Abstract
FTY720 (Fingolimod) is a novel type of immunosuppressive agent inhibiting lymphocyte egress from secondary lymphoid tissues thereby causing peripheral lymphopenia. FTY720 can inhibit macrophage infiltration into inflammatory lesions under pathological conditions. FTY720 has been clinically evaluated for prophylaxis of allograft rejection and treatment of multiple sclerosis, showing promising immunosuppressive effects. A robust inflammatory response after traumatic brain injury (TBI) plays an important role in the secondary or delayed injuries of TBI. Here we have investigated by immunohistochemistry in a rat TBI model the effects of FTY720 on early cell accumulation into the inflammatory tissue response and on expression of major histo-compatibility complex class II (MHC-II) and endothelial-monocyte activating polypeptide II (EMAP-II). Accumulation of MHC-II+ or EMAP-II+ cells became significant 1 day after injury and continuously increased during the early time periods. Further, double-staining experiments confirmed that the major cellular sources of MHC-II were reactive macrophages, however MHC-II+ cells only constituted a small subpopulation of reactive macrophages. Immediately after TBI, peripheral administration of FTY720 (1 mg/kg in 1 mL saline, every second day) significantly attenuated the accumulation of MHC-II+ macrophages from Day 1 to 4 and significantly attenuated the accumulation of EMAP-II+ macrophages/microglia at Day 4. Our findings show that FTY720 attenuates early accumulation of EMAP-II+ and MHC-II+ reactive monocytes following TBI, indicating that FTY720 might be a drug candidate to inhibit brain inflammatory reaction following TBI.
Keywords: traumatic brain injury, FTY720, MHC-II, EMAP-II, monocytes
Introduction
Following traumatic brain injury (TBI), a robust inflam-of glia and neurons as well as local accumulation of matory response develops that involves the activation leukocytes [1]. Accumulation of reactive microglia/ macrophages, polymorphonuclear granulocytes and lymphocytes has been observed in and around affected tissues [2–5]. Certain cytokines, like EMAP II, can promote cell activation and migration to lesional tissues and contribute to secondary brain damage following TBI [6]. The appearance of microglia clusters and infiltrating macrophages expressing cellular activation markers, e.g. MHC-II antigens, is associated with neuronal and axonal damage [7–9].
EMAP-II is a proinflammatory antiangiogenic cytokine, up-regulated and involved in inflammatory cascades of secondary damage following CNS injury [10, 11]. The expression of EMAP-II is a sensitive marker of microglia/macrophage activation and associated with the reactivation and accumulation /infiltration of microglia/macrophages after injury in the CNS [11]. MHC-II antigens are also an indicator of reactive microglia/macrophages [12]. Expression of MHC-II on microglia/macrophages is induced following a variety of pathological events, including brain trauma [2, 8, 13]. Clusters of microglia with up-regulated MHC-II antigens are observed not only in lesioned areas but also in regions without overt tissue damage and remote from the site of impact following TBI [7, 14]. However, expression pattern of MHC-II following weight-drop induced TBI remains unknown.
FTY720 (Fingolimod), 2-amino-2-(2-[4-octylphenyl] ethyl)-1, 3-propanediol hydrochloride, is the first of a new class of immunosuppressive agents: sphingo-sine 1-phosphate (S1P) receptor agonists and has shown promising immunosuppressive effects in vivo and in vitro[15–17]. FTY720 functions through a new mechanism, inhibiting lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at S1P receptors. It sequesters the circulating lymphocytes into lymph nodes, resulting in reduced peripheral lymphocyte counts, but does not alter major effector functions of lymphocytes. Therefore, FTY720 is a potent immunosuppressive agent with minor toxicity and is well tolerated by patients. Furthermore, FTY720 has a novel mechanism of action that has not been observed with other immunosuppressive agents and shows a synergism with currently applied immunosuppressive agents. FTY720 has been shown to be highly effective in experimental allotransplantation and autoimmune disease models. Interestingly FTY720 reduces the number of lesions detected by MRI and clinical disease activity in patients with multiple sclerosis [18]. In animal models of multiple sclerosis, FTY720 prevents the onset of disease and reduces established neurological deficits [19–21]. In addition administration of FTY720 can inhibit macrophage infiltration into inflammatory lesions under pathological conditions, like experimental autoimmune encephalomyelitis and amyloid β-protein stimulated monocyte infiltration [22, 23].
