Abstract
Transgenic rats with high expression of HLA-B27 and human β2-microglobulin (B27TR) develop a multisystem inflammatory disease resembling human inflammatory bowel disease (IBD) and spondyloarthropaties (SpA). Tumour necrosis factor α (TNF-α) has a crucial role in sustaining chronic inflammation in the gut and joints. The aim of this work was to evaluate whether TNF-α blockade could prevent or reduce the inflammation of peripheral joints in B27TR. A first group of 9-week-old B27TR received an anti-TNF-α monoclonal antibody (mAb) or an isotypic IgG2a,k up to the age of 18 weeks. An untreated group was monitored up to the age of 18 weeks and then randomly assigned to a 9-week treatment with anti-TNF-α mAb or IgG2a,k. Each rat was monitored for clinical IBD and peripheral joint manifestations. After sacrifice the colon and hind paws were examined for macroscopical and microscopical pathological changes. Early TNF-α blockade prevented, and late treatment improved IBD signs in B27TR. Erythema, oedema, inflammatory infiltrate close to the tendons and enthesis, proliferating chondrocyte-like cells, signs of new endochondral bone ossification and bone erosion were observed in peripheral joints of four out of six IgG2a,k-treated B27TR, both at 18 and 27 weeks. Immunopositivity for phosphorylated Smad1/5/8 indicated that the process of joint remodelling was activated in B27TR. Some entheses showed chondroid nodules. Anti-TNF-α treatment reduced inflammation and preserved the enthesis organization in most animals. Occasional and transient erythema and oedema were still present in three of six of the late anti-TNF-α-treated animals. Smad1/5/8 signalling was not inhibited by late anti-TNF-α treatment. In B27TR, articular involvement follows IBD onset and develops at entheses. Early TNF-α blockade prevents the onset of IBD and consequently the development of enthesitis in peripheral joints in the B27TR model of human SpA.
Keywords: HLA-B27 transgenic rats, TNF-α, enthesis, spondyloarthritis, SpA, IBD, Smad1/5/8
Introduction
The major histocompatibility complex (MHC) class I gene HLA-B27 has a striking association with a group of inflammatory human disorders that affect the bowel, the joints and the axial skeleton.
In an attempt to create an animal model of B27-associated disease, Taurog et al. produced transgenic rats bearing HLA-B27 and human β2-microglobulin (hβ2m) genes (B27TR) [1]. Among the different lines of rats, two of them, 21-4H on the inbred Lewis (LEW) background and 33-3 on the inbred Fisher 344 (F344) background, developed a spontaneous multisystemic inflammatory disease, resembling human spondyloarthropathies (SpA) [1, 2]. These rats show inflammatory lesions of peripheral and axial joints, gut, male genital tract, nails and skin [1]. The susceptibility to disease is clearly related to gene copy number and expression level of HLA-B27, with disease developing only in those lines having high levels of transgene expression [2]. Both 21-4H and 33-3 lines have the highest expression of HLA-B27 and hβ2m genes. The occurrence of disease in the high copy 21-4H and 33-3 lines is a result of high levels of HLA-B27 expression, which rises in aging and is not merely a consequence of an ongoing inflammatory state [2]. The 21-4H line carries the highest copy number of B27 genes and shows B27 protein expression consistently lower in young premorbid rats than in similarly aged rats of the disease-prone 33-3 line. The earlier rise in B27 protein expression in 33-3 rats, compared with 21-4H, correlates with the earlier onset of disease manifestations, both clinically and histologically [2]. In these rats, diarrhoea is the earliest clinical manifestation [1], appearing after 10 weeks of age. Within several weeks of the onset of intestinal inflammation, most affected rats develop peripheral arthritis [1–5]. In 21-4H, arthritis follows closely the onset of diarrhoea, whereas in 33-3 male B27TR diarrhoea appears earlier than in 21-4H and other manifestations appear later. In most cases, arthritis is characterized by swelling, erythema and tenderness of the tarsal joints of one or both hind limbs [1]. Arthritis persists from few days to several weeks, and in some cases shows a cyclical pattern of remission and exacerbation [1]. Involved joints show pathological modifications commonly seen in experimental arthritis in rats and peripheral arthritis in human beings. These modifications are characterized by synovial hyperplasia, pannus formation, inflammatory cell infiltrate and destruction of articular cartilage and bone [1]. Fibrotic ankylosis occurs where the articular cartilage on adjacent joint surface is completely replaced by pannus. Usually, chronic inflammation involves the joint capsule and the adjacent ligaments and tendons [1]. The vertebral lesion observed in the tail of the 21-4H rats closely resembles the enthesitis, inflammation at ligamentous attachments to bone [1].
Several mediators of inflammation were detected in B27TR colonic mucosa and these rats have been used for several years to evaluate the activity and mechanisms of action of anti-inflammatory molecules [6–9].
