Abstract
This study was to investigate the effect of oxidized low-density lipoprotein (ox-LDL) on the behaviour of bone marrow stem cells and their endothelial differentiation as well as the underlying mechanisms. Adult rat bone marrow multipotent progenitor cells (MAPCs) were incubated with ox-LDL for up to 2 weeks. Ox-LDL treatment resulted in a time- and dose-dependent reduction of MAPC population in culture through a combination of decreased cell proliferation and increased apoptosis. The expression of stem cell marker Oct-4 was significantly suppressed in MAPCs by ox-LDL in a dose- and time-dependant manner. Endothelial differentiation of MAPCs was substantially inhibited by ox-LDL with markedly decreased expression of endothelial markers vWF, Flk-1 and CD31, as well as impaired in vitro vascular structure formation. Ox-LDL-induced apoptosis and inhibition of Oct-4 expression, cell proliferation and endothelial differentiation of MAPCs were associated with significant inhibition of Akt phosphorylation. Akt overexpression in MAPCs transfected with a constitutively active Akt completely reversed the effects of ox-LDL on MAPCs including enhanced apoptosis, decreased cell proliferation, suppressed Oct-4 expression and endothelial differentiation as well as in vitro vascular structure formation. In conclusion, ox-LDL promotes apoptosis and inhibits Oct-4 expression and self-renewal of MAPCs, and impairs their endothelial differentiation via suppression of Akt signalling.
Keywords: bone marrow stem cell, ox-LDL, Oct-4, endothelial cell, apoptosis, Akt
Introduction
Bone marrow-derived circulating endothelial progenitor cells (EPCs) play a key role in vascular re-endothelialization and angiogenesis [1]. The number and function of EPCs are significantly reduced in patients with hyperlipidemia [2], indicating that the EPCs are defective both structurally and functionally in the setting of hyperlipidemia. Oxidized low-density lipoprotein (ox-LDL) has been shown to significantly inhibit the proliferation, migration, adhesion and endothelial differentiation of EPCs, as well as incorporation of EPCs into vasculature [3–5]. As EPCs originate from bone marrow stem cells, the number and function of EPCs could be intimately associated with the status of bone marrow stem cells and their differentiation into EPCs. Therefore, it is important to define the effect of ox-LDL on bone marrow stem cells.
Stem cells exhibit unique characteristics including pluripotency, self-renewal and expression of specific cell markers and genes like Oct-4 [6–8]. Oct-4 is a transcription factor, and is expressed at high level in embryonic stem cells (ESCs). It is critical to the pluripotency, self-renewal and differentiation of stem cells [6, 7, 9]. Multipotent adult progenitor cells (MAPCs) are isolated from postnatal human and rodent tissues including bone marrow, muscle and brain [10]. These cells have been well characterized and are able to differentiate into a variety of cell lineages of mesodermal, ectodermal and endodermal origins in vitro and in vivo including vascular endothelium, hepatocytes and neurons [11]. Similar to ESCs, MAPCs exhibit remarkable self-renewal capability and express Oct-4 abundantly [12, 13]. Recently, we demonstrated that nitric oxide enhanced Oct-4 expression and promoted endothelial differentiation of mouse MAPCs in vitro[14].
The outcomes of ox-LDL on its target cells are variable and complex depending on individual cell type. Ox-LDL inhibits proliferation and promotes apoptosis of vascular endothelial cells (ECs) and EPCs [15, 34]; whereas it induces cell proliferation of vascular smooth muscle cells, and attenuates apoptosis of macrophages and monocytes through activation of mitogen-activated protein kinase [16–18]. Ox-LDL interferes with the function of EPCs through multiple mechanisms including inhibition of endothelial nitric oxide synthase (eNOS), downregulation of E-selectin and integrin alpha(v)beta(5) expression, inactivation of telomerase and acceleration of cell senescence (33, 34, 41). The present study was designed to investigate the effect of ox-LDL on the behaviour of rat MAPCs including Oct-4 expression, self-renewal and their endothelial differentiation and the underlying mechanisms.
Materials and methods
LDL isolation and oxidative modification
Plasma from healthy volunteers was obtained from Hong Kong Red Cross (Kowloon, Hong Kong). LDL was prepared from plasma with the method as described [19]. Ox-LDL was prepared by exposure of LDL to CuSO4 in dark for 24 hrs; and the degree of LDL oxidation was monitored by measuring the production of thiobarbituric acid reactive substances (TBARS) as described [20]. The value for TBARS in ox-LDL was determined to be 30.8 ± 3.1 nmol malondialdehyde/mg protein by 24 hrs of LDL oxidation. No TBARS were detectable in native LDL as expected.
Cell culture
Rat MAPCs were isolated and cultured with the method as described [21]. To investigate the effect of ox-LDL on MAPCs, the cells were cultured at a density of 500 cells/cm2 in the presence of ox-LDL (from 0 to 40 μg/ml) for 24, 48 and 72 hrs. Native LDL was used as control. The cells were counted in each group, and collected for real-time PCR and Western blot analysis.
