Abstract
Aiming for regeneration of severed or lost parts of the body, the combined application of gene therapy and tissue engineering has received much attention by regenerative medicine. Techniques of molecular biology can enhance the regenerative potential of a biomaterial by co-delivery of therapeutic genes, and several different strategies have been used to achieve that goal. Possibilities for application are many-fold and have been investigated to regenerate tissues such as skin, cartilage, bone, nerve, liver, pancreas and blood vessels. This review discusses advantages and problems encountered with the different gene delivery strategies as far as they relate to tissue engineering, analyses the positive aspects of polymeric gene delivery from matrices and discusses advances and future challenges of gene transfer strategies in selected tissues.
Keywords: gene therapy, tissue engineering, scaffold, gene-activated matrix, regeneration growth factor
Introduction
Replacing lost or severed parts of the body or restoring their function is the goal of regenerative medicine. In recent years, two emerging new technologies have provided new and exciting potential towards that goal: Tissue Engineering and Gene Therapy. Tissue Engineering, according to the definition of Langer and Vacanti, is ‘an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue functions’[1]. The first efforts were mainly concerned with the generation of bioengineered skin substitutes by combination of fibroblasts with a collagen matrix [2] or collagen gel [3], or keratinocyte sheet grafts [4]. Over the past 15 years, tissue-engineering applications have been continuously extended to a wide variety of tissues and organs and the scaffolds employed for initiation of tissue regeneration have become more and more sophisticated. Given that initial expectations regarding the therapeutic application of tissue engineering could not completely be transferred into clinical reality, new strategies are being investigated to enhance its potential.The synthesis of tissue engineering with innovative methods of molecular biology appears very promising, and the delivery of therapeutic genes has become of particular interest in this context.
Gene Therapy can be defined as the introduction of genetic material into cells with the intent of altering cellular function or structure at the molecular level to improve a clinical outcome [5]. Growth factors are small proteins that can be synthesized by a wide variety of cells and play an important role in the regulation of cell proliferation, migration, differentiation and matrix synthesis [6]. Because of their crucial role in regenerative processes such as soft tissue repair, bone formation, cartilage healing and nerve regeneration, considerable efforts have been undertaken to administer a large number of different growth factors [6].Topical application of recombinant growth factors has shown some clinical benefit, resulting in an FDA approval for recombinant Platelet-derived growth factor Regranex® for the treatment of diabetic foot ulcers [7]. On the other hand, application of growth factors as recombinant proteins is associated with draw-backs such as short half-life, low bioavailability, enzymatic inactivation and high cost of production [8]. Using gene transfer techniques, the localized, long-term production of therapeutic levels of the desired cytokine may be achieved without the disadvantages of recombinant protein application. Given these reasons, gene transfer of cytokines for the purpose of tissue regeneration is of great interest. The aim of this review is to outline possible applications of gene therapy as far as they apply to tissue engineering as well as basic principles of gene therapy, vectors and gene delivery.
Methods of gene delivery
There are two fundamentally different gene delivery systems, viral and non-viral. Both approaches can be carried out in vivo or ex vivo[9]. The in vivo approach involves direct delivery of genes to the target cell with-in the living organism and is attractive for its technical straightforwardness, making possible clinical application much easier. Its drawbacks are that targeting is not completely specific and transfection efficiency is usually lower than with ex vivo gene transfer. The ex vivo approach requires removal of the cells from the host, in vitro cultivation, expansion and genetic modification followed by implantation back into the tissue. This approach is laborious and more expensive, but has the advantage that selective genetic manipulation of the desired cell type and quantification of the transfection efficiency in vitro is possible [10].
Non-viral gene transfer is referred to as ‘transfection’ and is dependent on chemical or physical delivery of the genetic material and therefore relies on cellular transport systems for uptake and expression in the host cell.The most important examples of such techniques include direct injection of naked DNA [12], electroporation [13], particle bombardment [14] and cationic liposomes [15]. Non-viral gene therapy offers several advantages over approaches that rely on viral vectors: the respective techniques are easy, simple, direct and, with the exception of cationic lipo-somes, inexpensive [11].
A number of different viruses have gained attention as vectors for gene delivery. To make them applicable for gene therapy, they undergo genetic modification before they are used to introduce the gene of interest into a host cell, a process referred to as viral ‘trans-duction’ or ‘infection’. In most cases, viral vectors exhibit a higher transfection efficiency compared with that of non-viral gene delivery systems. Some of the most common viruses used in gene therapy studies that will be discussed in this review are adenoviruses, adeno-associated viruses, retroviruses and herpes simplex virus (HSV). Other viral vector systems such as vaccinia virus and poxvirus have also been inves-tigated for gene therapy applications lately, but are less common and will not be part of this review.
Gene delivery from polymeric biomaterials constitutes an alternative strategy that attempts to circumvent disadvantages of the viral and non-viral gene delivery methods outlined earlier. The two basic principles are referred to as polymeric release or substrate-mediated delivery [16]. Polymeric release describes the release of a therapeutic protein (generated from the foreign DNA) in the most efficient way possible, which may be either rapidly or over an extended period of several days, weeks or even months, depending on the therapeutic setting and requirements. Substrate-mediated delivery, on the other hand, implicates that DNA is immobilized to a biomaterial, with the goal of stimulating adhesion and migration of cells that contribute to tissue regeneration. Gene delivery from polymeric biomaterials can be accomplished either through direct incorporation of the DNA into the scaffold or through cells genetically manipulated by viral or non-viral gene transfer to express a certain gene prior to seeding them onto the matrix. Gene delivery from polymeric biomaterials may provide better protection against DNA degradation, a better control of transgene and protein levels, reduction of systemic side effects and minimization of the inflammatory response when viral vectors are used in combination with the polymer [16].
Gene therapy has gained more and more attention as a therapeutic tool where new experimental protocols have emerged and long-existing vector systems have been constantly refined and improved. Particularities, advantages and drawbacks of common gene delivery strategies will be discussed in detail in the following paragraphs. Table 1 summarizes the most commonly used gene transfer techniques. While Figure 1 represents an illustration of the basic fundamentals of gene transfer, examples of gene delivery strategies from matrices for tissue regeneration are provided in Table 2.
1.
Overview of gene delivery techniques
| Gene Delivery Technique | Advantages | Disadvantages |
|---|---|---|
| Direct injection of naked DNA/Plasmid DNA | Simple, local delivery, unlimited gene size, non-toxic, most efficient in cardiac and skeletal muscle cells | Only applicable to tissues accessible by direct injection, very low transfection efficiency, transient gene expression only |
| Gene Gun | Can deliver large amounts of DNA, technically simple | Non-specific, physical damage to cell required for DNA uptake, low transfection efficiency |
| Microseeding | Can deliver large amounts and different types of DNA | Low transfection efficiency, cellular damage, limited experience |
| Electroporation | Technically simple, can deliver large amounts of DNA | Non-specific, complex equipment, damage to cell membrane required for DNA uptake, low transfection efficiency |
| Cationic Liposomes | Technically simple, local delivery, can transfect any cell type, no immunogenicity | Cannot target specific cell types, low transfection efficiency |
| Retrovirus | Transduces many different cell types, high efficiency of ex vivo transduction, long-term gene expression | Transduces dividing cells only, inefficient transduction in vivo, risk of insertional mutagenesis |
| Adenovirus | Transfects virtually all cell types, dividing and non-dividing cells, good transfection efficiency in vivo, no integration into host genome | Immune response, lack of permanent expression, potential wild-type breakthrough, small DNA insert size (8 kb) |
| Adeno-associated Virus | Transduces dividing and non-dividing cells, integrates to specific site at chromosome 19, long-term gene expression | Difficult to grow to high titres, risk of insertional mutagenesis, small DNA insert size (4.7 kb), possible immune response and inflammatory reaction |
| Herpes Simplex Virus 1 | Transduces wide variety of cell types, neurotropism, large DNA insert size (30 kb), long term expression feasible | Difficult to manipulate due to complex life cycle, risk of wild-type breakthrough |
1.