In the mucosa of B27TR with advanced gut disease, tumour necrosis factor α (TNF-α) is increased and, for this reason, its role in sustaining chronic mucosal inflammation has been suggested [10–12]. Furthermore, dense cellular infiltrate of T cells and macrophages with enhanced expression of TNF-α has been found in the joints of patients with ankylosing spondylitis [13]. Anti-TNF-α agents are now used in the treatment of ankylosing spondylitis and inflammatory bowel disease (IBD), although not all are equally effective [14]. Besides joint inflammation, increasing evidence suggests that, in animal models of SpA as well as in patients, new cartilage and bone formation is a major player in the pathological manifestations leading to ankylosis [15]. The relationship between inflammation and new bone formation in SpA is still unclear, but there is evidence that joint remodelling may be uncoupled from chronic inflammation. Recent animal model data and clinical observations indicate that control of inflammation by TNF-α blockers may not be sufficient to impede disease progression towards ankylosis in these patients [15].
In a previous study, we have shown that the early treatment of B27TR with an anti-TNF-α antibody, before the onset of any clinical manifestation, prevents mucosal inflammation effectively, while, a delayed treatment can only reduce clinical symptoms and partially ameliorate the pathological signs of gut damage [16].
The aim of the present work was to evaluate whether TNF-α blockade could ameliorate or prevent peripheral joint involvement in B27TR.
Materials and methods
Experimental design
Male HLA-B27/hb2m transgenic rats (B27TR, n = 24) of the 33-3 line and male non-transgenic control rats of the same breed (F344, n = 16) were purchased from Taconic Farms (Taconic Farms, Inc., Germantown, WI, USA). All rats were bred and housed under conventional conditions. Study procedures were approved by the animal care committee of the local government.
Twelve B27TR at the age of 9 weeks, which is prior to the onset of colitis, were randomly assigned to the treatment with an anti-rat TNF-α monoclonal antibody (mAb) or an isotype-matched negative control immunoglobulin IgG2a,k (n= 6 in each group), which were kindly supplied by Centocor (Centocor, Inc., Malvern, PA, USA). Each rat received a weekly intraperitoneal injection of anti-TNF-α mAb or isotype IgG2a,k (15 mg/kg) up to the age of 18 weeks.
Twelve B27TR were monitored up to the age of 18 weeks and then randomly assigned to the treatment with anti-TNF-α mAb (n= 6) or with isotypic IgG2a,k (n= 6) up to the age of 27 weeks.
Non-transgenic F344 littermates were used as control rats (n= 4 in each protocol arm).
Each rat was weighted weekly and monitored for clinical manifestations of colitis (diarrhoea; the rats were housed individually in metabolic cages), and arthritis (erythematous and swollen hind paws) by two independent observers who were blinded with regard to the treatment group. Stool character observations for each animal were performed assigning numerical scores of 3 for diarrhoea, 2 for soft stool and 1 for normal stool. The assessment of clinical signs of arthritis in the tarsal joints was performed visually twice a week with a scale for swelling (0 to 3) and for erythema (0 to 3) of the hind paws (normal = 0, mild = 1, moderate = 2, severe = 3). The maximum possible score for arthritis per animal per paw was 6 (total score per animal = 12 for both hind paws). Data obtained from individual rats in the different experimental groups were summarized by calculating the group mean and standard deviation (mean ± S.D.). At the end of the experiments the animals were killed by ether anaesthesia.
Sample collection and histological evaluation
The dissected colon was prepared for routine histology and paraffin sections (5 μm thick) were cut across the longitudinal axis, and stained with haematoxylin and eosin. The sections were scored blindly by two investigators for histological evidence of inflammation and structural damage, as previously described [16].
During necropsy, segments of the rear limbs (with the tarsal joints) were removed, fixed in 10% buffered formalin, decalcified (Electrolytic decalcifying solution, Bio-Optica, Milan, Italy) and embedded in paraffin wax. Histological sections (8 μm thick) were stained with haematoxylin and eosin, toluidine blue and Goldner’s Masson trichrome. Slides were coded so that the two examiners were blinded to the treatment groups. Synovial tissue from the tarsal joints was evaluated based on synovial hyperplasia, inflammatory cell infiltration and pannus formation. Articular cartilage was evaluated with the following histological scoring system: cartilage organization changes (normal = 0, surface irregularity = 1, clefts = 2, complete disorganization = 3), chondrocyte proliferation (none = 0, hypercellularity = 1, cloning = 2), chondrocyte alteration (none = 0, hypertrophy = 1, necrosis = 2) and tidemark integrity (intact = 0, disorganization = 1). Inflammatory infiltrate (none = 0, mild = 1, severe = 2), enthesis (normal = 0, disorganization = 1) and bone (normal = 0, erosion = 1) were also evaluated. An average score (based on five sections per hind paw) was determined. When there was interobserver disagreement, the particular specimen was re-reviewed by both observers and the disagreement resolved. Data obtained from individual rats in the different experimental groups were summarized by calculating the group median.
Immunohistochemistry
The joint sections were de-waxed and after blocking endogenous peroxidase activity, immunohistochemistry was performed with the Ultravision Detection System (LabVision Corporation, Fremont, CA, USA), according to the manufacturer’s protocol. After blocking nonspecific antibody binding, sections were incubated overnight at 4°C with the following anti-rat antibodies: mouse monoclonal anti-CD3/T cell marker (1:10 dilution; AbD Serotec, Oxford, UK), rabbit polyclonal anti-CD68/macrophage marker (1:100 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-TNF-α (1:100 dilution; Abcam, Cambridge, UK) and rabbit polyclonal anti-phosphorylated Smad1/5/8 (1:100 dilution; Cell Signalling Technology, Danvers, MA, USA). The immunopositive products were detected using 3,3′-diaminobenzidine tetrahydrochloride substrate (DAB kit; Vector Laboratories, Burlingame, CA, USA) as chromogen. The sections were lightly counterstained with haematoxylin, observed under a light microscope (Eclipse E400; Nikon, Tokyo, Japan) and photographed with a digital camera (Coolpix 2500; Nikon). Negative controls were obtained by omitting the primary antibodies.