Cell proliferation assay
MAPCs were seeded in a 96-well plate at a density of 1000 cells/well in the presence of ox-LDL (from 0 to 40 μg/ml) or native LDL for 24, 48 and 72 hrs. The cells were then prepared for cell proliferation assay using BrdU Cell Proliferation Assay Kit (Calbiochem, San Diego, CA) according to manufacturer's instructions. All samples were prepared in duplicates.
Cell apoptosis assay
MAPCs were seeded into 96-well plates with 1000 cells per well. After 6 hrs of incubation, the cells were exposed to ox-LDL (from 0 to 80 μg/ml) for additional 24 hrs. Native LDL served as control. The cells were then prepared for apoptotic cell death measurement with Cell Death Detection ELISAPLUS kit (R&D, Minneapolis, MN) as per manufacturer's protocol.
Endothelial differentiation
Endothelial differentiation of MAPCs was initiated with the method as described [13, 22]. To evaluate the effect of ox-LDL on their endothelial differentiation, ox-LDL (from 10 to 40 μg/ml) was added to the culture media. Native LDL was used as control. Cells were collected at day 0, 7, 10 and 14 of differentiation for RT-PCR, Western blot analysis and immunofluorescence staining to determine the expression of endothelial markers vWF Flk-1 and CD31.
Quantitative real-time PCR
Total RNA was extracted from MAPCs treated with ox-LDL or native LDL using RNeasy kit (Qiagen). Real-time PCR was performed as described [14]. mRNA levels were normalized by using GAPDH as housekeeping gene and compared to the mRNA levels in rat spleen. The detailed primer sequences for vWF, Oct-4, CD31, Flk-1 and GAPDH were published previously [13].
Immunofluorescence staining for vWF
Undifferentiated or differentiating MAPCs were plated in FN-coated chamber slides. At day 0, 7, 10 and 14 of differentiation, cells were prepared for vWF immunofluorescence staining as described [14]. The dilution factor was 1:100 for vWF primary Ab, and 1:200 for secondary Ab (anti-goat IgG-Cy-3, Sigma). Cells exposed only to secondary Ab served as negative controls. Cultured human umbilical vein ECs served as positive control.
In vitro tube formation assay
In vitro vascular tube formation from MAPCs-derived cells was evaluated in three-dimensional growth factor-reduced Matrigel (10 mg/ml; Collaborative Research, Bedford, MA) as described [14, 22].
Cell transfection with Akt plasmid
MAPCs were transfected with Myristoylated (Myr)-Akt plasmid using the Nucleofector kit (VPE-1001) (Amaxa Biosystems, Gaithersburg, MD) as described [23, 24]. The plasmid of a constitutively active Myr-Akt was kindly provided as a gift by Dr. Susheela Tridandapani at the Ohio State University Medical Center. A total of 3 × 106 cells in 100 ul solution (human MSC kit, program A-23) at room temperature were mixed with 5 ug Akt constructs or control vector or eGFP-encoding plasmids. Successful transfection was confirmed 24 hrs post-transfection by GFP fluorescence using a Nikon Eclipse TE 2000-S (Melville, NY) and with FACS. Dead cells were excluded by propidium iodide staining. The expression of constitutively active Akt in MAPCs was determined by Western blot.
Western blot analysis
Cells were collected and prepared as described for Western blot analysis [14, 22]. After preparation, the samples were blotted with primary Abs against Oct-4, Bax (Santa Cruz Biotechnology, Santa Cruz, CA), p-Akt, t-Akt, p-ERK1/2, t-ERK1/2 and actin (Cell Signal, Berley, MA) with dilution factors recommended by the manufacturers. The immunoreactive proteins were detected with horseradish peroxidase-linked secondary Abs and ECL System (Amersham Biosciences, Piscataway, NJ). All Western blot experiments were repeated for at least three times.
Statistical analysis
The data were expressed as mean ± S.D. and statistically analysed by independent sample T-test or one-way anova. Differences were considered statistically significant when P< 0.05.
Results
Ox-LDL down-regulated Oct-4 expression in MAPCs
As expected, Oct-4 was expressed abundantly in MAPCs as analysed by RT-PCR and Western blot (Fig. 1A and B). When MAPCs were exposed to ox-LDL for 24 hrs, Oct-4 transcriptional expression was significantly decreased with a reduction of mRNA level up to 42% in a concentration-dependent manner (Fig. 1A). Western blot analysis showed that Oct-4 protein content in MAPCs was also substantially decreased by up to 70% when incubated with ox-LDL for 24 hrs (Fig. 1B, P< 0.01, n= 4). Of note, Oct-4 expression in MAPCs was not affected by native LDL (data not shown).
Fig 1.