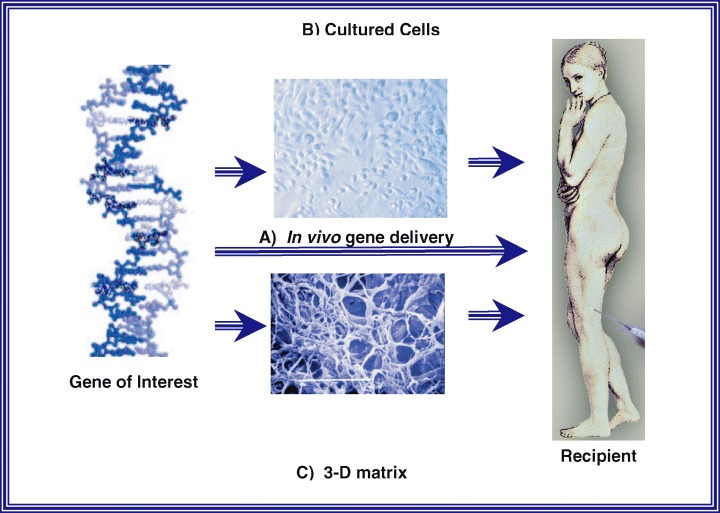
Genedelivery strategies in tissue engineering.ADNAsequence constituting the gene of interest which codes for the therapeutic protein is inserted into a suitable vector. Gene transfer into the host can now occur in four different ways: (A) Direct In vivo gene delivery or ex vivo gene transfer to either (B) cells or (C) Three-dimensional matrices, followed by transplantation of the genetically manipulated material into the host. Alternatively (B) and (C) can be combined whereby genetically modified cells are seeded onto threedimensional matrices followed by transplantation into the host.
2.
Gene delivery strategies from scaffolds for tissue regeneration
| Vector/gene/cell type and scaffold | Organ/Application | Result |
|---|---|---|
| Adenovirus/PDGF-B - Collagen/PVA | Skin (rat/subdermal) | Increased granulation tissue formation [101] |
| Granulation tissue and epithelialisation [102] | ||
| Plasmid/PDGF – Collagen | Skin (rabbit/ear dermal ulcer) | Prolonged EGF-expression in vivo[39] |
| Plasmid/EGF – Fibrin/Keratinocytes | Skin (mouse/full-thickness wounds) | Accelerated re-epithelialization [43] |
| Retrovirus/PDGF-B/fibroblasts - PGA | Skin (diabetic mouse/full thickness wound) | Epidermal hyperproliferation, increasedVEGF-secretion, accelerated vascularization[103] |
| Retrovirus/FGF-7/keratinocytes –acellular dermis | Skin (mouse/full-thickness wound) | |
| Plasmid/BMP-7/Periosteal MSC/ - PGA | Cartilage (rabbit/osteochondral defect) | Good transfection efficiency, therapeuticIGF-1 levels, improved cartilage repair [48] |
| Non-Liposomal Lipid/IGF-1/chondrocytes– alginate spheres | Cartilage (rabbit/osteochondral defect) | Increased bone formation [54] |
| Retrovirus/BMP-7/periosteal cells – PGA | Bone (rabbit/cranial defect) | New bone formation [31] |
| Plasmid/hPTH1-34 | Bone (dog/tibia and femur defects) | Significant bone regeneration after6 weeks [55] |
| Retrovirus/Sonic hedgehog/fibroblasts,MSC, fat-derived cells – alginate/collagen | Bone (rabbit/calvarial defect) | Improved survival of axotomized retinalganglion cells [104] |
| Plasmid/FGF-2, BDNF, NT-3 - PLL | Nerve (rat/optic nerve regeneration) | lacZ-expressing endothelial cells liningluminal graft surface 5 weeks afterimplantation [78] |
| Retrovirus/lacZ/endothelial cells – vessel | Blood Vessels(dog/carotid interposition grafts) | |
Non-viral techniques
Non-viral gene transfer techniques possess several advantages. The production of large amounts of non-viral vectors is much easier and the costs are lower. Most methods are simple and inexpensive, immunogenicity and toxicity are considered relatively low in most cases, while safety concerns are not an issue compared with viral vectors where the risk of recombination resulting in replication-competent virus that could cause disease needs to be addressed. Furthermore, non-viral gene transfer is usually characterized by transient gene expression and low-transfection efficiency. On the other hand, short-term gene expression may be desirable in clinical settings such as wound healing or bone regeneration. Long-term gene expression can be achieved by the selection of stable clones of cells transfected with plasmid DNA in vitro. This approach is more cumbersome and requires ex vivo manipulation, but it permits the selection of cell lines stably expressing a particular gene of interest over longer periods without the need to use a viral vector.
Naked DNA
Transduction using naked DNA likely represents the safest known method of gene delivery. Successful delivery and expression of genes in several different tissues has been achieved by injection using hypodermic needles. Even though the transduction efficiency is very low and the DNA is more susceptible to degradation, a clinical effect was seen nonetheless [12, 17], particularly for transduction of skeletal and cardiac muscle cells. Superior transduction of wounds was achieved using a ‘gene gun’ where DNA-coated gold particles are accelerated [18] or by micro-seeding which employs a set of oscillating needles to which DNA is delivered via an infusion pump [19]. These methods appear to improve trans-duction efficiency by increase of the surface area and induction of a micro-trauma of the treated tissue, thereby improving the DNA uptake. A disadvantage of the gene gun, however, is that foreign particles, that is, gold, are introduced into the tissue, while experiences with microseeding are very limited thus far. In electroporation, brief electric pulses are applied to cells to transiently create pores in the plasma membrane, thus allowing DNA diffusion into the cell. in vitro as well as in vivo gene delivery using electroporation has been reported [20]. The technique has been initially used for introduction of genes into plants and was later adapted for gene transfer to mammalian cells.
Cationic liposomes
These positively charged lipid vesicles form a complex with negatively charged DNA. Transfer of the DNA across the cell membrane appears to occur through an endocytosis-like process. Owing to their lack of immunogenicity, repeated deliveries in vivo are possible [11], and further improvement of transfection efficiency is possible by combination with Sendai virus fusion proteins [21]. Another advantage is the potential to deliver large amounts of DNA and that large transgenes can be incorporated. Nevertheless, the fact that transfection efficiency in vivo after gene transfer using cationic liposomes is low compared to that of gene transfer using viral vectors is a limiting factor to its usefulness.
Viral techniques
Numerous viruses are under investigation for use as gene transfer vectors, but the analysis provided in this review will be limited to some viruses commonly used in gene therapy: adenovirus, adeno-associated virus, retrovirus and HSV.Viral delivery systems have been demonstrated to be superior in terms of trans-duction efficiency because of their ability to efficiently infect cells. Their common drawbacks are increased technical demands in vector manufacturing and an increased risk of virus-associated toxicity. However, significant efforts have been made in recent years to generate vector systems employing replication-defective viruses with very little cytotoxicity. These altered viruses require propagation in cell lines that have been engineered to substitute absent viral functions.
Retrovirus
Retroviruses are particular in that they selectively infect proliferating cells. After entry into the cell through interactions between its envelope protein and receptor molecules on the target cell, the single-stranded RNA is reverse transcribed into double stranded DNA.The major advantage to retroviral vectors is integration into the host cell chromosome, offering stable transformation and sustained gene expression. Through non-specific integration into the host chromosome, however, there is a risk of insertional mutagenesis. Other drawbacks include a small size of possible DNA insert up to 8.5 kb and a lower yield of infectious viral titres, particularly in comparison to adenovirus. The most frequently used recombinant retroviral vectors are derived from murine leukaemia virus [5], where replication deficiency is achieved by replacing genes encoding essential viral proteins such as gag, pol and env with therapeutic genes. Retroviruses are the most common type of virus used in clinical trials investigating viral vectors for gene therapy thus far.
Adenovirus
In contrary to retrovirus, adenoviruses can infect dividing and non-dividing cells, and the viral genome stays episomal and is not integrated into the host genome. This feature and their ability to infect a broad range of cells such as skin, lung, liver, brain, muscle and blood vessels render adenoviruses promising and versatile vectors for gene therapy. Adenoviruses are linear double-stranded DNA viruses and can be rendered replication-defective by substitution of the essential E1 gene; further deletion of the E3 gene is commonly carried out to maximize the amount of transgene packaging capacity (up to 7.5 kb) without apparent effect on viral growth [8]. They can be produced to very high titres and therefore attain high levels of transgene expression. On the other hand, duration of transgene expression is often limited to short periods, potentially owing to a strong immune reaction that these viruses can induce in the host. This problem has been partially circumvented by the development of helper-dependent [22] and gutless [23] adenoviral vectors in which all viral coding sequences have been deleted.
Adeno-associated virus
These viruses have not been associated with human disease and can only grow in the presence of a helper virus such as adenovirus or herpes virus. The genome size is relatively small, measuring less than 5 kb, thereby allowing only insertion of small trans-genes up to 4.5 kb. Wild-type adeno-associated viruses are integrated into the host genome, display a unique tropism for chromosome 19q13.3 and can infect both dividing and non-dividing cells. These advantages make it a promising viral vector for long-term transgene expression [24].
Herpes simplex virus
HSV replicates in epithelial cells and establishes a life-long latent infection in neuronal cell bodies within the sensory ganglia of infected individuals, where the latent viral genome is maintained in an episomal state [25]. HSV infects a broad range of mitotic and post-mitotic cells and can effectively deliver transgenes not only to neurons but also to the heart, skeletal muscle, bladder, pituitary gland, articular joints, peripheral blood mononuclear cells and inguinal adipose tissue [26–30]. Because of its large genome, 30–50 kb of transgene can be introduced into recombinant HSV-1 vectors. At present, two major classes of HSV-1 vectors have been developed: replication-defective viruses and replication conditional mutants [31, 32].