Statistical analysis
Statistical differences among the experimental groups were evaluated by the non-parametric Mann-Whitney U-test using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). P-values less than 0.05 were considered statistically significant.
Results
IgG2a,k-treated and untreated B27TR
In control IgG2a,k-treated and untreated B27TR, the clinical signs of gastrointestinal involvement were evident around the 11th week of age. The disease progressively worsened, with diarrhoea affecting all animals at the 13th week. A high value of stool score persisted until the end of the experiments. In these rats, in the weeks following the onset of intestinal inflammation, episodes of joint erythema and metatarsal/tarsal oedema of the hind limbs started (Fig. 1A). At 18 weeks of age, IgG2a,k-treated rats showed erythema and oedema with a mean joint score of 6.9 6 1. Untreated B27TR showed the same symptoms, with a joint score of 6.5 ± 2. It should be noted that, in IgG2a,k-treated and untreated groups, 2 out of 6 and 3 out of 12 rats, respectively, did not develop clinical joint signs. The untreated group, at the age of 18 weeks, was randomly assigned to the late treatment with anti-TNF-α mAb or with isotypic IgG2a,k.
Fig 1.
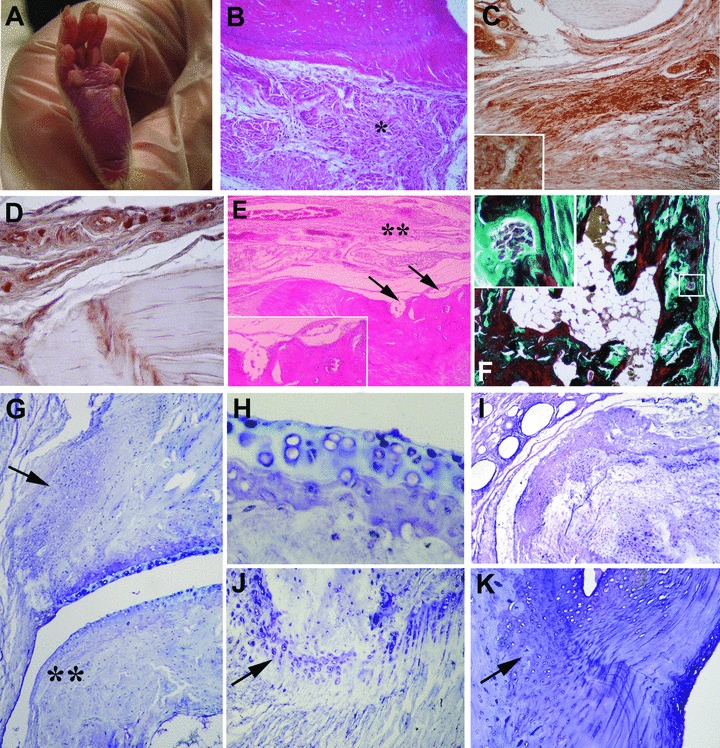
Macroscopic and microscopic features of joint disease in IgG2a,k-treated HLA-B27 transgenic rats (B27TR). (A) Oedema and erythema in the hind paw. (B)–(K) Representative histological sections of peripheral joints (B, E: haematoxylin and eosin staining, F: Goldner’s Masson trichrome staining, (G)–(K) toluidine blue staining). (B) Subcutaneous inflammatory infiltrate (asterisk) and oedema close to the enthesis and tendons in a 18-week-old rat. (C), (D) Representative microphotographs of immunostaining for CD3 and CD68 in the same rat showed in (B). (C) Immunohistochemistry showed that most of the infiltrating cells are CD3+ T cells; Inset: Higher magnification of CD3+ T cells. (D) Some CD68+ macrophages are present in the connective tissue. (E) Bone erosions (arrows) and inflammatory infiltrate (double asterisk) in a 18-week-old rat; Inset: Higher magnification of bone erosion areas. (F) Representative histological section of the peripheral joint of a 27-week-old rat; Inset: Higher magnification of the boxed area in F showing bone erosion with osteoclasts. (G) Reduced thickness of articular cartilage with hypertrophic chondrocytes, fibroblast-like cell proliferation (arrow) and irregular articular lining (double asterisk) in a 18-week-old rat. (H) Higher magnification of the articular cartilage shown in (G). Areas of superficial matrix pallor with necrosis and degeneration of chondrocytes are present. (I) Derangement of articular cartilage with proliferating chondrocyte-like cells and endochondral bone ossification in a 27-week-old rat. (J) Altered enthesis with proliferating chondrocyte-like cells and hypertrophic chondrocytes (arrow) in a 18-week-old rat. (K) Chondrocyte proliferation and endochondral bone ossification close to the tidemark (arrow) in a 27-week-old rat. Original magnification: (I) ×4; (F) ×10; (B–E), (G), (K): ×20; (J) ×40; (H) ×60; Insets: ×60.