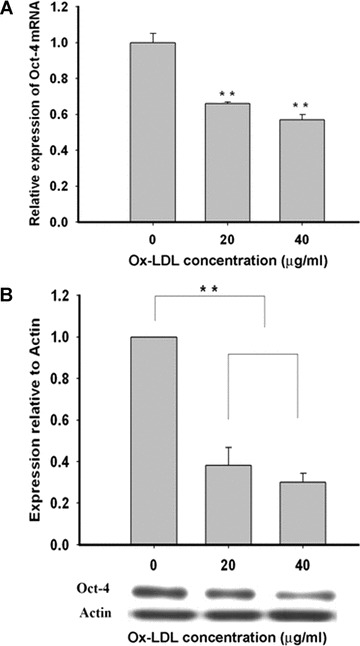
Ox-LDL inhibited Oct-4 expression. When MAPCs were exposed to ox-LDL (from 0 to 40 μg/ml) for 24 hrs, Oct-4 expression was significantly decreased with a concentration-dependent reduction of both mRNA and protein levels in the cells. Oct-4 expression was not affected by native LDL (data not shown). **P< 0.01 versus 0 μg/ml (n= 4). (A) Ox-LDL dose-dependently inhibited Oct-4 transcriptional expression in MAPCs with decreased mRNA level as measured by RT-PCR. (B) Oct-4 protein content was dramatically decreased dose-dependently by ox-LDL in MAPCs after 24 hrs of incubation as determined by Western blot.
Ox-LDL inhibited self-renewal of MAPCs
Under normal condition, MAPCs doubling time was 12.4 hrs. It was dramatically increased dose-dependently up to 34.2 hrs (P< 0.05, n= 4) by ox-LDL (Fig. 2A). Accordingly, the MAPC population in culture was dramatically decreased by ox-LDL in a dose- and time-dependent manner (Fig. 2B). When ox-LDL concentration was increased to 40 μg/ml, the cell number was reduced to less than 30% of control (P < 0.001, n= 4).
Fig 2.
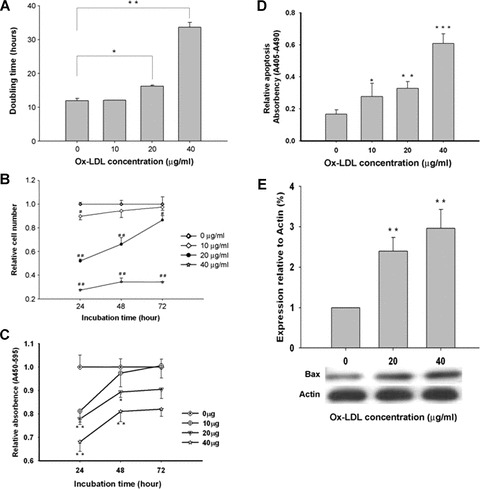
Ox-LDL inhibited self-renewal and induced apoptosis of MAPCs. When MAPCs were incubated with ox-LDL (from 0 to 40 μg/ml), cell proliferation was significantly decreased with increased doubling time and reduced cell population in culture in a time- and dose-dependent fashion. Cell apoptosis was also dramatically increased dose-dependently by ox-LDL as determined by ELISA along with enhanced expression of proapoptotic protein Bax as measured by Western blot. No effect of native LDL was observed on the self-renewal and apoptosis of MAPCs (data not shown). *P< 0.05 versus 0 μg/ml (n = 4); **P< 0.01 versus 0 μg/ml (n= 4); ***P< 0.001 versus 0 μg/ml (n= 4). (A) The doubling time was dose-dependently increased when MAPCs were exposed to ox-LDL. (B) The number of MAPCs was significantly reduced when incubated with ox-LDL in a time- and dose-dependent manner. (C) Ox-LDL dramatically inhibited MAPCs proliferation in a time- and dose-dependent manner as determined by BrdU proliferation assay. (D) Apoptotic cell death of MAPCs was significantly increased by ox-LDL as measured by ELISA. (E) Bax expression was substantially enhanced in MAPCs by ox-LDL as determined by Western blot.
Experiments were then performed to determine the mechanisms for the reduced cell number by ox-LDL. Ox-LDL significantly inhibited the proliferation of MAPCs in a concentration-and time-dependent manner (Fig. 2C). The inhibitory effect was most prominent at 24 hrs of incubation with 40 μg/ml ox-LDL (P< 0.001, n= 4). Of note, native LDL had no effect on the number and proliferation of MAPCs (data not shown). There was a low level of apoptosis in MAPCs cultured in normal condition (Fig. 2D). Cell apoptosis was significantly increased dose-dependently by up to 3.5-fold when MAPCs exposed to ox-LDL (from 0 to 40 μg/ml) for 24 hrs (P< 0.01, n= 4). When ox-LDL concentration was increased to 80 μg/ml, cell death occurred to over 90% of MAPCs as evaluated by Trypan blue exclusion assay (data not shown). In addition, the expression of proapoptotic protein Bax was significantly up-regulated by up to 3.0-fold in MAPCs incubated with ox-LDL for 24 hrs dose-dependently (from 0 to 40 μg/ml) as determined by Western blot (P< 0.01, n= 4; Fig. 2E). These data indicated that ox-LDL reduced MAPC population via a combination of decreased cell proliferation and enhanced cell apoptosis.