In this study, we have investigated the potential effects of FTY720 on expression patterns of MHC-II and EMAP-II in the first 96 hrs after weight-dropinduced TBI.
Materials and methods
Animal experiments
Lewis rats (8–9 weeks old, 350–400 g, Elevage Janvier, Le Genest-St-Isle, France) were housed with equal daily periods of light and dark and free access to food and water. All procedures were performed in accordance with the published International Health Guidelines under a protocol approved by the local Administration District Official Committee. The number of the rats used and their suffering were minimized.
TBI was induced using a weight-drop contusion model in anesthetized rats as described previously [24, 25]. Briefly, rats were randomly grouped and anesthetized with Ketamin (120 mg/kg)/Rompun (8 mg/kg) and underwent a craniotomy, in which a circular region of the skull (3.0 mm diameter, centered 2.3 mm caudal and 2.3 mm lateral to bregma) was removed over the right somatosensory cortex. A weight-drop device was placed over the dura and adjusted to stop an impact transducer (foot plate) at a depth of 2.5 mm below the dura. Then, a 20 g weight was dropped from 15 cm above the dura, through a guiding tube onto the foot plate. Body temperature was maintained using an overhead heating lamp during surgery. After injury the scalp was closed tightly. Immediately after surgery rats received an intraperitoneal injection of FTY720 (1 mg/kg, in 1 mL saline, once every second day) or 1 mL saline, respectively (vehicle control group). Rats were killed 24, 48 or 96 hrs after TBI. At the end of the experiment, rats (18 TBI rats and 5 normal adult control rats) were deeply anes-thetized with Ketamin (120 mg/kg)/Rompun (8 mg/kg) and perfused intracardially with 4°C 4% paraformaldehyde (PFA) in PBS. Brains were quickly removed and post-fixed in 4% PFA overnight at 4°C. A cortical coronal slice containing the contusion site was embedded in paraffin, serially sectioned (3 μm) and mounted on silan-covered slides. The sections were numbered and during the following immunostaining the same antibody was applied to sections with the same number.
Immunohistochemistry
After dewaxing, brain sections were boiled (in an 850 W microwave oven) for 15 min in citrate buffer (2.1 g citric acid monohyrate/L, pH 6) (Carl Roth, Karlsruhe, Germany). Endogenous peroxidase was inhibited by 1% H2O2 in pure methanol (Merck, Darmstadt, Germany) for 15 min. Sections were incubated with 10% normal pig serum (Biochrom, Berlin, Germany) to block non-specific binding of immunoglobulins and then with the mouse monoclonal antibodies: OX6 for MHC-II (1:100; Camon, Wiesbaden, Germany) or EMAP-II (1:100; BMA, Augst, Switzerland) overnight at 4°C. Antibody binding to tissue sections was visualized with a biotinylated rabbit antimouse IgG F(ab)2 antibody fragment (1:400; DAKO, Hamburg, Germany). Subsequently, sections were incubated with a horseradish peroxidase-conjugated Streptavidin complex for 30 min (1:100; DAKO, Hamburg, Germany), followed by development with diaminobenzidine (DAB) substrate (Fluka, Neu-Ulm, Germany). Finally, sections were counterstained with Maier's Hemalaun. As negative controls, the primary anti-bodies were omitted. Blocking studies have been described [26]. To evaluate the lesion histologically, additional sections were stained with Hematoxylin and Eosin (HE).