From the 18th to the 27th week, IgG2a,k-treated B27TR still showed diarrhoea and an increase of the joint score from 6.5 ± 2 to 8.0 ± 2, as recorded at the end of the experiment. Two animals manifested no signs of arthritis during the treatment. When animals were killed, no joint deformity was observed, neither at 18 nor at 27 weeks of age.
At the time of sacrifice, both at 18 and 27 weeks, the macroscopical examination of the gut showed colon inflammation with oedematous and hyperaemic wall and a prominent vascular network. The pathological examination revealed that gut inflammation was more severe in the late phase. In the colon, a derangement of the mucosal folds with altered tubular glands and a prominent inflammatory infiltrate in the lamina propria were evident. In these rats, pathological modifications of the joints were similar, both at 18 and 27 weeks. The severity of the joint damage was not directly related to aging, as usually observed in the gut [16].
In each animal, pathological parameters in peripheral joints were blindly evaluated and scored by two investigators, as above described, and the results are reported in Fig. 3. In IgG2a,k-treated rats, peripheral joints showed diffuse subcutaneous oedema and inflammatory infiltrate in the plantar fascia, whereas the overlying skin appeared normal, without signs of hyperkeratosis or dermal hyperplasia. Around tendons a diffuse inflammatory infiltrate was evident, and cell infiltration extended into the entheseal region (Fig. 1B–E), while no inflammatory infiltrate was detected in the synovium. Immunohistochemical analysis showed that most of the infiltrating cells were CD3+ T cells (Fig. 1C), while only some CD68+ macrophages were observed (Fig. 1D). Sometimes, undulating cartilage surface with variable degree of fibrillation, reduced thickness of the cartilage layer and clefting were observed (Fig. 1G–I). Occasionally, derangement of articular cartilage with proliferating chondrocyte-like cells and endochondral bone ossification areas was detected (Fig. 1G, I). In the cartilage, extensive areas of superficial matrix pallor were observed using toluidine blue staining, indicating necrosis and degeneration of chondrocytes (e.g. piknotic nuclei, absence of nuclei or pale ‘ghost’ cells) (Fig. 1G, H). In the examined entheses, proliferating chondrocytes and chondrocyte-like cells were clearly visible (Fig. 1J, K), leading to the formation of chondroid nodules and new endochondral bone ossification areas (Fig. 1K). In two animals, the tidemark between the calcified fibrocartilage and the bone appeared with an irregular border. In most affected ankles, the formation of periarticular enthesophytes was observed. Bone erosions were detected under the periostium (Fig. 1E, F).
Fig 3.
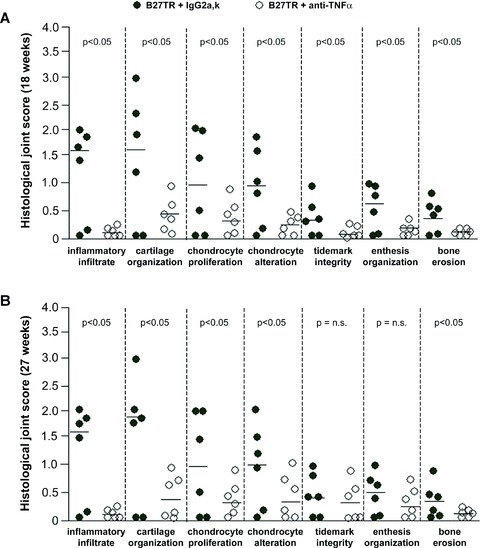
Effects of anti-TNF-α mAb treatment on peripheral joint disease in HLA-B27 transgenic rats (B27TR). Results of histological score of inflammation, cartilage, enthesis and bone changes in 18-week-old B27TR (A) and 27-week-old B27TR (B) are shown. Each dot represents the value for an individual rat. Horizontal lines indicate the group medians. P < 0.05: anti-TNF-α-treated B27TR versus IgG2a,k-treated B27TR. n.s., not significant.
Immunohistochemistry was performed to identify cells expressing TNF-α in the affected joints of IgG2a,k-treated B27TR. In both 18- and 27-week-old rats, chondrocyte-like cells showed immunopositivity for TNF-α in the proliferating zone of the articular cartilage and at the fibrocartilaginous point of attachment of the entheses (Fig. 4A–C). Some vessels close to the entheses showed positive cells for TNF-α. In addition, immunostaining for nuclear phosphorylated Smad 1/5/8 (p-Smad1/5/8), a marker for active bone morphogenetic protein (BMP) signalling [17], was performed. Activation of Smad1/5/8 signalling was detected in spindle-shaped entheseal fibroblast-like cells, inflammatory cells and in round chondroblast-like cells (Fig. 5A, B). Moreover, proliferating and prehypertrophic chondrocyte-like cells showed strong p-Smad1/5/8-immunopositivity in the fibrocartilage at the point of entheseal attachment (Fig. 5C, D). Instead, hypertrophic chondrocytes were negative (Fig. 5D). Chondrocytes in the articular cartilage were also found positive for p-Smad1/5/8 (Fig. 5E, F). No difference in p-Smad1/5/8 expression was found between 18- and 27-week-old IgG2a,k-treated B27TR.
Fig 4.