Ox-LDL attenuated endothelial differentiation of MAPCs
As expected, the differentiating MAPCs started to express endothelial markers vWF, CD31 and Flk-1 by day 7 of differentiation (Fig. 3). The differentiated cells also stained positive for vWF, and formed capillary structures on growth factor reduced-Matrigel by day 14 of differentiation (Fig. 6G), suggesting that the MAPCs-derived cells are indeed functional ECs. When treated with ox-LDL, expressions of vWF, CD31 and Flk-1 were all significantly decreased dose-dependently in the cells with dramatic reduction in mRNAs and proteins (Fig. 3), indicating that ox-LDL significantly impaired the endothelial differentiation of MAPCs. There was no vascular structure formation by ox-LDL-treated cells after 2 weeks of differentiation on the Matrigel (Fig. 6G), further suggesting that the cells were not ECs. Native LDL did not change the endothelial differentiation of MAPCs (data not shown).
Fig 3.
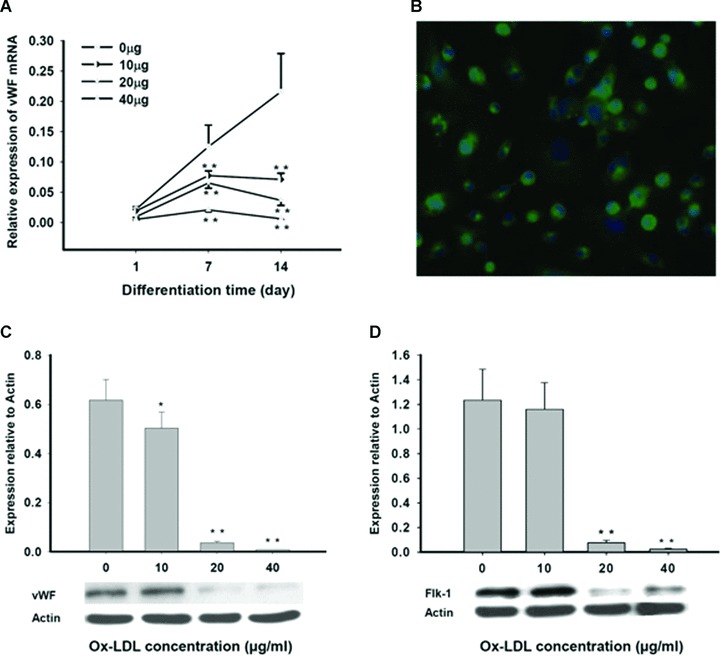
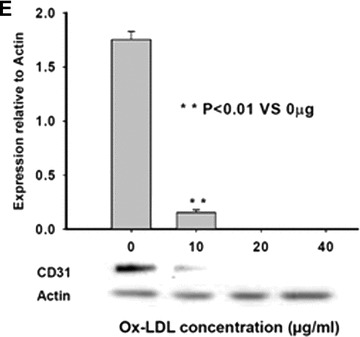
Ox-LDL suppressed endothelial differentiation of MAPCs. Endothelial differentiation of MAPCs was dose-dependently inhibited in the presence of ox-LDL with decreased expression of endothelial markers including vWF, Flk-1 and CD31, as well as impaired vascular structure formation. Native LDL had no impact on endothelial differentiation of MAPCs (data not shown). *P< 0.05 versus control (n= 4); **P< 0.01 versus control (n= 4). (A) The transcriptional expression of vWF (mRNA) in MAPCs during their endothelial differentiation was dramatically inhibited by ox-LDL as analysed by RT-PCR. (B) The majority of the cells stained positive for vWF after 2 weeks of endothelial differentiation under normal condition. (C) vWF protein content in the differentiating MAPCs was significantly decreased by ox-LDL as analysed by Western blot at week 2 of differentiation. (D) Ox-LDL substantially suppressed Flk-1 expression in the differentiating cells as analysed by Western blot at week 2 of differentiation. (E) CD31 expression in the differentiating cells was dramatically reduced by ox-LDL as analysed by Western blot at week 2 of differentiation.
Fig 6.
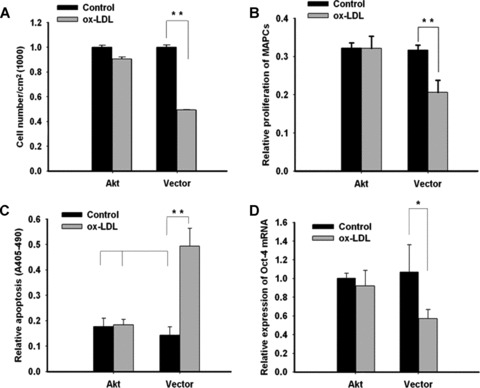
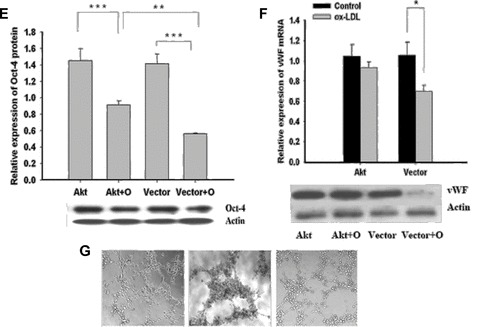
Effects of Akt over-expression on MAPCs in the presence of ox-LDL. Experiments were performed to evaluate the behaviour of MAPCs that overexpressed active Akt in the presence of ox-LDL (40 ug/ml). Akt overex-pression completely protected the cells against the actions of ox-LDL (40 ug/lm) on MAPCs. (A and B) Ox-LDL-induced inhibition of cell proliferation was completely reversed after Akt transfection. (C) Ox-LDL-induced apoptosis was effectively prevented in MAPCs after Akt trasnfection. (D and E) Suppressed Oct-4 expression by ox-LDL was significantly enhanced in MAPCs after Akt transfection. (F) Impaired vWF expression by ox-LDL was completely restored in MAPCs at week 2 of endothelial differentiation after Akt transfection. (G) Vascular structure formation by MAPC-derived cells at week 2 of differentiation was observed on Matrigel under normal condition in the presence of native LDL (left-hand panel). When MAPCs were treated with ox-LDL, no vascular structures were generated on Matrigel at week 2 of differentiation (middle panel). When MAPCs were transfected with Myr-Akt, the diminished capability of the MAPC-derived cells to form vascular structures on Matrigel by ox-LDL was completely recovered (right-hand panel). Akt, MAPCs transfected with active Akt; Akt+O, Cells were transfected with active Akt and treated treated with ox-LDL; Vector, MAPCs transfected with control vector; Vector+O, MAPCs transfected with control vector and treated with ox-LDL. *P < 0.05 versus control (n= 4); **P < 0.01 versus control (n= 4); ***P < 0.001 versus control (n= 4).
