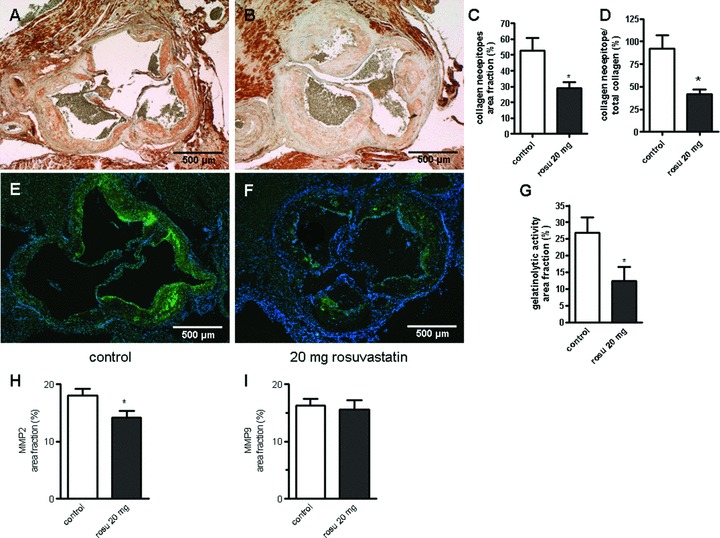
Rosuvastatin inhibits collagen degradation and gelatinolytic activity in atherosclerotic plaque of ApoE−/− mice. Collagen cleavage was demonstrated by staining of collagen neoepitopes originating from MMP mediated cleavage in aortic root plaques. (A) control and (B) rosuvastatin. (C) quantitative image analysis, n= 8; *P < 0.05. (D) ratio between collagen neoepitopes and total collagen as determined by picrosirius red staining (Fig. 1). (E, F) MMP activity as detected by in situ zymography on cryosections in control versus rosuvastatin. (G)quantitative image analysis. (H, I)expression of MMP-2 (H) and MMP-9 (I) was analysed by immunostaining, quantitative data from the image analysis is provided; n= 8–9, *, P < 0.05.
Regulation of SLRP expression by rosuvastatin and polymeric collagen in vitro
In order to investigate whether direct effects of rosuvastatin on biglycan and decorin expression in VSMC could account for the increased accumulation of these proteoglycans in atherosclerotic plaques, VSMC were cultured on plastic and treated with 1 μmol/l rosuvastatin for 5 days in vitro. As determined by real-time RT-PCR analysis rosuvastatin caused no significant change in the expression of decorin, biglycan, collagen type 1 and collagen type 3 (Fig. 5A, B). Alternatively, we suggested that the rosuvastatin induced collagen-rich ECM might induce up-regulation of SLRPs in VSMC. To test this hypothesis VSMC were cultured in a three dimensional polymeric collagen gel and the expression of biglycan and decorin was compared to VSMC on plastic. Indeed both biglycan and decorin were up-regulated in VSMC cultured in a polymeric collagen gel as compared to monolayer cultures on plastic (Fig. 5C). In addition, we analysed if rosuvastatin affected the SLRP expression in collagen gels, but detected no further increase (Fig. 5D) compared to polymeric collagen gel alone.
Fig 5.
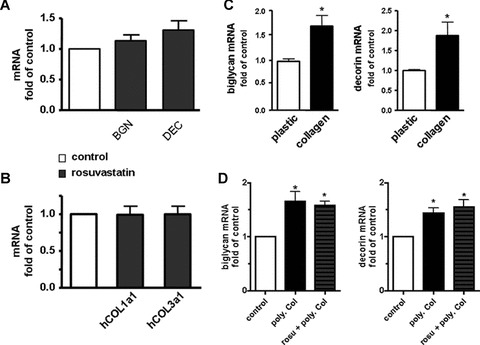
Three dimensional collagen gels cause increased bigly-can and decorin expression. To investigate whether rosuvastatin or three dimensional collagen type 1 matrix affect SLRP expression in vitro experiments were performed. (A)–(B) human coronary SMC were cultured on plastic and incubated with or without 1 μM rosuvastatin in normal growth medium (5% FCS) for 5 days. Subsequently mRNA expression of (A) biglycan, decorin and (B) human collagen type 1 and 3 were determined by real-time RT-PCR, n= 10. (C), human coronary SMC were cultured either on plastic under routine conditions or in a gel of polymeric collagen type 1 (20,000 cells/400 mm3), after 24 days mRNA expression of biglycan and decorin was determined by real-time RT-PCR and expressed as fold of the monolayer cultures on plastic; n= 4; *, P < 0.05. (D), to investigate whether the SLRP expression in collagen gels is further influenced by rosu-vastatin, the experiments shown in C were repeated in the presence or absence of 1 μM rosuvastatin. n= 8–11; *, P < 0.05 versus control.