Double-staining
In double-staining experiments, brain sections were immunolabeled as described above. Then they were once more irradiated in a microwave for 15 min in citrate buffer and were incubated with 10% normal pig serum (Biochrom, Berlin, Germany). Subsequently the sections were incubated with the appropriate second primary monoclonal anti-bodies for 1 h at room temperature. Three monoclonal anti-bodies were used: ED1 (1:100; Serotec, Oxford, UK) to localize activated microglia/macrophages, W3/13 (1:50; Serotec, Oxford, UK) to identify T-lymphocytes and OX22 (1:100; Serotec, Oxford, UK) to detect CD45RC expressed by B lymphocytes and a T cell subset. Consecutively, visu-alization was achieved by adding secondary antibody (biotinylated rabbit antimouse IgG) at a dilution of 1:400 in TBS-BSA for 30 min and then alkaline phophatase-conjugated Avidin complex diluted 1:100 in Tris-BSA for another 30 min. Finally immunostaining was developed with Fast Blue BB salt chromogen-substrate solution, but by omission of counterstaining with Hemalaun.
Evaluation and statistical analysis
After immunostaining, brain sections of each time point after TBI were examined by light microscopy. EMAP-II and MHC-II (OX6) expression was evaluated at the lesioned areas.The numbers of EMAP-II+ and MHC-II+ cells of every rat brain section were counted in 3 non-overlapping high-power fields (HPFs, × 400 magnification) for each section. The HPFs were selected from lesional areas that had a maximum of positive cells. In each field studied, only positive cells with the nucleus at the focal plane were counted. Results were given as arithmetic means of MHC-II+ cells per HPF and standard errors of means (SEM). Statistical analysis was performed by one-way ANOVA followed by Dunnett's multiple comparison tests or non-parametric t test (Graph Pad Prism 4.0 software). For all statistical analyses, significance levels were set at P < 0.05.
Results Traumatic brain injury
TBI by weight drop is a well-established animal model for brain trauma studies and was used to generate a reproducible cortical injury. In brains of control TBI rats receiving no FTY720, pannecrosis, where no neuronal structures can be observed, emerged 2 days after injury in the ipsilateral somatosensory cortex and selective neuronal loss ventrally adjacent the pan-necrosis extended to near the subcortical white matter. Leukocyte infiltration and hemorrhage were observed soon in those pannecrotic areas. A similar pattern of injury was seen in brains of FTY720 treated rats.
MHC-II expression pattern in rat brains following experimental TBI
Expression of MHC-II was detected by OX6 antibody. In normal adult rat brains, only few MHC-II+ cells (2.0 ± 0.3 per HPF, n = 5, Fig. 1A) were observed in subarachnoid and perivascular spaces and no MHC-II immunoreactivity was detected in the parenchyma of normal adult rat brains (Fig. 2A), which is in accordance with previous reports [27] A significant accumulation of MHC-II+ cells was detected at Day 1 after TBI (6.1 ± 0.4 per HPF, n = 3, P < 0.01; Fig. 1A) and increased over time up to the end of our observed period, 96 hrs post-TBI (16.3 ± 2.3 per HPF, n = 3, P < 0.01; Fig. 1A). Accumulated MHC-II+ cells were observed mainly in lesional areas and in subarachnoid and perivascular spaces near lesioned areas as early as Day 1 post-TBI, but MHC-II+ cells were rarely seen in perilesional and remote areas.
1.