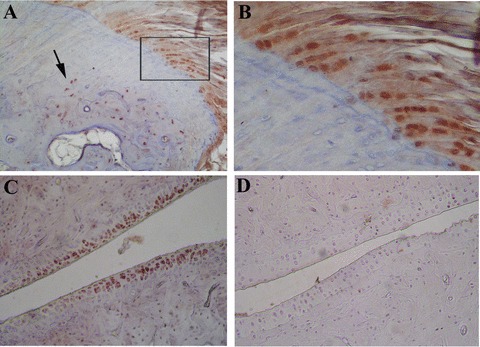
Representative immunostaining for TNF-α in the peripheral joints of HLA-B27 transgenic rats (B27TR). (A), (B) 18-week-old IgG2a,k-treated B27TR. (B) is a higher magnification view of boxed area in (A). Immunopositive chondrocytes in the fibrocartilaginous point of entheseal attachment (B) and in the proliferating zone at the border between bone and cartilage (arrow). (C) 27-week-old IgG2a,k-treated B27TR: immunopositive proliferating chondrocytes in the articular cartilage. (D) 18-week-old anti-TNF-α treated B27TR: absence of TNF-α immunopositivity in the cartilage. Original magnification: (A), (C), (D) × 20; (B) × 60.
Fig 5.
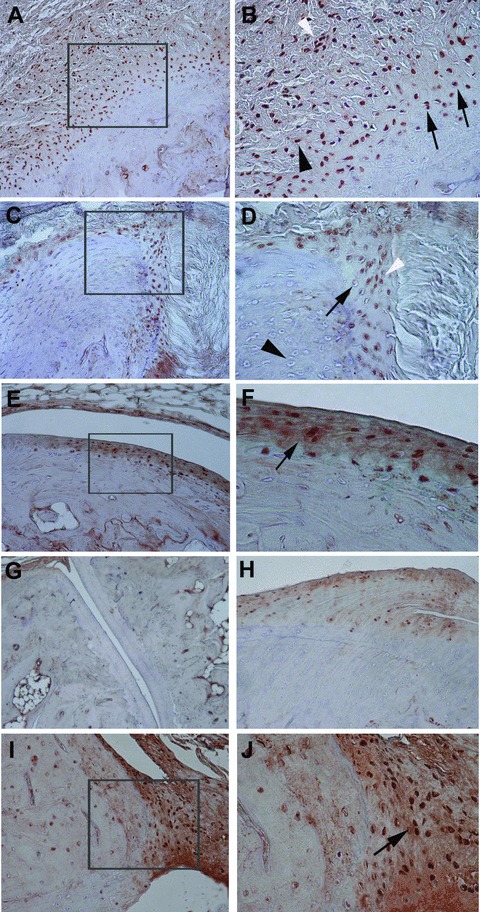
Representative immunohistochemical results of phosphorylated Smad1/5/8 in the peripheral joints of HLA-B27 transgenic rats (B27TR). (A), (B) 18-week-old IgG2a,k treated B27TR. (B) is a higher magnification view of boxed area in (A). Smad1/5/8 signalling is detected in spindle-shaped entheseal fibroblast-like cells (black arrowhead), inflammatory cells (white arrowhead), and in round chondroblast-like cells (black arrows) at entheseal level. (C), (D) 27-week-old IgG2a,k treated B27TR. (D) is a higher magnification view of boxed area in (C). Proliferating (white arrowhead) and prehypertrophic (black arrow) chondrocyte-like cells show phosphorylated Smad1/5/8-immunopositivity in the fibrocartilage at the point of entheseal attachment. Hypertrophic chondrocytes are negative (black arrowhead). (E), (F) 27-week-old IgG2a,k treated B27TR. (F) is a higher magnification view of boxed area in (E). Immunopositive proliferating chondrocytes are evident in the articular cartilage (black arrow). (G), (H) 18-week-old anti-TNF-α treated B27TR. Absence of phosphorylated Smad1/5/8 immunopositivity in the articular cartilage (G) and at the entheseal level (H). (I), (J) 27-week-old anti-TNF-α treated B27TR. (J) is a higher magnification view of boxed area in (I). Proliferating chondrocytes in the fibrocartilaginous point of entheseal attachment show strong immunopositivity for phosphorylated Smad1/5/8 (black arrow). Original magnification: (G) ×10; (A, C, E, H, I) ×20; (B, D, F, J): ×40.
Early anti-TNF-α treatment
The early TNF-α blockade, started at the age of 9 weeks before the onset of any symptoms, prevented the appearance of diarrhoea and the stool score remained normal. During the treatment no clinical signs of oedema and erythema were observed in the peripheral joints. In these rats there was no evidence of inflammatory infiltrate within the colonic mucosa and submucosa, and we did not observe the architectural damage seen in the IgG2a,k-treated rats (Fig. 2A). No inflammatory infiltrate was detected into the joints and periarticular tissue, and the organization of the articular cartilage and entheses, with regular tidemarks and aligned chondrocyte rows towards the ligament, was maintained (Fig. 2B–D). In these rats, bone erosion was never detected. Results of the histological joint score are reported in Fig. 3A. TNF-α was not detected in the peripheral joints of early treated rats, neither at the cartilage nor at the enthesis (Fig. 4D). Activation of Smad1/5/8 signalling was not found in these rats (Fig. 5G, H).
Fig 2.