Rosuvastatin does not affect lipid retention
Because proteoglycans play an important role in the retention of lipids during the initial stages of atherosclerosis we determined whether the observed changes in matrix composition in response to rosuvastatin affected also lipid retention. Oil Red O staining revealed a trend towards reduced lipid accumulation at the aortic root of rosuvastatin treated animals (Fig. 6). Furthermore, LDL in the plaque matrix was detected by immunohistochemical staining of murine ApoB-48 and showed no change (Fig. 6). This finding appears to be important because it argues against the possibility that accumulation of biglycan and decorin in late stage atherosclerosis could cause increased retention of LDL in the collagenous plaque matrix.
Fig 6.
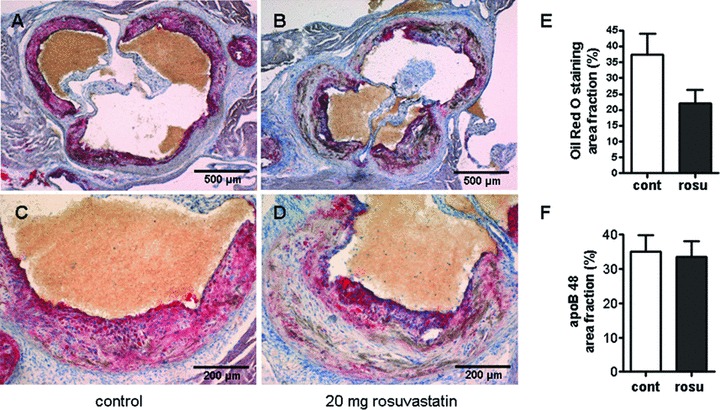
(A–D) Retention of lipids was not affected by rosuvastatin in aortic roots of ApoE−/− mice. Oil Red O staining and immunostaining of apoB48 of plaques at the aortic root was performed in order to analyse whether the changes in matrix composition were associated with effects on lipid retention. (A)(C) control; (B, D) rosuvastatin, (E) quantification of lipid staining by image analysis. (F) quantification of Apo B48 immunostaining; n= 7; *, P < 0.05.
Statins induce SLRPs in human atherosclerosis
Atherectomy samples of patients treated with statins were compared with atherectomy samples of patients not receiving statins. Immunostaining for decorin and biglycan revealed only a trend to increased accumulation of both decorin and biglycan in the ECM of atherosclerotic plaques of patients receiving statins (not shown). To address the possibility that the cumulative amounts of decorin and biglycan were affected co-immunostaining of both SLRPs on the same section was performed. Indeed the co-immunostaining revealed a significant increase of decorin and biglycan in the group receiving statins (Fig. 7). Furthermore, co-localization of SLRPs to the collagenous matrix was observed and the packing of collagen was increased in these samples as demonstrated by birefringence analysis of sirius red stained sections.
Fig 7.
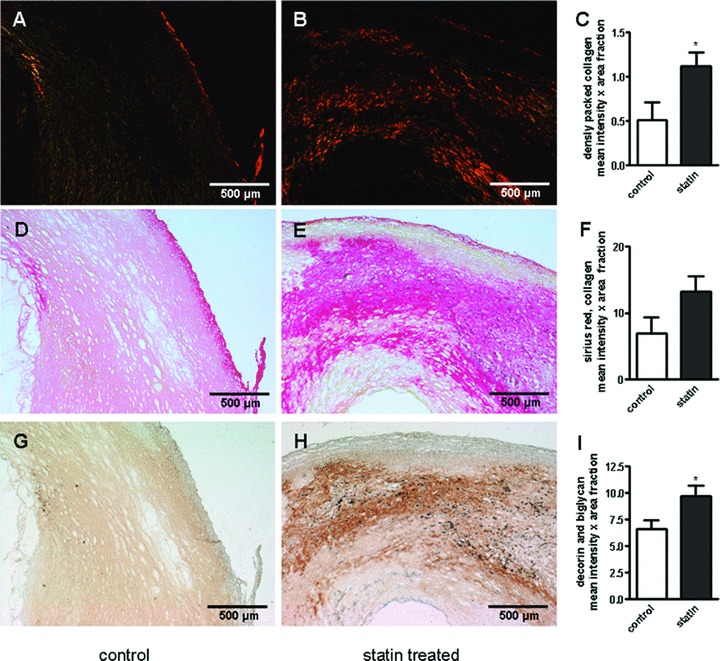
Condensed collagen matrix and increased SLRPs in statin treated endarterectomies. Decorin, biglycan and collagen in human atherosclerosis after statin treatment. (A, D, G) control and (B, E, H) specimens derived from patients treated with statins for at least one month. (A–F) picrosirius red staining of human carotid endarterectomies. (A, B) viewed under polarized light. (C, F) quantitative image analysis. (G, H) co-immunostaining of bigly-can and decorin, (I) quantitative image analysis; n= 10; *, P < 0.05.