Ox-LDL selectively suppressed Akt phosphorylation
Serine/threonin protein kinase Akt (Akt) and extracellular signal-regulated kinase (ERK1/2) signalling are important to cell proliferation and survival. In this study, a detectable level of Akt and ERK1/2 phosphorylation was observed in MAPCs under normal condition (Fig. 4). Akt phosphorylation was significantly decreased dose-dependently by up to 97.6% (n= 4, P< 0.01) in MAPCs when incubated with ox-LDL for 24 hrs (Fig. 4A). No change in ERK1/2 phosphorylation by ox-LDL was observed in MAPCs (Fig. 4B), indicating that x-LDL selectively interrupted Akt signalling in MAPCs. Native LDL had no impact on either Akt or ERK1/2 activation in MAPCs.
Fig 4.
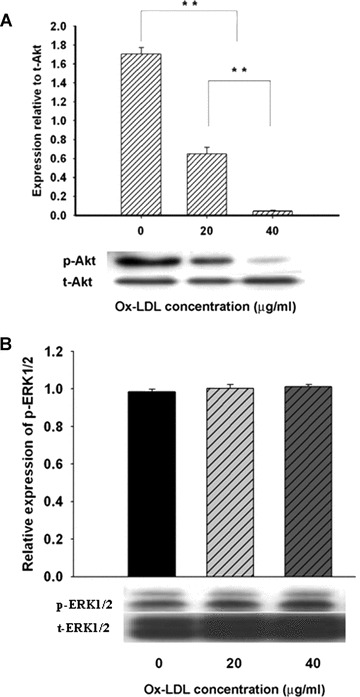
Effect of ox-LDL on Akt and ERK1/2 phosphorylation in MAPCs. When MAPCs were incubated with ox-LDL (from 0 to 40 μg/ml) for 24 hrs, Akt phosphorylation was dramatically inhibited in the cells (A) in a dose-dependent manner; although ERK1/2 activation was not affected (B). Native LDL had no effect on Akt or ERK1/2 phosphorylation (data not shown). p-Akt, phosphorylated Akt; t-Akt, total Akt; p-ERK1/2, phosphorylated ERK1/2; t-ERK1/2, total ERK1/2; **P< 0.01 versus 0 μg/ml (n= 4).
Akt overexpression reversed the effect of ox-LDL on MAPCs
To investigate the role of Akt signalling in mediating the effect of ox-LDL on MAPCs, the cells were transfected with a constitutively active Akt (Myr-Akt). After 24 hrs of transfection, over 60% MAPCs were successfully transfected with Akt plasmids as determined by fluorescence microscopy and cytometry (Fig. 5). The transfected cells exhibited a dramatic increase of over 30-time in phosphorylated Akt over control (n= 4, P< 0.001).
Fig 5.
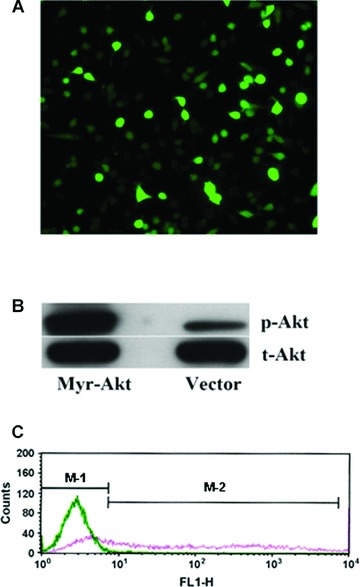
Transfection of MAPCs with Akt plasmids. After 24 hrs of transfection, the majority of MAPCs (over 60%) were successfully transfected with the constitutively active Akt plasmids as determined by fluorescence microscopy and cytometry as well as Western blot. (A) A fluorescence image of the transfected cells (20×) showing the majority of the cells are positive for the plasmids. (B) Phosphorylated Akt level was dramatically increased in the cells after transfection with Akt vectors as determined by Western blot. (C) Akt transfection efficiency in MAPCs was evaluated by flow-cytometry. The blue line presented the cells in the control group (in the M-1 Zone) that showed no positive expression of eGFP; whereas the red line demonstrated that over 60% of the cells (in the M-2 zone) were positive for eGFP after Akt transfection.