Using the tetracycline gene switch technology [33], T-REx™ (Invitrogen, CA), Yao et al. constructed the first HSV-1 recombinant, CJ83193, capable of inhibiting its own replication and that of wild-type HSV-1 in normal cells as well as non-tetracycline repressor (tetR) expressing cells [34]. Using the lacZ gene as reporter, it was demonstrated that CJ83193 can be translated into a new class of HSV-based vector system for gene transfer to various tissues (Theopold et al., manuscript submitted for publication). In an effort to further explore the utility of regulated gene expression in gene therapy application, Yao et al. recently constructed a novel HSV-1 replication-defective virus that can lead to 300- to 1000-fold of tetracycline-regulated gene expression in the target cells [35]. This newly developed tet-regulatable HSV-1 vector system should be useful for studying gene function in the nervous system and delivering regulated gene expression in therapeutic applications, particularly in the treatment of CNS diseases.
Gene delivery from scaffolds for tissue engineering
The combined use of biomaterial scaffolds, drug delivery technology and gene therapy has great potential to provide improved tissue replacements [36]. In general, the DNA of interest, which is encoded either by a viral or non-viral vector, is positioned together with a biomaterial scaffold at the site of desired tissue regeneration to provide conduction and induction. Conduction describes the purpose of a matrix to maintain a space and provide physical support for new tissue to be generated [16]. Induction defines the role of the matrix as a drug delivery vehicle to stimulate tissue formation [16]. Drug delivery, that is, introduction of a gene of interest, can occur through plasmid DNA incorporated into the scaffold or through cells genetically manipulated in vitro before they are seeded onto a scaffold followed by transplantation into the host. Interaction with progenitor cells from the surrounding tissue plays a key role, where these cells can support tissue regeneration by uptake of DNA released from the scaffold, thereby assuming the role of a ‘bioreactor’ that produces a desired trans-gene such as a growth factor [37].
Numerous natural and synthetic materials have been investigated for potential use as scaffolds in tissue engineering [38], which can be categorized as either hydrophilic (e.g. hyaluronic acid [HA], collagen and poly(ethylene glycol) [PEG]) or hydrophobic (e.g. poly[lactide-co-glycolide]). Collagen and hyaluronan occur naturally and are involved in numerous physiological processes and are advantageous due to their safety. Synthetic polymers, on the other hand, can be designed specifically in terms of material, mechanical properties, porosity and degradation properties. Scaffolds can either be formed as a mesh of fibres or processed into a porous structure [16].
Viral and non-viral gene delivery through scaffolds has a number of advantages, including increased residence time within the tissue, superior protection against degradation and a reduced inflammatory response, particularly in the case of adenoviral vectors. Molecular interactions between vector and polymer determine whether the DNA is released from the polymer or bound to it. Polymeric release involves an encapsulation and release of DNA into the local microenvironment. Alternatively, substrate-mediated delivery employs immobilization of DNA to the scaffold surface.
A multitude of clinical trials involving gene therapy have been carried out for many different clinical conditions such as monogenic diseases, cancer, cardio-vascular disease and others. Lack of success has been frequently attributed to inefficient delivery, lack of stable gene expression, inappropriate levels of gene expression and immune clearance of either the vector or the cells expressing the foreign gene [5]. Polymeric-based gene delivery may be useful to circumvent these problems and thereby improve clinical outcome. Matrix-based delivery of non-viral and viral DNA has been employed for tumour therapy [39], bone [40], cartilage [41] and nerve regeneration [42] as well as wound healing [43] and muscle repair [44] and demonstrated the potential for extended local production of growth factors.
Gene therapy applications in tissue engineering
Skin and wound healing
Delayed wound healing caused by diabetes, steroid therapy, peripheral vascular disease as well as impaired function and aesthetics due to excessive scarring caused by burns are among the most common conditions treated by the plastic surgeon. Wound repair is a complex cascade of events that occurs in distinct phases and involves multiple interactions between cytokines, cells and extra-cellular matrix [45]. Skin is an easily accessible tissue, and the high turnover of the epidermis and the fact that a multitude of cytokines and growth factors crucial to the regener-ation process undergo short-term up- and down-regulation make it an ideal candidate for gene therapy: short-term gene expression, which is frequently a problem with gene therapy vectors, is desirable when trans-duction of the skin for wound repair is intended. Keratinocytes and Fibroblasts, the predominant cells of the skin, can easily be harvested and cultured, allowing for in vitro amplification and genetic manipulation. Many different protocols combining gene delivery, sometimes cell-based using ex vivo manipulated skin cells, with a tissue-engineered matrix have been tested. A thorough review on tissue engineering of cultured skin substitutes has recently been published as a part of this review series [46]. Briefly, a wide array of skin replacements have been under clinical investigation, including cultured autologous or allogenic keratinocyte grafts, autologous or allogenic composites, acellular
(e.g. dermal) biological matrices and cellular matrices including substances such as fibrin sealant and collagen, hyaluronic acid and others [46]. Here, we will focus on skin repair methods employing gene transfer techniques. Some of the earliest studies in growth factor gene delivery to wounds have been conducted by the Eriksson group in a porcine wound-healing model. Gene delivery of epidermal growth factor (EGF) using particle bombardment to partial-thickness wounds resulted in a dramatic increase in EGF protein concentration and significantly accelerated healing [18]. Transplantation of fibroblast suspensions transiently transfected with EGF plasmid could accelerate wound re-epithelialization [47]. Another study investigated the combination of a matrix for cell transplantation with a transfection system to release therapeutic proteins in vitro and in vivo. A hEGF-expressing plasmid was incorporated in a fibrin matrix, which was re-suspended with human keratinocytes. After transplantation to full thickness wounds on athymic mice, EGF expression was 180-fold increased compared to controls and persisted for 7 days [48]. Several other growth factors, including IGF-1 and platelet-derived growth factor BB (PDGF-BB) have been delivered to skin by means of gene therapy to stimulate wound healing. Retrovirally transduced human keratinocytes expressing IGF-1 showed an increased proliferative capacity after trans-plantation into nude mice, but failed to speed up wound healing [49]. Liposomal IGF-1 cDNA delivery showed a therapeutic effect in a rat burn wound model [50].
Matrices are useful not only as a carrier for ker-atinocyte or fibroblast transplantation to wounds but also as a vehicle for gene transfer [51]. Retroviral transduction of dermal fibroblasts from diabetic mice with the human PDGF-B gene was followed by seeding of these transgenic cells onto a PGA scaffold matrix, which was then applied to full-thickness skin wounds in diabetic mice. Re-epithelialization was accelerated by 40% compared with controls [52]. High-level long-term delivery of proteins in vivo can be achieved by in vitro transduction and selection of cells that constitutively express the gene of interest, but unregulated gene expression may also result in adverse effects. We recently transplanted fibroblasts stably transfected with a PDGF-B expressing plasmid into porcine full-thickness wounds. High wound fluid levels of PDGF-BB in vivo were associated with retarded wound healing compared with the wounds receiving untransfected fibroblasts only (Petrie et al., manuscript submitted for publication), emphasizing the need for regulatable gene expression.