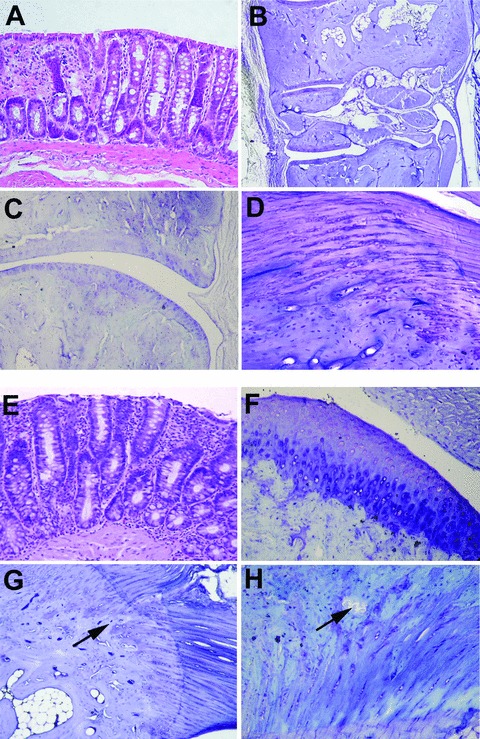
Microscopic features of disease in anti-TNF-α-treated HLA-B27 transgenic rats (B27TR). (A)–(D) Early anti-TNF-α treatment. (A) Histological section of colon in early-treated B27TR (haematoxylin and eosin staining). (B), (C) Representative histological sections of peripheral joint, with well preserved articular components. (D) Normal organization of the enthesis, with regular tidemarks and aligned chondrocyte rows towards the ligament. (B–D: toluidine blue staining). Original magnification: (A, C) ×10; (B) ×4; (D) ×40. (E)–(H) Late anti-TNF-α treatment. (E) Histological section of colon in late-treated B27TR (haematoxylin and eosin staining). (F)–(H) The histopathological joint features of three out of six late anti-TNF-α treated B27TR are shown. The other three late treated-B27TR showed no significant joint alterations as in the early treated group (B–D). (F) Areas of superficial matrix pallor, with hypertrophic chondrocytes and undulating articular surface. (G) Irregular tidemark between fibrocartilage and bone and endochondral bone ossification (arrow). (H) Bone erosion at the enthesis (arrow). [(F–H) toluidine blue staining]. Original magnification: (E–G) ×20; (H) ×40.
Late anti-TNF-α treatment
In rats treated late with the anti-TNF-α mAb, clinical signs of gut inflammation and stool score decreased after the first week of treatment, and then normalized. Three out of six animals showed only episodes of transient erythema and oedema in the hind paws, with a mean joint score of 6.0 ± 1. Two out of six rats had a complete remission, while one animal manifested no signs of arthritis during the experiments. In the colon, the late anti-TNF-α treatment did not significantly reduce the inflammatory features and did not lead to the repair of the architectural damages (Fig. 2E). Instead, the treatment effectively reduced joint inflammatory infiltrate in the subcutaneous tissue. The three B27TR with clinical episodes of erythema and oedema during treatment developed areas of superficial matrix pallor due to degeneration/necrosis of chondrocytes in the articular cartilage (Fig. 2F). In these rats, bone erosion was seldom detected. In the entheses, irregular tidemark between fibrocartilage and bone, chondrocytes not aligned in rows, areas of chondrocyte proliferation and endochondral bone ossification were found (Fig. 2G, H). Proliferation of chondrocytes was occasionally seen in tendons far from the entheses. The histological joint score is reported in Fig. 3B. In the areas where chondrocyte proliferation was observed, immunopositivity for TNF-α was found. Positivity for p-Smad1/5/8 was also observed in the articular cartilage and at the entheseal level. In particular, proliferating chondrocytes at the fibrocartilaginous point of entheseal attachment showed strong immunopositivity for p-Smad1/5/8 (Fig. 5I, J). Staining for p-Smad1/5/8 was also observed in the treated rats that showed complete remission.
In B27TR synovial involvement was never observed.
F344 control rats did not show any clinical and/or histological sign of inflammation, affecting the gut or peripheral joints.
Discussion
Our data confirm that in B27TR gastrointestinal involvement always precedes joint involvement [1–5, 11, 12], and clearly show that TNF-α blockade can prevent the development of gut and subsequently joint inflammation. Indeed, the present data identify in B27TR the enthesis as the earliest structure that is mainly targeted by the inflammatory process.
Previously, we reported that treatment of B27TR with an anti-TNF-α mAb, before the development of any clinical IBD manifestation, prevented the onset of colon inflammation, while, when the disease is established, TNF-α blockade was significantly less effective, improving clinical gastrointestinal signs but not significantly reducing the pathological features [16].