Discussion
The increase in collagen accumulation as reported here in response to rosuvastatin is in line with data obtained in hypercho-lesterolemic rabbits treated with pravastatin for 52 weeks [14] and in patients treated with atorvastatin and pravastatin [27, 28]. The increase in collagen has been attributed mainly to decreased collagen turnover as a consequence of reduced MMP activity.
The key finding of the present study is however that in addition to the effect on collagen both decorin and biglycan were markedly increased by rosuvastatin. The increase in accumulation of SLRPs was detected in lesions of the aortic root, the innominate artery and in the aorta in close association with the dense collagen matrix. This is the first demonstration of induction of SLRPs in response to pharmacologic treatment. We analysed also SLRP accumulation in plaques from a small group of patients and detected increased collagen content and moderately increased decorin and biglycan. However, these data were obtained from a small group of patients that received different statins and represent therefore only preliminary evidence suggesting that SLRPs may also increase in response to statins in human beings. This conclusion is in line with findings from Underhill et al. showing by MRI that 24 months of treatment with rosuvastatin caused coronary artery plaque remodelling in human beings and reduced lipid rich necrotic cores without reducing lesion volume [29].
Possible mechanisms responsible for the accumulation of decorin and biglycan and possible functional consequences are discussed below. Decorin has been shown to be cleaved by MMP-2, -3, -7 and MMP-13 whereas biglycan has been shown to be cleaved by MMP-13 [30, 31]. Rosuvastatin reduced activity of MMPs in atherosclerotic plaques of ApoE−/− mice as shown here in line with previous studies showing inhibition of e.g. MMP-1, -3 and -9 in response to statin treatment [14]. Therefore, it is conceivable that decreased breakdown of decorin and biglycan by MMPs contributes to the increased accumulation of these SLRPs in the present study. Alternatively, decorin and biglycan expression might be up-regulated by rosuvastatin. However, no significant effects were detected after treatment of human VSMC with rosuvastatin in vitro. Therefore, a direct effect of rosuvastatin on SLRP expression is unlikely. SLRPs are often prominent in collagen-rich tissues including the vascular intima [32]. As presented here the expression of decorin and biglycan by VSMC was increased in polymeric collagen gels in vitro. Therefore, it might be speculated that increased fibrillar collagen matrix in response to rosuvastatin activates expression of decorin and biglycan by VSMC. However, future studies are needed to unravel the molecular mechanism responsible for up-regulation of SLRPs in collagen-rich matrices.
Could increased amounts of decorin and biglycan also be causally involved in the establishment of the collagen rich matrix in response to rosuvastatin? Decorin and biglycan play an important role in post-transcriptional and post-translational regulation of collagen fibrillogenesis in skin, heart and bone [17, 33, 34]. Cell-mediated gene transfer of decorin caused increased intimal collagen deposition and collagen density in the rat model of balloon injury [35]. Consistent with this ability of decorin to compact the three dimensional organization of collagen matrices in vivo is the finding that decorin causes collagen type 1 gel contraction in vitro[36]. Biglycan deficient mice suffer from frequent aortic ruptures if bred on a BALB/cA background [37]. The aortic ruptures are again due to disturbed collagen fibril assembly in the adventitia, providing the first in vivo evidence that biglycan is an important regulator of collagen fibril formation in the vasculature. It is therefore conceivable that within atherosclerotic plaques increased deposition of biglycan and decorin as a consequence of rosuvastatin treatment compacts and stabilizes the collagen matrix. Of note, in addition to their role in collagen fibrillogenesis, decorin and biglycan control proteolytic cleavage of collagen. in vitro the interaction of decorin and biglycan with fibrilar collagen type I inhibits collagenase-1 (MMP-1) and collagenase-3 (MMP-13) mediated cleavage of collagen type I [38]. Decreased collagen cleavage was also detected in this study in the rosuvastatin treated group by decreased collagen neoepitope staining. Therefore, in the context of the literature on SLRPs, these findings support the hypothesis that decorin and biglycan, cause contraction of collagenous ECM and/or directly inhibit collagen turnover. Taken together SLRP accumulation may act as a positive feedback mechanism to further increase collagen accumulation in response to statins.