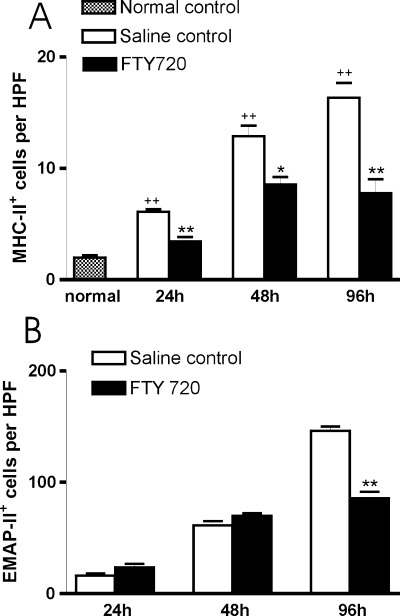
MHC-II+ leukocyte accumulation in rat brain after TBI: FTY720 reduces the early accumulation of MHC-II+ and EMAP-II+ cells. (A) Bar graph showing numbers of MHC-II+ leukocytes in normal rat brain and accumulation in brain lesions of rats treated with saline or FTY720 (1 mg/kg, i.p. injected immediately after TBI and once every second day after injury). For each section, numbers of MHC-II+ cells of every rat brain coronal section were counted in three non-overlapping high-power fields (HPFs, × 400 magnification), which were selected from lesional areas that have a maximum of positive cells. Results were given as arithmetic means of positive cells per HPF and standard errors of means (SEM). Statistical analysis was performed by non-parametric t test (Graph Pad Prism 4.0 software). *P < 0.05 and **P < 0.01 compared with their respective saline controls and ++ P < 0.01 compared with the normal control. (B) Bar graph showing accumulation of EMAP-II+ leukocytes in brain lesions of saline or FTY720 (1 mg/kg, i.p. injected immediately after TBI and once per day) treated TBI rats at days 1, 2 and 4 after injury. The numbers of EMAP-II+ cells of each rat brain coronal section were counted in 3 HPF in the lesioned areas. Results were given as arithmetic means of positive cells per HPF and standard errors of means (SEM). Statistical analysis was performed by non-parametric t test (Graph Pad Prism 4.0 software). **P < 0.01 compared with their respective saline controls.
2.
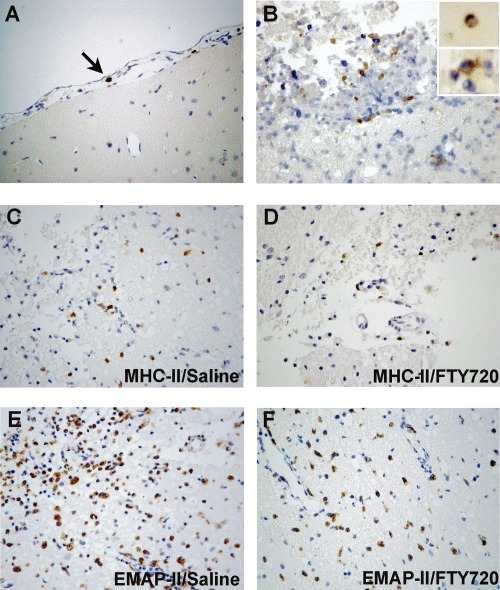
FTY720 attenuates the accumulation of EMAP-II+ and MHC-II+ cells in the early lesion of rat brains following experimental TBI. (A) In normal rat brain, only few MHC-II+ cells (arrow indicated) were occasionally seen in the subarachnoid space. (B) Cells double-stained with MHC-II+ (brown) and ED1+ (blue) were found in the lesioned area at Day 2 after TBI and almost all MHC-II+ cells co-expressed ED1 but only a small portion of ED1+ cells co-expressed MHC-II. (C-F) Representative photomicrographs showing that expressions of MHC-II (B, C and D) and EMAP-II (E and F) in brain lesions of TBI rats treated by FTY720 was attenuated as compared to brains of saline treated rats at Day 2 (MHC-II) or Day 4 (EMAP-II) after injury. C and E: saline treated rats; D and F: FTY720 treated rats. Original magnification: A-F× 400.
We further characterized MHC-II+ cells by double-staining with monoclonal antibodies against activated microglia/macrophages (ED1), T cells (W3/13) or B cells (OX22). MHC-II+ cells almost all co-expressed ED1, but only 2–5% ED1+ cells were double-stained by MHC-II (Fig. 2B). At the same time, co-labelling by the pan-Tmarker W3/13 or the B cell-marker OX22 with MHC-II was rarely seen. In addition, we have not observed any MHC-II+ cells with ramified morphology, which is a necessary intermediate phenotype during microglial activation. Therefore, the major cellular sources of MHC-II in the weight-drop TBI model appear to be reactive macrophages. Nevertheless, MHC-II expression only defines a small subpopulation of activated macrophages.
FTY720 treatment reduces MHC-II+ and EMAPII+ cell accumulation in TBI rat brains
In brains of FTY720 treated TBI rats, numbers of MHC-II+ cells was significantly lower (Fig. 2D) than of saline treated TBI rats (Fig. 2C) as early as Day 1 (3.4 ± 0.7 per HPF, n = 3, P < 0.01) and up to Day 4 (7.8 ± 2.2 per HPF, n = 3, P < 0.01; Fig. 1A).