Cartilage
Since cartilage is composed of a single cell type, the chondrocyte and an avascular tissue, one might expect it to be an easier tissue to regenerate, given that there is no need to generate a vascular supply or a multilayered tissue composed of multiple cell types. On the other hand, the need for nourishment through diffusion from adjacent tissue limits regeneration capacity of damaged cartilage. On the basis of different matrix compositions, cartilage can be divided into three major types: Hyaline cartilage (e.g. articular surfaces), elastic cartilage (e.g. external ear) and fibrocartilage (e.g. intervertebral discs). Fibrin and collagen matrices, synthetic hyaluronic acid sponges, hydroxylapatite and numerous polymers have all been employed as scaffolds in cartilage regeneration, providing a three-dimensional back-bone, which is necessary to preserve cell morphology. Chondrocytes are not accessible to direct in vivo vector DNA delivery due to the extra-cellular matrix surrounding them. Therefore the cells usually under-go genetic manipulation in vitro to express the desired gene and then seeded onto the scaffold prior to implantation. Autologous chondrocytes lose their chondrocytic phenotype when grown in vitro in monolayer, assuming a fibroblastic phenotype, thus limiting the number of cells that can be used for gene transfer and transplantation [53], even though several different protocols have been described to induce re-differentiation [54]. Mesenchymal stem cells (MSCs) may constitute a remarkable alternative as carriers of therapeutic genes. Different populations of multipotent MSCs can be harvested in large quantities from the iliac crest, periosteum or blood and can be stimulated with chondrogenic growth factors including BMP-2 and IGF-1. Bone morphogenic proteins (BMPs), IGF-1 and TGF- have emerged as some of the most promising candidate genes for induction of chondrogenesis via gene therapy. Intervertebral disc cells transfected with TGF-1 plasmid responded with a fourfold increase in proteoglycan synthesis compared with pellets containing cells transfected with the empty vector only when grown in three-dimensional pellet cultures [55]. BMP-2 and BMP-7 have also been successfully tested for their chondrogenic potential in cartilage tissue engineering. Periosteal MSCs transfected with BMP-7 cDNA embedded in Poly(glycolic acid) (PGA) scaf-folds contributed to formation of a larger amount of hyaline cartilage than untransfected cells [56]. Madry et al. performed gene transfer of IGF-1 using FuGene 6, a non-liposomal lipid formulation, and encapsulated the transfected articular chondrocytes into alginate spheres, resulting in a 35% transfection efficiency and therapeutic levels of IGF-1 expression for 32 days [57]. Despite these results, adenoviral and retroviral vectors are still considered superior in terms of transduction efficiency and long-term gene expression. Since direct articular injection of viral vectors was shown to result in inflammatory response and owing to concerns about systemic spread, ex vivo transduction of extracted cells has emerged as a promising alternative. This cell-mediated gene transfer has been shown to significantly increase the duration of protein expression in mouse joint compared with the direct intra-articular injection of the viral vector [58]. Ex vivo transduction of chon-drocytes using a GFP-expressing adenovirus resulted in expression of the marker gene for more than 60 days [59], while retroviral gene transfer to rabbit synovial cells could maintain gene expression for 6 weeks [60].
Bone
In contrary to cartilage, bone relies on direct nutrition by a vascular network and has great potential for spontaneous regeneration, even though this is fre-quently limited in the clinical situation through non-unions or critical-size segmental defects [61]. Many of the cytokines discussed with regard to cartilage repair also directly and indirectly affect bone growth. Evans and co-workers report healing of critical-sized segmental defects in immunocompetent rabbits and rats by direct injection of an adenovirus carrying the BMP-2 gene [62]. The same author investigated the distribution of the marker gene luciferase after injection of a recombinant adenovirus (Ad.luc) into a critical-sized defect in rabbits and found expression in the surrounding muscle, the scar in the defect, the cut ends of the bone and marrow cells. Even though these studies demonstrate that osseous lesions can be healed by direct injection of adenoviral vectors carrying osteogenic cDNAs, ex vivo manipulation of autologous osteogenic cells has the advantage of specifically transducing only the desired cell type.
Meinel et al. transduced MSCs with adenovirus containing a human BMP-2 gene at clinically reasonable viral concentrations and cultured for 4 weeks. Cells secreted a matrix that underwent mineralization on the silk fibroin scaffolds, forming clusters of osseous material and the expression of osteogenic marker proteins, and alkaline phosphates was significantly higher in the Ad-BMP-2 MSC group than in the control group that had received exogenous protein BMP-2. The results demonstrate that transfection resulted in higher levels of expression of osteogenic marker genes, no change in proliferation rate and did not impact the capacity of the cells to calcify tissues on these protein scaffolds, suggesting that exogenous addition of growth factors or morphogens can be replaced with transfected MSCs [63, Fig. 2, printed with permission from Elsevier].
2.
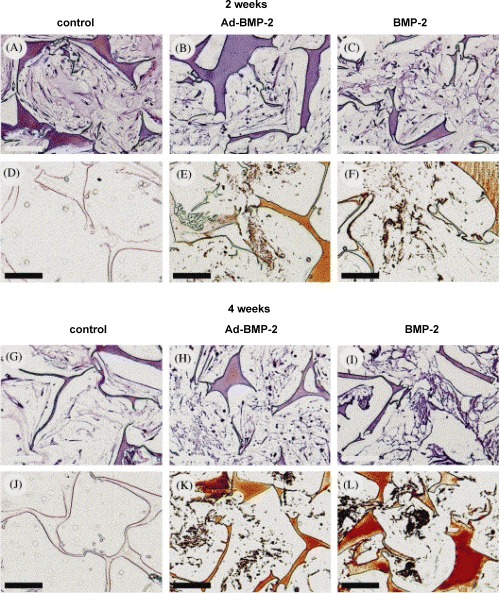
Combination of bone tissue engineering, gene therapy based on human mesenchymal stem cells (MSCs) and silk fibroin biomaterials to study the impact of viral transfection on MSC osteogenic performance. MSCs were transduced with adenovirus containing a human BMP-2 (Ad-BMP-2) gene at clinically reasonable viral concentrations and cultured for 4 weeks. Controls with non-transfected MSCs, but exposed to exogenous BMP-2 concentrations on an analogous time profile as that secreted by the Ad-BMP-2 group, were compared. Both the Ad-BMP-2 MSC group and the exogenous protein BMP-2 group strongly expressed osteopontin and bone sialoprotein. Cells secreted a matrix that underwent mineralization on the silk fibroin scaffolds, forming clusters of osseous material, as determined by micro-computed tomography. The expression of osteogenic marker proteins and alkaline phosphatase was significantly higher in the Ad-BMP-2 MSC group than in the exogenous protein BMP-2 group, and no significant differences in mineralization were observed in two of the three MSC sources tested.The results demonstrate that transfection resulted in higher levels of expression of osteogenic marker genes, no change in proliferation rate and did not impact the capacity of the cells to calcify tissues on these protein scaffolds.These findings suggest additional options to control differentiation where exogenous additions of growth factors or morphogens can be replaced with transfected MSCs. Light microscopy of construct cross sections after 2 weeks (A–F) or 4 weeks (G–L) of cultivation in osteogenic medium (B, C, E, F, H, I, K, L) or control medium (A, D, G, J). MSC were either transduced with Ad-BMP-2 (center column) or exposed to BMP-2 concentrations as secreted and measured for the Ad-BMP-2 transduced cells (right column) or cultivated in control medium (left column). Sections were stained with H&E (A–C; G–I) or with von Kossa (D–F; J–L). Bar length is 100 μm. Reprinted from [63], with permission from Elsevier.
Retroviral transduction of periosteal cells with BMP-7 and subsequent transplantation of these cells using PGA matrices significantly stimulated bone formation in rabbit cranial defects [64]. Despite the limitations of non-viral vectors for gene delivery to bone, Bonadio proposed a new approach described as a gene-activated matrix (GAM) where plasmids are incorporated in a collagen sponge, which is then inserted into a critical-sized bone defect [36]. Using this principle of substrate-mediated delivery, cells entering the GAM became transfected by its DNA and subsequently functioned as bioreactors, expressing transgenes locally for several weeks and leading to formation of significant amounts of bone. There are several different cell types suitable for ex vivo genetic manipulation and subsequent transfer into a defect with or without a scaffold. Bone-forming osteoblasts appear to be the most obvious choice, but they are mature cells with a decreased ability to proliferate. Hence, focus has shifted towards stem cells, highly proliferative cells capable of osteogenic differentiation and potential for bone formation. Since the use of omnipotent embryonic stem cells is limited because of ethic concerns, adult MSCs have become of particular interest. Edwards et al. delivered MSCs retrovirally transduced with the transcription factor Sonic hedgehog (Shh) to rabbit cranial bone defects in an alginate/collagen matrix. After 6 weeks, significant bone regeneration was detected [65]. Transcription factors are currently considered a very promising tool to induce bone formation. Bone marrow stromal cells engineered to constitutively express the osteoblastic transcription factor Runx2/Cbfa1 were integrated into three-dimensional polymeric scaffolds and displayed significantly up-regulated osteoblastic differentiation and mineralization in vitro and in vivo in an ectopic, non-osseous subcutaneous site. in vitro construct development to create a mineralized template before implantation significantly enhanced subsequent in vivo mineralized tissue formation [66].