The current results may also be of interest with respect to the response to retention hypothesis of atherosclerosis, which points out the contribution of proteoglycans including biglycan to early LDL retention [39]. The binding and retention of LDL by the polyanionic glycosaminoglycan chains takes place in the loose permissive matrix of early pathological intimal thickening [40]. However, here we show that even massive accumulation of decorin and biglycan in the highly collagenous matrix of mature statin treated plaques do evidently not increase lipid retention in an ApoE deficient mice on Western diet. For lipid retention length and sulfation pattern of GAG chains is critical. These modifications of GAG chains could present valuable targets for therapeutic intervention (for review see [41]). From the present results it might be suggested that rosuvastatin treatment reduces the LDL-binding capacity of GAG-chains, which would explain why increased lipid retention was not observed despite of increased biglycan and decorin accumulation. Clearly analysis of GAG-chain modifications in response to rosuvastatin treatment in vivo would be very interesting in the future.
In conclusion, the key findings of the present study are that the collagen-binding proteoglycans, decorin and biglycan, are increased in association with increased collagen deposition and collagen density. Similar changes of the ECM are observed in human atherosclerosis after statin treatment. Therefore, the present study identifies SLRPs as novel targets of chronic rosuvastatin treatment which might have implications for the development of a stable plaque phenotype.
Acknowledgments
A.M.-B. has been supported by the Deutsche Forschungsgemeinschaft (GRK 1089). The study has been financially supported by AstraZeneca.
References
- 1.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 2.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 3.Maron DJ, Fazio S, Linton MF. Current perspectives on statins. Circulation. 2000;101:207–13. doi: 10.1161/01.cir.101.2.207. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM, Rifai N, Pfeffer MA, et al. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–5. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 5.Sparrow CP, Burton CA, Hernandez M, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler Thromb Vasc Biol. 2001;21:115–21. doi: 10.1161/01.atv.21.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Olsson AG, McTaggart F, Raza A. Rosuvastatin: a highly effective new HMG-CoA reductase inhibitor. Cardiovasc Drug Rev. 2002;20:303–28. doi: 10.1111/j.1527-3466.2002.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 7.Kleemann R, Princen HM, Emeis JJ, et al. Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-leiden transgenic mice. Evidence for antiinflammatory effects of rosuvastatin. Circulation. 2003;108:1368–74. doi: 10.1161/01.CIR.0000086460.55494.AF. [DOI] [PubMed] [Google Scholar]
- 8.Crouse JR, 3rd, Grobbee DE, O’Leary DH, et al. Measuring effects on intima media thickness: an evaluation of rosuvastatin in subclinical atherosclerosis–the rationale and methodology of the METEOR study. Cardiovasc Drugs Ther. 2004;18:231–8. doi: 10.1023/B:CARD.0000033645.55138.3d. [DOI] [PubMed] [Google Scholar]
- 9.Pelat M, Dessy C, Massion P, et al. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107:2480–6. doi: 10.1161/01.CIR.0000065601.83526.3E. [DOI] [PubMed] [Google Scholar]
- 10.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Sasiela W. Plaque stabilization: Can we turn theory into evidence? Am J Cardiol. 2006;98:26–33. doi: 10.1016/j.amjcard.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Newby AC, Zaltsman AB. Fibrous cap formation or destruction–the critical importance of vascular smooth muscle cell proliferation, migration and matrix formation. Cardiovasc Res. 1999;41:345–60. [PubMed] [Google Scholar]
- 13.Shah PK, Falk E, Badimon JJ, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–9. [PubMed] [Google Scholar]
- 14.Fukumoto Y, Libby P, Rabkin E, et al. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipi-demic rabbits. Circulation. 2001;103:993–9. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- 15.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–74. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 16.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12:107–16. doi: 10.1093/glycob/cwf065. [DOI] [PubMed] [Google Scholar]
- 17.Danielson KG, Baribault H, Holmes DF, et al. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–43. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu T, Bianco P, Fisher LW, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20:78–82. doi: 10.1038/1746. [DOI] [PubMed] [Google Scholar]