Interestingly, Akt overexpression had no effect on cell proliferation, apoptosis and Oct-4 expression in MAPCs under normal conditions (Fig. 6), suggesting that baseline Akt signalling was adequate to maintain their normal function. However, decreased cell proliferation and enhanced apoptosis by ox-LDL were completely prevented in MAPCs overexpressing active Akt (Fig. 6A-C). With Akt overexpression, the cell population was only decreased by 7.3 ± 4.6% (n= 4, P> 0.05) by 40 ug/ml ox-LDL; whereas the cell number in control group (transfected with empty vector) was decreased by 50.7 ± 2.8% (n= 4, P< 0.01) (Fig. 6A). Similar results were obtained for cell proliferation and apoptosis in MAPCs that overexpressed active Akt (Fig. 6B and C). Surprisingly, the inhibitory effect of ox-LDL on Oct-4 transcriptional expression (mRNA) was completely reversed in MAPCs transfected with Myr-Akt (Fig. 6D); whereas Oct-4 protein level was only partially recovered (Fig. 6E). Ox-LDL treatment (40 ug/ml) for 24 hrs lead to 37.5 ± 3.1% reduction in Oct-4 protein (n= 4, P< 0.001) in MAPCs transfected with Myr-Akt; whereas Oct-4 protein content was decreased by 59.3 ± 1.2% in control (n= 4, P< 0.001) (Fig. 6E).
Akt overexpression also completely restored the potential of MAPCs to differentiate into functioning ECs impaired by ox-LDL as reflected by normalization of diminished expression of endothelial marker vWF and in vitro vascular structure formation (Fig. 6F and G).
Discussion
In this study, we reported that ox-LDL significantly downregulated Oct-4 expression in MAPCs, inhibited proliferation, promoted apoptosis and suppressed endothelial differentiation of MAPCs in association with selective suppression of Akt phosphorylation. Akt overexpression reversed the effects of ox-LDL on MAPCs. We demonstrated for the first time that ox-LDL modified the behaviour of bone marrow stem cells with suppression of Oct-4 expression and inhibition of self-renewal as well as endothelial differentiation through disruption of Akt signalling.
Oct-4 expression in stem cells is tightly regulated, and critical to maintaining the cells in an undifferentiated state, their self-renewal capability and regulation of their differentiation [7, 9, 25]. However, the regulatory mechanisms for Oct-4 expression are poorly understood. A number of factors are involved in Oct-4 expression including leukemia inhibitory factor (LIF), serum and retinoic acid [26]. LIF and serum are required for Oct-4 expression in mouse ESCs; whereas retinoic acid suppresses Oct-4 expression. Oct-4 is also expressed abundantly in rat MAPCs [13, 21]. This was confirmed in the present study at both protein and transcriptional levels. Oct-4 expression was significantly decreased by ox-LDL in MAPCs, suggesting that ox-LDL could modify the behaviour of bone marrow stem cells.
One of the important features for stem cells is self-renewal, a process that the cells divide to produce two identical daughter cells. Oct-4 is important in stem cell self-renewal through a complex and sophisticated transcriptional network of genes and growth factors [27–29]. As ox-LDL decreases Oct-4 expression in MAPCs, it may impair their capability of self-renewal. Indeed, the number of MAPCs was dramatically decreased with significant increase in their doubling time when exposed to ox-LDL, indicating that self-renewal of MAPCs was suppressed by ox-LDL. Ox-LDL-induced decrease in cell population was a combination of decreased proliferation and increased apoptosis of MAPCs. Increased apoptosis by ox-LDL was further supported by enhanced expression of proapoptotic protein Bax in ox-LDL-treated cells.
The effect of ox-LDL on its target cells is very variable, depending on the cell type. Ox-LDL promotes proliferation of macrophages and vascular smooth muscle cells, and inhibits apoptosis of macrophages and monocytes through MAPK activation [16–18, 30–32]. On the other hand, ox-LDL inhibits proliferation and promotes apoptosis of vascular ECs and EPCs [4, 15]. In this study ox-LDL inhibited cell proliferation, induced apoptosis of MAPCs, and caused cell necrosis at high concentration (80 μg/ml), as well as suppressed endothelial differentiation of MAPCs. The EPC number and function are known to be reduced in hyperlipidemic patients. Ox-LDL inhibits EPC proliferation and differentiation, and suppress their function and survival [4, 5, 33, 34], suggesting that ox-LDL negatively affect EPCs through multiple mechanisms. The data from this study suggest that ox-LDL reduces EPC number and function by reducing stem cell pool available for differentiation into EPCs, and decreasing differentiation of bone marrow stem cells into EPCs.