Nerve
Nerve regeneration is a slow process that often results in less than desirable outcomes. The factors determining neuroregenerative capacity are still under investigation, and they include neurotrophic factors, neuronal-associated cells and nerve scaf-folds functioning as guidance channels. The composition of the ideal conduit is still unknown, despite intensive research investigating the properties of biodegradable and permanent, synthetic and natural scaffolds, in order to find an acceptable alternative to the current gold standard, the autologous nerve graft. The various options for guidance channels include synthetic substances such as lactate polymer, polyglactin mesh, polyethylene, silicone and silicone polymer tubes. Biologic conduits include autologous collagen, arterial and venous grafts and acellular muscle grafts [67]. Recently, the use of spider silk fibres as an innovative material for construction of a nerve conduit has been reported [68]. For nerve regeneration to occur, the presence of cells secreting neurotrophic factors such as nerve growth factor (NGF) is a necessity. Schwann cells appear to be important in this context, apart from their presumptive mechanical role in that they bridge the gap between axonal growth cone migration and basement membrane [69]. Genetically engineered fibroblasts as well as HEK-293 cells were shown to function as alternative sources of NGF [70, 71]. Neuronal progenitor cells [72] and neural stem cells [73] have recently gained interest for their presumptive neu-roregenerative potential, in part due to secretion of neurotrophic factors. These include NGF, brain-derived neurotrophic factor (BDNF), IGF-1, PDGF and others which act directly to promote survival and indirectly on regenerating axons via non-neuronal cells [67]. Traditional ways of growth factor delivery as a recombinant protein characteristically suffer from lack of dosage control, resulting in excessively high or subtherapeutic levels, while variable degradation is a common problem in microsphere technology. Gene therapy may constitute a promising attempt to provide controlled gene expression of growth factors in this context. Adenoviral gene delivery of BDNF was carried out in newborn rats that underwent unilateral facial nerve transaction 24 hrs after vector injection into nasolabial and lower lip muscles. Twice as many facial motoneurons had survived in the treatment group after 1 week (34.5% versus 18.2%) [74]. HSV-1 is particularly suitable for genetic manipulation of neurons. In a rat model of crush-traction injury, lumbar motor neurons were labelled by fluorogold injection into the sciatic nerve. Thirty minutes after spinal nerve crush combined with mechanical traction to produce a proximal axonal injury, a recombinant herpesvirus co-expressing the neurotrophic factor GDNF and the anti-apoptotic gene bcl-2 was stereotactically inoculated into the ventral horn. Recovery of distal nerve function was measured using the sciatic function index calculated from toe spread and print length. Five months after injury, animals that had received injections of the therapeutic vector showed substantially better functional recovery than control-injected animals [75]. The merit of HSV-1 vectors for neuro-regeneration could be further enhanced if one could control trans-gene expression from the virus. To this end, Yao et al. constructed a replication-defective HSV-1 recombinant where transgene expression can be finely regulated by tetracycline over three orders of magnitude in several different cell lines in vitro. Moreover, this vector demonstrated great safety in an in vivo mouse model where intra-cerebral injection did not result in any morbidity, while efficient tetracycline-dependent intra-cerebral expression of the lacZ reporter gene was shown [35].
Liver and endocrine pancreas
Hepatic cells have an excellent regenerative potential, enabling regeneration of the liver after secretion of up to 70% of the organ [76, 77]. Given this promising feature, Vacanti and Langer started to apply tissue-engineering principles to deliver hepatocytes using biodegradable polymers as a scaffold as early as almost 20 years ago [78]. Transplantation of hepatocytes may offer a promising therapeutic approach for patients with liver-based defects or fulminant hepatic failure, given the scarcity of available donor organs. More patients could benefit from a treatment adapted to their particular liver disease by making better use of the available donor tissue or using part of their own liver as a cell source for the treatment of hepatocyte-based metabolic disorders after ex vivo gene therapy of their hepatocytes [79]. Indeed, adenoviral, AAV, and lentiviral vectors have all been shown to efficiently transducer hepatocyte cultures. Follenzi et al. demonstrated efficient and long-lasting ex vivo and in vivo transduction of hepatocytes using a lentiviral vector. Expression could be restricted to hepatocytes after systemic injection of the virus by employing the promoter of the albumin gene in lentiviral vector construction [80]. Given these results, a combined approach of tissue engineering and gene transfer may be a promising strategy to treat liver diseases. There is also ongoing research investigating the potential use of liver stem cells or hepatic progenitor cells for tissue-engineering applications [81].
Pancreatic islet cell transplantation has been investigated as a method to provide insulin and thereby control blood glucose and potentially cure type 1 diabetes [82], but the low survival rate of transplanted cells and the shortage of donor pancreata constitutes a major problem. Since expansion of primary β-cells by growth factors is hampered by cell senescence and immortalization by genetic manipu-lation induces loss of differentiated function of the cells [83], Narushima created a human β-cell line functionally equivalent to primary β-cells by genetic manipulation. A reversibly immortalized human β-cell clone was established by retroviral transduction of primary β-cells with a retroviral vector containing human telomerase reverse transcriptase (hTERT) cDNA flanked by paired loxP recombination targets allowing deletion of TERT by Cre recombinase. After reversion, these cells could maintain normoglycemia in streptozotocin-induced immunodeficient mice for longer than 30 weeks [84]. The Morrison group created a ‘pancreatic organoid’ by seeding Matrigel-suspended islets into a vascularized chamber in order to optimize survival and demonstrated significantly improved glycemic control in SZT-induced diabetic mice for a 7-week follow-up period [85].
Blood vessels
The generation of functional blood vessels and a vascular network is of paramount importance towards the generation of a viable bioartificial organ or tissue. Different ex vivo and in vivo methods have been employed for diverse applications, which will be described in this paragraph. Ex vivo methodology includes seeding of endothelial cells or its precursors, which may or may not be genetically manipulated to express angioinductive factors onto a porous scaffold to generate a vascularized matrix. Generation of prosthetic grafts seeded with endothelial cells has been a major research effort in vascular and cardiac surgery. Cultured endothelial cells seeded onto the lumina of engineered blood vessels have been suggested to exhibit a pro-inflammaotry and procoagulant phenotype, leading to premature graft thrombosis [86]. To alter endothelial cell phenotype, genetic modification has been employed. In one of the earliest studies endothelial cells retrovirally trans-duced to express the lacZ gene were seeded into grafts that were subsequently implanted as carotid interposition grafts in dogs [87]. LacZ expressing endothelial cells lining the luminal surface were demonstrated 5 weeks later. Later studies showed successful retroviral transduction of endothelial cells with proangiogenic genes such tissue-plasminogen activating (TPA) factor [88]. In a similar context, saphenous vein grafts could be successfully trans-duced using an adenoviral vector [89].
Vascularization of large bioartificial constructs arguably remains the key challenge in tissue engineering and various strategies towards it have been investigated. Endothelial lineage cells such as differ-entiated endothelial cells, endothelial progenitor cells (EPCs) and embryonic stem cells have all been used for seeding of polymer matrices for in vitro fabrication of vascularized tissue-engineered constructs. For example, Wu et al. seeded endothelial progenitor cells onto a PGA-PLLA matrix, resulting in the formation of tube-shaped vascular structures in vitro[90]. After construct implantation, interconnections should be established with the host vasculature. The ingrowth of blood vessels to the centre of the scaffold could be significantly accelerated. Difficulties of this approach concern the homogenous distribution of angiogenic cells in large constructs and the selection of the optimal endothelial cell type. Moreover, it remains to be determined in detail how material, pore size and degradation properties of the scaffold influence phenotype, proliferation and gene expression of the endothelial cells [91]. Another option con-stitutes the implantation of a matrix without prior ex vivo vascularization, instead aiming for vascular ingrowth from the surrounding host tissue. This process can be supported by incorporation of angioinductive genes into the scaffold, a concept known as gene-activated matrix (36). Based on this principle, researchers demonstrated that both aden-oviral [92] and plasmid [93] gene delivery of PDGF through collagen and PLG scaffolds could stimulate vascularization rodent wound healing models.
Guided in vivo vascularization of a bioartificial material in a separation chamber can be induced by microsurgical construction of an arteriovenous (AV) loop. The method was first described by the Morrison group [94] and further developed by our group [95]. We and others have shown efficient in vivo vascularization of different biomaterials or tissue such as fibrin (Arkudas et al., manuscript submitted for publication), dermis [96] and processed bovine cancellous bone [95]. The angioinductive potential of this model could be further enhanced by introduction of proan-giogenic growth factors. Arkudas et al. recently showed that fibrin-immobilization of VEGF and basic fibroblast growth factor (bFGF) can further enhance vascularization of a fibrin matrix in this model (manuscript submitted for publication).
Outlook
Gene transfer strategies in combination with tissue engineering are under intense investigation due to the therapeutic potential. Viral vectors in particular have the advantage of superior transduction efficiency, but their use is limited by safety concerns. Large-scale transduction of target cells is most efficiently achieved using adenovirus a high titres, which involves the risk of vector toxicity [97]. Long-term gene expression can be achieved using retrovirus, but insertional remains a major concern. Polymer-based gene delivery systems offer promising advantages over traditional gene delivery systems by prolonging gene expression, avoiding distribution to distant tissues and systemic circulation, reduced toxicity and decreased immune response [98].