- 19.Meyers CD, Tannock LR, Wight TN, et al. Statin-exposed vascular smooth muscle cells secrete proteoglycans with decreased binding affinity for LDL. J Lipid Res. 2003;44:2152–60. doi: 10.1194/jlr.M300252-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Dai G, Freudenberger T, Zipper P, et al. Chronic ultraviolet B irradiation causes loss of hyaluronic Acid from mouse dermis because of down-regulation of hyaluronic Acid synthases. Am J Pathol. 2007;171:1451–61. doi: 10.2353/ajpath.2007.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang F, Thompson JC, Wilson PG, et al. Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res. 2008;49:521–30. doi: 10.1194/jlr.M700329-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Galis ZS, Sukhova GK, Libby P. Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995;9:974–80. doi: 10.1096/fasebj.9.10.7615167. [DOI] [PubMed] [Google Scholar]
- 23.Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–40. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 24.Kinsella MG, Fischer JW, Mason DP, et al. Retrovirally mediated expression of decorin by macrovascular endothelial cells. Effects on cellular migration and fibronectin fibrillogenesis in vitro. J Biol Chem. 2000;275:13924–32. doi: 10.1074/jbc.275.18.13924. [DOI] [PubMed] [Google Scholar]
- 25.Vernon RB, Gooden MD. An improved method for the collagen gel contraction assay. In Vitro Cell Dev Biol Anim. 2002;38:97–101. doi: 10.1290/1071-2690(2002)038<0097:AIMFTC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 26.Glossl J, Beck M, Kresse H. Biosynthesis of proteodermatan sulfate in cultured human fibroblasts. J Biol Chem. 1984;259:14144–50. [PubMed] [Google Scholar]
- 27.Schartl M, Bocksch W, Koschyk DH, et al. Use of intravascular ultrasound to compare effects of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation. 2001;104:387–92. doi: 10.1161/hc2901.093188. [DOI] [PubMed] [Google Scholar]
- 28.Crisby M, Nordin-Fredriksson G, Shah PK, et al. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–33. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 29.Underhill HR, Yarnykh VL, Hatsukami TS, et al. Carotid plaque morphology and composition: initial comparison between 1.5- and 3.0-T magnetic field strengths. Radiology. 2008;248:550–60. doi: 10.1148/radiol.2482071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai K, Hiramatsu A, Fukushima D, et al. Degradation of decorin by matrix metallo-proteinases: identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J. 1997;322:809–14. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monfort J, Tardif G, Reboul P, et al. Degradation of small leucine-rich repeat proteoglycans by matrix metallo-protease-13: identification of a new bigly-can cleavage site. Arthritis Res Ther. 2006;8:R26. doi: 10.1186/ar1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riessen R, Isner JM, Blessing E, et al. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. Am J Pathol. 1994;144:962–74. [PMC free article] [PubMed] [Google Scholar]
- 33.Weis SM, Zimmerman SD, Shah M, et al. A role for decorin in the remodeling of myocardial infarction. Matrix Biol. 2005;24:313–24. doi: 10.1016/j.matbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Corsi A, Xu T, Chen XD, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are syner-gized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–9. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 35.Fischer JW, Kinsella MG, Clowes MM, et al. Local expression of bovine decorin by cell-mediated gene transfer reduces neointimal formation after balloon injury in rats. Circ Res. 2000;86:676–83. doi: 10.1161/01.res.86.6.676. [DOI] [PubMed] [Google Scholar]
- 36.Jarvelainen H, Vernon RB, Gooden MD, et al. Overexpression of decorin by rat arterial smooth muscle cells enhances contraction of type I collagen in vitro. Arterioscler Thromb Vasc Biol. 2004;24:67–72. doi: 10.1161/01.ATV.0000107026.98626.3b. [DOI] [PubMed] [Google Scholar]
- 37.Heegaard AM, Corsi A, Danielsen CC, et al. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115:2731–8. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 38.Geng Y, McQuillan D, Roughley PJ. SLRP interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol. 2006;25:484–91. doi: 10.1016/j.matbio.2006.08.259. [DOI] [PubMed] [Google Scholar]
- 39.Skalen K, Gustafsson M, Rydberg EK, et al. Subendothelial retention of athero-genic lipoproteins in early atherosclerosis. Nature. 2002;417:750–4. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 40.Nakashima Y, Fujii H, Sumiyoshi S, et al. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol. 2007;27:1159–65. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- 41.Little PJ, Osman N, O’Brien KD. Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr Opin Lipidol. 2008;19:448–54. doi: 10.1097/MOL.0b013e32830dd7c4. [DOI] [PubMed] [Google Scholar]