PI3K/Akt signalling is involved in the regulation of self-renewal of mouse embryonic and spermatogonial stem cells [35, 36]. Akt overexpression is reported to adequately maintain the pluripotency of mouse and monkey ESCs in undifferentiated phenotypes, reduces the apoptosis of mesenchymal stem cells, and enhances cardiac protection during cell therapy [37, 38]. Ox-LDL selectively suppressed Akt phosphorylation in MAPCs in parallel to decreased Oct-4 expression, reduced cell proliferation, enhanced apoptosis and endothelial differentiation in our study. Akt overexpression effectively reversed the effects of ox-LDL on MAPCs, suggesting that Akt signalling is critical to the action of ox-LDL on MAPCs.
This is the first time to demonstrate that Akt signalling is involved in regulating Oct-4 expression in stem cells. However, baseline Akt signalling is sufficient to maintain adequate Oct-4 expression in MAPCs under normal conditions because its expression was unchanged with Akt overexpression. Impaired Akt signalling is also important to ox-LDL-mediated actions in other cells including EPCs and vascular smooth muscle cells [33, 39, 40]. Our data also indicate that maintaining an intact Akt signalling (such as Akt over-expression) may provide an effective way to protect the cells like MAPCs against the toxicity of ox-LDL.
In conclusion, our study demonstrated that ox-LDL modified the behaviour of bone marrow stem cells including Oct-4 expression, self-renewal and endothelial differentiation through inhibition of Akt signalling. The data from this study may provide a molecular explanation for decreased number and function of EPCs in the setting of hyperlipidemia. These data may also have important clinical impact on patient selection for cell therapy with bone marrow stem cells especially for those with poorly controlled hyperlipidemia.
Acknowledgments
This work was supported by NIH K08 HL075410 (ZL). The authors thank Dr. Susheela Tridandapani in the Davis Heart and Lung Research Institute at the Ohio State University Medical Center for her expertise assistance on Akt transfection.
References
- 1.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 2.Chen JZ, Zhang FR, Tao QM, et al. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci. 2004;107:273–80. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B, Ma FX, Liu PX, et al. Impaired therapeutic vasculogenesis by transplantation of OxLDL-treated endothelial progenitor cells. J Lip Res. 2007;48:518–27. doi: 10.1194/jlr.M600251-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Chen J, Tao Q, et al. Effects of ox-LDL on number and activity of circulating endothelial progenitor cells. Drug Chem Toxicol. 2004;27:243–55. doi: 10.1081/dct-120037505. [DOI] [PubMed] [Google Scholar]
- 5.Imanishi T, Hano T, Matsuo Y, et al. Oxidized low-density lipoprotein inhibits vascular endothelial growth factor-induced endothelial progenitor cell differentiation. Clin Exp Pharm Phys. 2003;30:665–70. doi: 10.1046/j.1440-1681.2003.03894.x. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shushan E, Thompson JR, Gudas LJ, et al. Rex-1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct-3/4 and Oct-6 binding to an octamer site and a novel protein, Rox-1, binding to an adjacent site. Mol Cell Biol. 1998;18:1866–78. doi: 10.1128/mcb.18.4.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 8.Vassilieva S, Guan K, Pich U, et al. Establishment of SSEA-1- and Oct-4-expressing rat embryonic stem-like cell lines and effects of cytokines of the IL-6 family on clonal growth. Exp Cell Res. 2000;258:361–73. doi: 10.1006/excr.2000.4940. [DOI] [PubMed] [Google Scholar]
- 9.Ivanova N, Dobrin R, Lu R, et al. Dissecting self-renewal in stem cells with RNA interference. Nature. 2006;442:533–8. doi: 10.1038/nature04915. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Vaessen B, Lenvik T, et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 11.Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann NY Acad Sci. 2001;938:231–33. doi: 10.1111/j.1749-6632.2001.tb03593.x. [DOI] [PubMed] [Google Scholar]
- 12.Reyes M, Dudek A, Jahagirdar B, et al. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Inves. 2002;109:337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulloa-Montoya F, Kidder BL, Pauwelyn KA, et al. Comparative transcriptome analysis of embryonic and adult stem cells with extended and limited differentiation capacity. Genome Biol. 2007;8:1–20. doi: 10.1186/gb-2007-8-8-r163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu L, Jiang J, Hao H, et al. Nitric oxide enhances Oct-4 expression in bone marrow stem cells and promotes endothelial differentiation. Eur J Pharmacol. 2008;591:59–65. doi: 10.1016/j.ejphar.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Mehta JL, Haider N, et al. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–6. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- 16.Yang CM, Chiu CT, Wang CC, et al. Activation of mitogen-activated protein kinase by oxidized low-density lipoprotein in canine cultured vascular smooth muscle cells. Cell Signal. 2000;12:205–14. doi: 10.1016/s0898-6568(99)00087-x. [DOI] [PubMed] [Google Scholar]