Optimization and control of gene expression remains a challenge that needs to be addressed given the deleterious effects of inadequately high levels of transgene expression [55]. To address these issues, Yao et al. developed a Tetracycline-repressor based highly sensitive tetracycline-dependent tran-scription switch (T-Rex™ System, Invitrogen). The T-Rex™ system was integrated into a replication deficient HSV-1 vector. It could be demonstrated that in vitro infection of different cell types using the tet-conditional virus resulted in tetracycline dependent 300-to 1000-fold regulation of expression [35]. Another promising direction is the use of stem cells and pro-genitor cells as a vehicle for gene delivery. Currently, the ethical issues associated with the use of embryonic stem cells makes adult stem cells and progenitor cells a particularly attractive choice. Adult tissues require these cell types for continuous self-renewal [99, 100]. Multipotent stem cells are found in most adult tissues and can generate a certain spectrum of differentiated cell lineages dependent on their location. Progenitor cells, on the other hand, are unipotent, capable of generating one specific cell type. In the case of shortage of an autologous cell source, allogenic or xenogeneic sources become an option, even elimination by the host immune system is the key obstacle to xenotranspantation that must be solved in order to guarantee success. Taken together, gene delivery in combination with tissue-engineering applications may greatly enhance therapeutic options to (re-)generate tissue severed or lost by disease or trauma. Polymeric release and substrate-mediated gene delivery from natural or synthetic scaffolds can be carried out through both viral and non-viral vector systems. The efficacy of gene delivery systems in tissue engineering could be further enhanced by employing gene expression regulation, co-transplantation of stem cells or progenitor cells and use of xenogenic tissue or cell sources.
Acknowledgments
This work was funded in part by grants for the Yue-Hong and Hans Georgbers foundation and for the ELAN fund of the Friedrich-Alexander University Erlangen-Nurnberg.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–23. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Burke JF, Yannas IV, Quinby WC, Bondoc CC, Jung WK. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann Surg. 1981;194:413–28. doi: 10.1097/00000658-198110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green H. Cultured cells for the treatment of disease. Sci Am. 1991;254:96–102. doi: 10.1038/scientificamerican1191-96. [DOI] [PubMed] [Google Scholar]
- 4.Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–4. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 5.Anderson WF. Human gene therapy. Nature. 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- 6.Trippel SB. Growth factors as therapeutic agents. Instr Course Lect. 1997;46:473–6. [PubMed] [Google Scholar]
- 7.Steed Dl. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers: diabetic ulcer study group. J Vasc Surg. 1995;21:71–80. doi: 10.1016/s0741-5214(95)70245-8. [DOI] [PubMed] [Google Scholar]
- 8.Tepper OM, Mehrara BJ. Gene therapy in plastic surgery. Plast Reconstr Surg. 2002;109:716–34. doi: 10.1097/00006534-200202000-00047. [DOI] [PubMed] [Google Scholar]
- 9.Crystal RG. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–10. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 10.Cheng L, Ziegelhoffer PR, Yang NS. In vivo promoter activity and transgene expression in mammalian somatic tissues evaluated by using particle bombardment. Proc Natl Acad Sci USA. 1993;90:4455–9. doi: 10.1073/pnas.90.10.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeschke MG, Barrow RE, Hawkins HK, Yang K, Hayes RL, Lichtenbelt BJ, Perez-Polo JR, Herndon DN. IGF-I gene transfer in thermally injured rats. Gene Ther. 1999;6:1015–20. doi: 10.1038/sj.gt.3300923. [DOI] [PubMed] [Google Scholar]
- 12.Wolff JA, Malone RW, Willams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 13.Neumann E, Kakorin S, Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg. 1999;48:3–16. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- 14.Yang NS, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci USA. 1990;87:9568–71. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felgner PL, Ringold GM. Cationic liposome-mediated transfection. Nature. 1989;337:387–8. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 16.Jang JH, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev Med Devices. 2004;1:127–38. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 17.Takeshita S, Zheng PL, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis: a single intraarterial bolus of vascular endothelial growth factor augmentsrevascularization in a rabbit ischemic hindlimb model. J Clin Invest. 1994;93:662–70. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andree C, Swain WF, Page CP, Macklin MD, Slama J, Hatzis D, Eriksson E. In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci USA. 1994;91:12188–92. doi: 10.1073/pnas.91.25.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson E, Yao F, Svensjo T, Winkler T, Slama J, Macklin MD, Andree C, McGregor M, Hinshaw V, Swain WF. In vivo gene transfer to skin and wound by microseeding. J Surg Res. 1998;78:85–91. doi: 10.1006/jsre.1998.5325. [DOI] [PubMed] [Google Scholar]
- 20.Rizzuto G, Cappelletti M, Maione D, Savino R, Lazzaro D, Costa P, Mathiesen I, Cortese R, Ciliberto G, Laufer R, La Monica N, Fattori E. Efficient and regulated erythropoetin production by naked DNA injection and muscle electroporation. Proc Natl Acad Sci USA. 1999;96:6417–22. doi: 10.1073/pnas.96.11.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeki Y, Matsumoto N, Nakano Y, Mori M, Awai K, Kaneda Y. Development and characterisation of cationic liposomes conjugated with HVJ (Sendai virus): reciprocal effects of cationic lipid for in vitro and in vivo gene transfer. Hum Gene Ther. 1997;8:2133–41. doi: 10.1089/hum.1997.8.17-2133. [DOI] [PubMed] [Google Scholar]
- 22.Mitani K, Graham FL, Caskey CT, Kochanek S. Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc Natl Acad Sci USA. 1995;92:3854–8. doi: 10.1073/pnas.92.9.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haecker SE, Stedman HH, Balice-Gordon RJ, Smith DB, Greelish JP, Mitchell WA, Wells A, Sweeney HL, Wilson JM. In vivo expression of fulllength human dystrophin from adenoviral vectors deleted of all viral genes. Hum Gene Ther. 1996;7:1907–14. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 24.Kotin RM, Linden RM, Berns KI. Characterisation of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–8. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rock DL, Fraser NW. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983;302:523–5. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- 26.Huard J, Goins WF, Glodioso JC. Herpes simplex virus type 1 vector mediated gene transfer to muscle. Gene Ther. 1995;2:385–92. [PubMed] [Google Scholar]
- 27.Coffin RS, Howard MK, Cumming DV, Dollery CM, McEWAn J, Yellon DM, Marber MS, MacLean AR, Brwon SM, Latchman DS. Gene delivery to the heart in vivo and to cardiac myocytes and vascular smooth muscle cells in vitro using herpes virus vectors. Gene Ther. 1996;3:560–6. [PubMed] [Google Scholar]
- 28.Oligino T, Ghivizzani S, Wolfe D, Lechman E, Krisky D, Mi Z, Evans C, Robbins P, Glorioso J. Intra-articular delivery of a herpes simplex virus IL-1Ra gene vector reduces inflammation in a rabbit model of arthritis. Gene Ther. 1999;6:1713–20. doi: 10.1038/sj.gt.3301014. [DOI] [PubMed] [Google Scholar]
- 29.Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, Bennett N, Jr, De Groat WC, Glorioso JC, Chancellor MB. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol. 2001;165:1748–54. [PubMed] [Google Scholar]
- 30.Wolfe D, Goins WF, Kaplan TJ, Capuano SV, Fradette J, Murphey-Corb M, Robbins PD, Cohen JB, Glorioso JC. Herpesvirus-mediated systemic delivery of nerve growth factor. Mol Ther. 2001;3:61–9. doi: 10.1006/mthe.2000.0225. [DOI] [PubMed] [Google Scholar]
- 31.Roizman B. The function of herpes simplex virus genes: a primer for genetic engineering of novel vectors. Proc Natl Acad Sci USA. 1996;93:11307–12. doi: 10.1073/pnas.93.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glorioso JC, Goins WF, Schmidt MC, Oligino T, Krisky DM, Marconi PC, Cavalcolli JD, Ramakrishnan R, Poliani PL, Fink DJ. Engineering herpes simplex virus vectors for human gene therapy. Adv Pharmacol. 1997;40:103–36. [PubMed] [Google Scholar]