It is important to remark that our animals did not develop synovitis, but mainly enthesitis. In both IgG2a,k-treated and untreated B27TR, we observed oedema and erythema of the hind limbs, but the histopathological analysis of tarsal/metatarsal joints showed that this stage of the disease was not due to synovitis, but rather due to an enthesitis. These findings conflict with results previously described in these rats [1, 2, 4, 6]. The type and severity of damage of the enthesis and bone were not linked to age, in contrast with what was found in the gastrointestinal tract [16]. It should be noted that the duration of our experiments was relatively short in comparison with other studies in this animal model [1, 2, 5], and our work was mainly designed to treat B27TR with an anti-TNF-α antibody, both as early or late treatment, with a comparable number of follow-up weeks. According to the literature, 18 weeks of age represent, for this model, sufficient time for the development of early symptoms and initial damage and should allow for testing the preventive effect of TNF-α blockade, both at gastrointestinal and joint level. Later on, ankylosing enthesitis or synovial involvement could have been seen. This is corroborated by our observations of early features represented by diffuse subcutaneous oedema and inflammatory infiltrate around tendons and entheses, in which T cells predominate, together with foci of cartilage and bone remodelling. The findings of activation of Smad1/5/8, a downstream signalling cascade shared by different BMPs [17, 18], in the affected entheseal region and in the articular cartilage of IgG2a,k-treated rats support this hypothesis.
It is known that entheseal involvement is a pivotal event in the development of SpA in human beings [19, 20], although the functional relationship between the enthesis and the adjacent synovium is not yet fully understood [19]. Previous studies on 33-3 lines on Fisher 344 background rats showed a synovial involvement with an arthritis pattern, but not entheseal involvement [4, 6]. In 33-3 line on LEW background rats, Tran et al. found that the additional hβ2m promotes arthritis and spondylitis, in the absence of gut inflammation [21]. Clinical features of arthritis were found in the hind paws, while enthesitis was observed in the tail vertebral joints from 31-week-old rats. Thus, the authors suggested that the genetic background may have a strong influence on the incidence, pattern and severity of the disease [21]. B27TR on LEW background are more prone to joint involvement than B27TR on F344 background. Despite this, pathological features of enthesitis in the hind limbs were already observed at 18 weeks of age in our B27TR on F344 background.
In early treated rats, TNF-α blockade clearly prevented gastrointestinal manifestations and the development of enthesitis, while in the late treated rats it led to an improvement of macroscopical signs, observed before starting the therapy, with rare manifestations of acute joint inflammation. This suggests that TNF-α blockade may prevent joint alterations from evolving to irreversible damage, even in rats with already established gut inflammation. Moreover, the effect of late anti-TNF-α blockade on peripheral joints seems to be more efficacious than on gastrointestinal inflammation. This may be because, at 18 weeks, the gastrointestinal mucosa showed chronic inflammatory features since it was involved from the age of 13 weeks with a progressive worsening course [16]. At joint level, however, the occurrence of episodes of erythema and oedema did not lead to severe modification of the articular architecture. The articular involvement observed in the IgG2a,k-treated rats was more severe than in late anti-TNF-α treated animals. The persistence of peripheral joint inflammation in the IgG2a,k-treated rats was in accordance also with the persistence of the disease at gastrointestinal level. The most important effect obtained with TNF-α blockade was the prevention or reduction, with early or late anti-TNF-α treatment, respectively, of the joint inflammatory infiltrate. Besides inflammation, it is known that new cartilage and bone formation is a critical part of the pathological process in SpA [15]. Therefore, we further analysed the expression of TNF-α in the involved tissues and whether molecules related to the bone remodelling process were modulated by anti-TNF-α treatment in our experimental model. A strong expression of TNF-α was observed in the proliferating chondrocyte areas of IgG2a,k-treated B27TR, indicating that this cytokine may in part be involved in new cartilage formation and bone remodelling processes, as it has also been suggested in previous studies [22]. Phosphorilated Smad1/5/8 was also clearly observed in IgG2a,k-treated B27TR, both at 18 and 27 weeks of age. In particular, activation of Smad1/5/8 signalling was found in fibrocartilage and in cells undergoing chondrocytic differentiation near the entheses. In early anti-TNF-α treated B27TR, neither TNF-α nor p-Smad1/5/8 expression was observed, whereas a weak TNF-α immunopositivity was present in the areas of proliferative cartilage found in the late anti-TNF-α treated animals still showing histopathological damages. Interestingly, Smad1/5/8 signalling was found activated in late treated rats, even in those that showed complete remission. Thus, the early anti-TNF-α treatment seems more effective in inhibiting both inflammation and formation of new cartilage and bone than the delayed treatment, which may resolve inflammation without influencing the activation of signalling pathways involved in joint remodelling. These results are in agreement with recent data on animal models of SpA and clinical observations on human ankylosing spondylitis indicating that control of inflammation may not be sufficient to impede disease progression towards ankylosis in these patients [22, 23]. The role of TNF-α and anti-TNF-α strategies on disease evolution in enthesitis-related disorders is an important and debated topic. In human SpA, TNF-α blockers effectively inhibit inflammation, as shown by signs and symptoms, and will probably prevent erosive structural damage [23]. However, the ossification of already-damaged bone cannot be influenced by TNF-α blockers, because these drugs do not inhibit chondrogenesis [23]. It has also been suggested that inflammation and ankylosis may be linked in some manner, but both are largely independent processes and are probably controlled by different genetic susceptibility factors [22, 23]. The present study cannot solve this important debate, which seems in part related to the specific animal models used in different studies [22], but supports the theory of the uncoupling between inflammation and joint remodelling in enthesitis-related disorders. Our results clearly indicate that signalling pathways leading to bone remodelling are activated in B27TR, and are likely to have a role in disease progression. The episodes of acute inflammation that occurred in the late treated rats before starting the treatment may have induced complex mechanisms of bone remodelling which cannot subsequently be modulated by TNF-α blockade.