- 17.Hundal RS, Salh BS, Schrader JW, et al. Oxidized low density lipoprotein inhibits macrophage apoptosis through activation of the PI 3-kinase/PKB pathway. J Lip Res. 2001;42:1483–91. [PubMed] [Google Scholar]
- 18.Namgaladze D, Kollas A, Brüe B. Oxidized LDL attenuates apoptosis in monocytic cells by activating ERK signaling. J Lipid Res. 2008;49:58–65. doi: 10.1194/jlr.M700100-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZY, Wong IY, Leung MW, et al. Characterization of antioxidants present in bitter tea (Ligustrum pedunculare) J Agric Food Chem. 2002;50:7530–5. doi: 10.1021/jf0206421. [DOI] [PubMed] [Google Scholar]
- 20.Cominacini L, Garbin U, Davoli A, et al. A simple test for predisposition to LDL oxidation based on the fluorescence development during copper-catalyzed oxidative modification. J Lip Res. 1991;32:349–58. [PubMed] [Google Scholar]
- 21.Breyer A, Estharabadi N, Oki M, et al. Multipotent adult progenitor cell isolation and culture procedures. Exp Hematol. 2006;34:1596–601. doi: 10.1016/j.exphem.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Jiang Y, Hao H, et al. Endothelial nitric oxide synthase is dynamically expressed during bone marrow stem cell differentiation into endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1760–5. doi: 10.1152/ajpheart.01408.2006. [DOI] [PubMed] [Google Scholar]
- 23.Lakshmipathy U, Pelacho B, Sudo K, et al. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004;22:531–43. doi: 10.1634/stemcells.22-4-531. [DOI] [PubMed] [Google Scholar]
- 24.Ganesan LP, Wei G, Pengal RA, et al. The serine/threonine kinase Akt Promotes Fc gamma receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J Biol Chem. 2004;279:54416–25. doi: 10.1074/jbc.M408188200. [DOI] [PubMed] [Google Scholar]
- 25.Pesce M, Scholer HR. Oct-4: gatekeeper in the beginnings of mammalian development. Stem cells. 2001;19:271–8. doi: 10.1634/stemcells.19-4-271. [DOI] [PubMed] [Google Scholar]
- 26.Faherty S, Kane MT, Quinlan LR. Self-renewal and differentiation of mouse embryonic stem cells as measured by Oct 4 gene expression: effects of lif, serum-free medium, retinoic acid, and dbcAMP. In Vitro Cell Dev Biol Anim. 2005;41:356–63. doi: 10.1007/s11626-005-0008-0. [DOI] [PubMed] [Google Scholar]
- 27.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–94. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oatley JM, Avarbock MR, Telaranta AI, et al. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103:9524–9. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Babaie Y, Herwig R, Greber B, et al. Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells. 2007;25:500–10. doi: 10.1634/stemcells.2006-0426. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton JA, Myers D, Jessup W, et al. Oxidized LDL can induce macrophage survival, DNA synthesis, and enhanced prolif-erative response to CSF-1 and GM-CSF. Arterioscler Thromb Vasc Biol. 1999;19:98–105. doi: 10.1161/01.atv.19.1.98. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura T, Sakai M, Kobori S, et al. Two intracellular signaling pathways for activation of protein kinase C are involved in oxidized low-density lipoprotein-induced macrophage growth. Arterioscler Thromb Vasc Biol. 1997;17:3013–20. doi: 10.1161/01.atv.17.11.3013. [DOI] [PubMed] [Google Scholar]
- 32.Eto H, Miyata M, Kume N, et al. Expression of lectin-like oxidized LDL receptor-1 in smooth muscle cells after vascular injury. Biochem Biophys Res Comm. 2006;341:591–8. doi: 10.1016/j.bbrc.2005.12.211. [DOI] [PubMed] [Google Scholar]
- 33.Ma FX, Zhou B, Chen Z, et al. Oxidized low density lipoprotein impairs endothelial progenitor cells by regulation of endothe-lial nitric oxide synthase. J Lip Res. 2006;47:1227–37. doi: 10.1194/jlr.M500507-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Imanishi T, Hano T, Sawamura T, et al. Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction. Clin Exp Pharmacol Physiol. 2004;31:407–13. doi: 10.1111/j.1440-1681.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 35.Paling NR, Wheadon H, Bone HK, et al. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–70. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 36.Lee J, Kanatsu-Shinohara M, Inoue K, et al. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–9. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe S, Umehara H, Murayama K, et al. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- 38.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 39.Chavakis E, Dernbach E, Hermann C, et al. Oxidized LDL inhibits vascular endothelial growth factor-induced endothelial cell migration by an inhibitory effect on the Akt/endothelial nitric oxide synthase pathway. Circulation. 2001;103:2102–7. doi: 10.1161/01.cir.103.16.2102. [DOI] [PubMed] [Google Scholar]
- 40.Chien MW, Chien CS, Hsiao LD, et al. OxLDL induces mitogen-activated protein kinase activation mediated via PI3-kinase/Akt in vascular smooth muscle cells. J Lip Res. 2003;44:1667–75. doi: 10.1194/jlr.M300006-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Di Santo S, Diehm N, Ortmann J, et al. Oxidized low density lipoprotein impairs endothelial progenitor cell function by downregulation of E-selectin and integrin alpha(v)beta5. Biochem Biophys Res Commun. 2008;373:528–32. doi: 10.1016/j.bbrc.2008.06.066. [DOI] [PubMed] [Google Scholar]