- 33.Yao F, Svensjo T, Winkler T. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum Gene Ther. 1998;9:1939–50. doi: 10.1089/hum.1998.9.13-1939. [DOI] [PubMed] [Google Scholar]
- 34.Yao F, Eriksson E. A novel anti-herpes simplex type 1-specific herpes simplex type 1 recombinant. Hum Gene Ther. 1999;10:1811–8. doi: 10.1089/10430349950017491. [DOI] [PubMed] [Google Scholar]
- 35.Yao F, Theopold C, Hoeller D, Bleiziffer O, Lu Z. Highly efficient regulation of gene expression by tetracycline in a replication-defective herpes simplex viral vector. Mol Ther. 2006;13:1133–41. doi: 10.1016/j.ymthe.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Bonadio J. Tissue engineering via local gene delivery. J Mol Med. 2000;78:303–11. doi: 10.1007/s001090000118. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Yang ST, Kniss DA. Three-dimensional cellscaffold constructs promote efficient gene transfection: implications for cell-based gene therapy. Tissue Eng. 2001;7:585–98. doi: 10.1089/107632701753213200. [DOI] [PubMed] [Google Scholar]
- 38.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 39.Siemens DR, Austin JC, Hedican SP, Tartaglia J, Ratliff TL. Viral vector delivery in solid-state vehicles: gene expression in a murine prostate cancer model. J Natl Cancer Inst. 2000;92:403–12. doi: 10.1093/jnci/92.5.403. [DOI] [PubMed] [Google Scholar]
- 40.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–9. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 41.Pascher A, Palmer GD, Steinert A, Oligino T, Gouze E, Gouze JN, Betz O, Spector M, Robbins PD, Evans CH, Ghivizzini SC. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11:133–41. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 42.Berry M, Gonzalez AM, Clarke W, Greenlees L, Barrett L, Tsang W, Seymour L, Bonadio J, Logan A, Baird A. Sustained effects of gene-activated matrices after CNS injury. Mol Cell Neurosci. 2001;17:706–16. doi: 10.1006/mcne.2001.0975. [DOI] [PubMed] [Google Scholar]
- 43.Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, Nesbit M, Crombleholme TM, Herlyn M, Sosnowski BA, Pierce GF. FGF2- Targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000;2:153–60. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- 44.Doukas J, Blease K, Craig D, Ma C, Chandler LA, Sosnowski BA, Pierce GF. Sustained effects of gene-activated matrices after CNS injury. Mol Cell Neurosci. 2001;17:706–16. doi: 10.1006/mcne.2001.0975. [DOI] [PubMed] [Google Scholar]
- 45.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 46.Horch RE, Kopp J, Kneser U, Beier J, Bach AD. Tissue engineering of cultured skin substitutes. J Cell Mol Med. 2005;9:592–608. doi: 10.1111/j.1582-4934.2005.tb00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Svensjo T, Yao F, Pomahac B, Winkler T, Eriksson E. Cultured autologous fibroblasts augment epidermal repair. Transplantation. 2002;73:1033–41. doi: 10.1097/00007890-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 48.Andree C, Voigt M, Wenger A, Erichsen T, Bittner K, Schaefer D, Walgenbach KJ, Borges J, Horch RE, Eriksson E, Stark GB. Plasmid gene delivery to human keratinocytes through a fibrin-mediated transfection system. Tissue Eng. 2001;7:757–66. doi: 10.1089/107632701753337708. [DOI] [PubMed] [Google Scholar]
- 49.Eming SA, Snow RG, Yarmush ML, Morgan JR. Targeted expression of insulin-like growth factor to human keratinocytes: modification of the autocrine control of keratinocyte proliferation. J Invest Dermatol. 1996;107:113–20. doi: 10.1111/1523-1747.ep12298351. [DOI] [PubMed] [Google Scholar]
- 50.Jeschke MG, Herndon DN, Baer W, Barrow RE, Jauch KW. Possibilities of Non-viral gene transfer to improve cutaneous wound healing. Curr Gene Ther. 2001;1:267–78. doi: 10.2174/1566523013348571. [DOI] [PubMed] [Google Scholar]
- 51.Theopold C, Yao F, Eriksson E. Gene Therapy in the treatment of lower extremity wounds. Int J Low Extrem Wounds. 2004;3:69–79. doi: 10.1177/1534734604265431. [DOI] [PubMed] [Google Scholar]
- 52.Breitbart AS, Laser J, Parrett B, Porti D, Grant RT, Grande DA, Mason JM. Accelerated diabetic wound healing using cultured dermal fibroblasts retrovirally transduced with the platelet-derived growth factor B gene. Ann Plast Surg. 2003;51:409–14. doi: 10.1097/01.SAP.0000084461.83554.71. [DOI] [PubMed] [Google Scholar]
- 53.Saraf A, Mikos AG. Gene delivery strategies for cartilage tissue engineering. Adv. Drug Deliv Rev. 2006;58:592–603. doi: 10.1016/j.addr.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang KG, Saris DB, Geuze RE, Helm YI, Rijen MH, Verbout AJ, Dhert WJ, Creemers LB. Impact of expansion and redifferentiation conditions on chondrogenic capacity of cultured chondrocytes. Tissue Eng. 2006;12:2435–47. doi: 10.1089/ten.2006.12.2435. [DOI] [PubMed] [Google Scholar]
- 55.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 56.Grande DA, Mason J, Light E, Dines D. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am. 2003;85:111–6. doi: 10.2106/00004623-200300002-00015. [DOI] [PubMed] [Google Scholar]
- 57.Madry H, Kaul G, Cucchiarini M, Stein U, Zurakowski D, Remberger K, Menger MD, Kohn D, Trippel SB. Enhanced repair of articular cartilage defects in vivo by transplanted chondrocytes overexpressing insulin-like growth factor I (IGF-I) Gene Ther. 2005;12:1171–9. doi: 10.1038/sj.gt.3302515. [DOI] [PubMed] [Google Scholar]
- 58.Gelse K, Jiang QJ, Aigner T, Ritter T, Wagner K, Poschl E, Von Der Mark K, Schneider H. Fibroblastmediated delivery of growth factor complementary DNA into mouse joints induces chondrogenesis but avoids the disadvantages of direct viral gene transfer. Arthritis Rheum. 2001;44:1943–53. doi: 10.1002/1529-0131(200108)44:8<1943::AID-ART332>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 59.Nixon AJ, Brower-Toland BD, Bent SJ, Saxer RA, Wilke MJ, Robbins PD, Evans CH. Insulin-like growth factor-I gene therapy applications for cartilage repair. Clin Orthop Relat Res. 2000;379:S201–13. doi: 10.1097/00003086-200010001-00026. [DOI] [PubMed] [Google Scholar]
- 60.Ghivizzini SC, Lechman ER, Tio C, Mule KM, Chada S, JE McCormack, Evans CH, Robbins PD. Direct retrovirus-mediated gene transfer to the synovium of the rabbit knee: implications for arthritis gene therapy. Gene Ther. 1997;4:977–82. doi: 10.1038/sj.gt.3300486. [DOI] [PubMed] [Google Scholar]
- 61.Kneser U, Schaefer DJ, Polykandriotis E, Horch RE. Tissue engineering of bone: the reconstructive surgeon's point of view. J Cell Mol Med. 2006;10:7–19. doi: 10.1111/j.1582-4934.2006.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baltzer AW, Lattermann C, Whalen JD, Wooley P, Weiss K, Grimm M, Ghivizzini SC, Robbins PD, Evans CH. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–9. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 63.Meinel L, Hofmann S, Betz O, Fajardo R, Merkle HP, Langer R, Evans CH, Vunjak-Novakovic G, Kaplan DL. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials. 2006;27:4993–5002. doi: 10.1016/j.biomaterials.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 64.Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP- 7 gene. Ann Plast Surg. 1999;42:488–95. [PubMed] [Google Scholar]
- 65.Edwards PC, Ruggiero S, Fantasia J, Burakoff R, Moorji SM, Paric E, Razzano P, Grande DA, Mason JM. Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene Ther. 2005;12:75–86. doi: 10.1038/sj.gt.3302386. [DOI] [PubMed] [Google Scholar]
- 66.Byers BA, Guldberg RE, Garcia AJ. Synergy between genetic and tissue engineering: Runx2 overexpression and in vitro construct development enhance in vivo mineralization. Tissue Eng. 2004;10:1757–66. doi: 10.1089/ten.2004.10.1757. [DOI] [PubMed] [Google Scholar]
- 67.Fansa H, Keilhoff G, Wolf G, Schneider W. Tissue engineering of peripheral nerves: a comparison of venous and acellular muscle grafts with cultured Schwann cells. Plast Reconstr Surg. 2001;107:485–94. doi: 10.1097/00006534-200102000-00026. [DOI] [PubMed] [Google Scholar]