In conclusion, our data indicate that TNF-α blockade, started in the early phase of the disease, significantly prevents the development of inflammation, early at gut and later at peripheral joint level. Moreover, our data show that in B27TR peripheral joints disease starts at entheseal level in accordance with what has been already suggested in the case of human beings [20]. This may suggest that anti-TNF-α treatment should be started as early as possible to prevent the disease evolution and the development of enthesitis and later synovitis.
Acknowledgments
This study was supported by an unrestricted grant from the Schering-Ploug, Italy.
References
- 1.Hammer RE, Maika SD, Richardson JA, et al. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human beta2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 2.Taurog JD, Maika SD, Simmons WA, et al. Susceptibility to inflammatory disease in HLA-B27 transgenic rat lines correlates with the level of B27 expression. J Immunol. 1993;150:4168–78. [PubMed] [Google Scholar]
- 3.Breban M. Animal models and in vitro models for the study of aetiopathogenesis of spondyloarthropathies. Baillieres Clin Rheumatol. 1998;12:611–26. doi: 10.1016/s0950-3579(98)80040-x. [DOI] [PubMed] [Google Scholar]
- 4.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–53. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–64. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keith JC, Jr, Sainz IM, Isordia-Salas I, et al. A monoclonal antibody against kininogen reduces inflammation in the HLA-B27 transgenic rat. Arthritis Res Ther. 2005;7:R769–76. doi: 10.1186/ar1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peterson RL, Wang L, Albert L, et al. Molecular effects of recombinant human interleukin-11 in the HLA-B27 rat model of inflammatory bowel disease. Lab Invest. 1998;78:1503–12. [PubMed] [Google Scholar]
- 8.Makino J, Andoh A, Hata K, et al. Inhibitory effects of the new anti-inflammatory agent, IS-741, on spontaneous colitis in HLA-B27/β2-microglobulin transgenic rats. J Gastroenterol Hepatol. 2002;17:854–60. doi: 10.1046/j.1440-1746.2002.02815.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertrand V, Quéré S, Guimbaud R, et al. Effects of murine recombinant interleukin-10 on the inflammatory disease of rats transgenic for HLA-B27 and human beta 2-microglobulin. Eur Cytokine Netw. 1998;9:161–70. [PubMed] [Google Scholar]
- 10.Taurog JD, Maika SD, Satumtira N, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–23. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 11.Hacquard-Bouder C, Ittah M, Breban M. Animal models of HLA-B27-associated diseases: new outcomes. Joint Bone Spine. 2006;73:132–8. doi: 10.1016/j.jbspin.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Breban M, Hacquard-Bouder C, Falgarone G. Animal models of HLA-B27-associated diseases. Curr Mol Med. 2004;4:31–40. doi: 10.2174/1566524043479347. [DOI] [PubMed] [Google Scholar]
- 13.Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38:499–505. doi: 10.1002/art.1780380407. [DOI] [PubMed] [Google Scholar]
- 14.Braun J, Baraliakos X, Listing J, et al. Differences in the incidence of flares or new onset of inflammatory bowel diseases in patients with ankylosing spondylitis exposed to therapy with anti-tumor necrosis factor alpha agents. Arthritis Rheum. 2007;57:639–47. doi: 10.1002/art.22669. [DOI] [PubMed] [Google Scholar]
- 15.Lories RJ, Luyten FP, de Vlam K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther. 2009;11:221. doi: 10.1186/ar2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milia AF, Manetti M, Generini S, et al. TNFalpha blockade prevents the development of inflammatory bowel disease in HLA-B27 transgenic rats. J Cell Mol Med. 2009;13:164–76. doi: 10.1111/j.1582-4934.2008.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lories RJ, Luyten FP. Bone morphogenetic protein signalling in joint homeostasis and disease. Cytokine Growth Factor Rev. 2005;16:287–98. doi: 10.1016/j.cytogfr.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–9. doi: 10.1172/JCI23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGonagle D, Lories RJ, Tan AL, et al. The concept of a “synovio-entheseal complex” and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 2007;56:2482–91. doi: 10.1002/art.22758. [DOI] [PubMed] [Google Scholar]
- 20.McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352:1137–40. doi: 10.1016/S0140-6736(97)12004-9. [DOI] [PubMed] [Google Scholar]
- 21.Tran TM, Dorris ML, Satumtira N, et al. Additional human beta2-microglobulin curbs HLA-B27 misfolding and promotes arthritis and spondylitis without colitis in male HLA-B27-transgenic rats. Arthritis Rheum. 2006;54:1317–27. doi: 10.1002/art.21740. [DOI] [PubMed] [Google Scholar]
- 22.Lories RJ, Derese I, De Bari C, et al. Evidence for uncoupling of inflammation and joint remodelling in a mouse model of spondylarthritis. Arthritis Rheum. 2007;56:489–97. doi: 10.1002/art.22372. [DOI] [PubMed] [Google Scholar]
- 23.Sieper J, Appel H, Braun J, et al. Critical appraisal of assessment of structural damage in Ankylosing Spondylitis: Implications for treatment outcomes. Arthritis Rheum. 2008;58:649–56. doi: 10.1002/art.23260. [DOI] [PubMed] [Google Scholar]