- 68.Allmeling C, Jokuszies A, Reamers K, Kall S, Vogt PM. Use of spider silk fibers as an innovative material in a biocompatible artificial nerve conduit. J Cell Mol Med. 2006;10:770–7. doi: 10.1111/j.1582-4934.2006.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chalfoun CT, Wirth GA, Evans GR. Tissue engineered nerve constructs: where do we stand? J Cell Mol Med. 2006;10:309–17. doi: 10.1111/j.1582-4934.2006.tb00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patrick CW, Zheng B, Schmidt M, Herman PS, Chauvin BP, Fan Z, Stark B, Evans GR. Dermal fibroblasts genetically engineered to release NGF. Ann Plast Surg. 2001;47:660–5. doi: 10.1097/00000637-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 71.McConnell MP, Dhar S, Naran S, Nguyen T, Bradshaw RA, Evans GR. In vivo induction and delivery of nerve growth factor, using HEK-293 cells. Tissue Eng. 2004;10:1492–501. doi: 10.1089/ten.2004.10.1492. [DOI] [PubMed] [Google Scholar]
- 72.Murakami T, Fujimoto Y, Yasunaga Y, Ishida O, Tanaka N, Ikuta Y, Ochi M. Transplanted neuronal progenitor cells in a peripheral nerve gap promote nerve repair. Brain Res. 2003;974:17–24. doi: 10.1016/s0006-8993(03)02539-3. [DOI] [PubMed] [Google Scholar]
- 73.Heine W, Conant K, Griffin JW, Hoke A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189:231–40. doi: 10.1016/j.expneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Gimenez y Ribotta M, Revah F, Pradier L, Loquet I, Mallet J, Privat A. Prevention of motoneuron death by adenovirus-mediated neurotrophic factors. J Neurosci Res. 1997;48:281–5. doi: 10.1002/(sici)1097-4547(19970501)48:3<281::aid-jnr11>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 75.Natsume A, Wolfe D, Hu J, Huang S, Puskovic V, Glorioso JC, Fink DJ, Mata Enhanced functional recovery after proximal nerve root injury by vectormediated gene transfer. Exp Neurol. 2003;184:878–86. doi: 10.1016/S0014-4886(03)00334-0. [DOI] [PubMed] [Google Scholar]
- 76.Chen MF, Hwang TL, Hung CF. Human liver regeneration after major hepatectomy. A study of liver volume by computed tomography. Ann Surg. 1991;213:227–9. doi: 10.1097/00000658-199103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Selden C, Jones M, Wade D, Hodgson H. Hepatotropin mRNA expression in human foetal liver development and in liver regeneration. FEBS Lett. 1990;270:81–4. doi: 10.1016/0014-5793(90)81239-k. [DOI] [PubMed] [Google Scholar]
- 78.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J Pediatr Surg. 1988;23:3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 79.Fiegel HC, Kaufmann PM, Kneser U, Kluth D, Rogiers X. Priming of hepatocytes for cell culture by partial hepatectomy prior to cell isolation. Tissue Eng. 2000;6:619–26. doi: 10.1089/10763270050199569. [DOI] [PubMed] [Google Scholar]
- 80.Follenzi A, Sabatino G, Lombardo A, Boccaccio C, Naldini L. Efficient gene delivery and targeted expression to hepatocytes in vivo by improved lentiviral vectors. Hum Gene Ther. 2002;13:243–60. doi: 10.1089/10430340252769770. [DOI] [PubMed] [Google Scholar]
- 81.Fiegel HC, Lange C, Kneser U, Lambrecht W, Zander AR, Rogiers X, Kluth D. Fetal and adult liver stem cells for liver regneration and tissue engineering. J Cell Mol Med. 2006;10:577–87. doi: 10.1111/j.1582-4934.2006.tb00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ricordi C, Strom TB. Clinical islet transplantation: advances and immunological challenges. Nat Rev Immunol. 2004;4:259–68. doi: 10.1038/nri1332. [DOI] [PubMed] [Google Scholar]
- 83.De la Tour D, Halvorsen T, Demeterco C, Tyrberg B, Itkin-Ansari P, Loy M, Yoo SJ, Hao E, Bossie S, Levine F. Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol Endocrinol. 2001;15:476–83. doi: 10.1210/mend.15.3.0604. [DOI] [PubMed] [Google Scholar]
- 84.Narushima M, Kobayashi N, Okitsu, Tanaka Y, Li SA, Chen Y, Miki A, Tanaka K, Nakaji S, Takei K, Gutierrez AS, Rivas-Carrillo JD, Navarro-Alvarez N, Jun HS, Westerman KA, Noguchi H, Lakey JR, Leboulch P, Tanaka N, Yoon JW. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol. 2005;23:1274–82. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- 85.Knight KR, Uda Y, Findlay MW, Brown DL, Cronin KJ, Jamieson E, Tai T, Keramidaris E, Penington AJ, Rophael J, Harrison LC, Morrison WA. Vascularized tissue-engineered chambers promote survival and function of transplanted islets and improve glycemic control. FASEB J. 2006;20:565–7. doi: 10.1096/fj.05-4879fje. [DOI] [PubMed] [Google Scholar]
- 86.Fields RC, Solan A, McDonagh KT, Niklason LE, Lawson JH. Gene therapy in tissue-engineered blood vessels. Tissue Eng. 2003;9:1281–7. doi: 10.1089/10763270360728198. [DOI] [PubMed] [Google Scholar]
- 87.Wilson JM, Birinyi LK, Salomon RN, Libby P, Callow AD, Mulligan RC. Implantation of vascular grafts lined with genetically modified endothelial cells. Science. 1989;244:1344–6. doi: 10.1126/science.2734614. [DOI] [PubMed] [Google Scholar]
- 88.Dunn PF, Newman KD, Jones M, Yamada I, Shayani V, Virmani R, Dichek DA. Seeding of vascular grafts with genetically modified endothelial cells. Secretion of recombinant TPA results in decreased seeded cell retention in vitro and in vivo. Circulation. 1996;93:1439–46. doi: 10.1161/01.cir.93.7.1439. [DOI] [PubMed] [Google Scholar]
- 89.Fernandez HA, Kallenbach K, Seghezzi G, Mehrara B, Apazidis A, Baumann FG, Grossi EA, Colvin S, Mignatti P, Galloway AC. Modulation of matrix metalloproteinase activity in human saphenous vein grafts using adenovirus-mediated gene transfer. Surgery. 1998;124:129–36. [PubMed] [Google Scholar]
- 90.Wu X, Rabkin-Aikawa E, Guleserian KJ, Perry TE, Masuda Y, Sutherland FW, Schoen FJ, Mayer JE, Jr, Bischoff J. Tissue-engineered microvessels on three-dimensional biodegradable scaffolds using human endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2004;287:H480–7. doi: 10.1152/ajpheart.01232.2003. [DOI] [PubMed] [Google Scholar]
- 91.Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rucker M, Junker D, Haufel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–104. doi: 10.1089/ten.2006.12.2093. [DOI] [PubMed] [Google Scholar]
- 92.GU DL, Nguyen T, Gonzalez AM, Printz MA, Pierce GF, Sosnowski BA, Philllips ML, Chandler LA. Adenovirus encoding human platelet-derived growthfactor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Mol Ther. 2004;9:699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 93.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–4. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 94.Mian RA, Knight KR, Penington AJ, Hurley JV, Messina A, Romeo R, Morrison WA. Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng. 2000;6:595–603. doi: 10.1089/10763270050199541. [DOI] [PubMed] [Google Scholar]
- 95.Kneser U, Polykandriotis E, Euler S, Grabinger L, Heidner K, Amann M, Hess A, Stuerzl M, Horch RE. Induction of axial vascularization in processed bovine cancellous bone matrices using a microsurgically created arteriovenous loop. Tissue Eng. 2006;12:1721–31. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka Y, Tsutsumi A, Crowe DM, Tajima S, Morrison WA. Generation of an autologous tissue (matrix) flap by combining an arteriovenous shunt loop with artificial skin in rats: preliminary report. Br J Plast Surg. 2000;53:51–7. doi: 10.1054/bjps.1999.3186. [DOI] [PubMed] [Google Scholar]
- 97.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–8. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 98.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10:19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 99.Horch RE. Future perspectives in tissue engineering. J Cell Mol Med. 2006;10:4–6. doi: 10.1111/j.1582-4934.2006.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–76. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandler LA, Gu DL, Ma C, Gonzalez AM, Doukas J, Nguyen T, Pierce GF, Phillips ML. Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair Regen. 2000;8:473–9. doi: 10.1046/j.1524-475x.2000.00473.x. [DOI] [PubMed] [Google Scholar]
- 102.Tyrone JW, Mogford JE, Chandler LA, Ma C, Xia Y, Pierce GF, Mustoe TA. Collagen-embedded plateletderived growth factor DNA plasmid promotes wound healing in a dermal ulcer model. J Surg Res. 2000;93:230–6. doi: 10.1006/jsre.2000.5912. [DOI] [PubMed] [Google Scholar]
- 103.Erdag G, Medalie DA, Rakhorst H, Krueger GG, Morgan JR. FGF-7 expression enhances the performance of bioengineered skin. Mol Ther. 2004;10:76–85. doi: 10.1016/j.ymthe.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 104.Berry M, Gonzalez AM, Clarke W, Greenlees L, Barrett L, Tsang W, Seymour L, Bonadio J, Logan A, Baird A. Sustained effects of gene-activated matrices after CNS injury. Mol Cell Neurosci. 2001;17:706–16. doi: 10.1006/mcne.2001.0975. [DOI] [PubMed] [Google Scholar]