EMAP-II expression was as well analyzed in TBI rats. Significant accumulation of EMAP-II+ cells in lesional areas was seen at Day 1 after injury (17.7 ±4.6 per HPF, n = 3, P < 0.05, compared to normal controls, data submitted and not shown here) and reached maximal numbers by Day 4 (146.3 ± 6.6 per HPF, n = 3, P < 0.01). In brains of FTY720 treated TBI rats, inhibition of EMAP-II+ cell accumulation (Fig. 2E and 2F) was observed at Day 4 compared to saline treated group (85.6 ±10.2 per HPF in FTY720 group and 146.3 ± 6.6 per HPF in saline control group, n = 3, P < 0.01; Fig. 1B).
Discussion
Here we have analyzed the early expression pattern of MHC-II in rat TBI induced by a weight-drop model and studied effects of FTY720 on MHC-II and EMAP-II expression. Significant MHC-II+ cell accumulation was observed early at 24 hrs after TBI and increased steadily up to 96 hrs during our observation period. The major cellular sources of MHC-II were identified as reactive macrophages by double-staining experiments. FTY720 significantly attenuated accumulation of MHC-II+ cells and EMAP-II+ cells in the initial stages of lesion development in rat brains following TBI.
MHC-II molecules expressed together with processed antigen and additional co-stimulatory molecules at the surface of antigen-presenting cells are essential to antigen presentation [28]. In normal brains, a subpopulation of roundly shaped macrophages, usually present in the subarachnoid space, Virchow-Robin's space and the choroid plexus, are constitutively MHC-II positive [27, 29]. Under a variety of pathogenic events, like intrathecal administration of TNF-α and TNF-γ, peripheral nerve axotomy and CNS infection, MHC-II expression is rapidly induced in microglia [12, 30–36]. Here a slight but significant increase in expression of MHC-II was also observed early following the weight-drop trauma and was restricted to a small sub-population of activated macrophages. Up-regulation of MHC-II has been reported in other TBI models but its expression pattern is different from that of the weight-drop model. In a stab wound TBI model, which is characterized by severe invasive mechanical lesions, a much stronger accumulation of MHC-II+ cells was observed compared with the weight-drop model and most reactive macrophages/microglia were MHC-II positive at any given time point [37]. In a closed TBI model, which is characterized with diffuse lesion, a much wider distribution of MHC-II+ cells was reported [27]. Therefore, MHC-II up-expression is confirmed in different TBI models but its expression patterns are different from each other, indicating that MHC-II defined cell population might play distinguishing roles in different TBI models.
An interesting finding in this investigation is that FTY720 attenuated early accumulation of MHC-II+ and EMAP-II+ cells after TBI. As mentioned above, MHC-II+ cells are reactive macrophages. Further, EMAP-II is a proinflammatory, antiangiogenic cytokine and expressed by activated microglia/macrophages in the parenchyma of the CNS. Increased expression of EMAP-II is considered a sensitive marker of microglia/macrophage activation [10, 11]. Therefore, FTY720 actually suppressed accumulation of reactive microglia/macrophages following TBI. FTY720 is a promising and potent immunosuppressive agent and acts through S1P signalling pathway to sequester lymphocytes into secondary lymphatic tissues and thus away from inflammatory lesions and graft sites [38, 39]. For monocytes/macrophages, depletion of circulating monocytes and alteration of macrophage functions following FTY720 treatment has not been reported. However, under pathological conditions, like experimental autoimmune encephalomyelitis and amyloid β-protein stimulated monocyte infiltration, administration of FTY720 inhibits macrophage infiltration to inflammatory lesions [22]. Here we have shown that FTY720 also suppressed reactive microglia/ macrophages accumulation to early lesions of TBI. The general, these suppressive effects of FTY720 on the accumulation of inflammatory cells following TBI could provide a new option to inhibit brain inflammatory reaction following TBI.
Pathological EMAP-II expression triggers the recruitment of macrophages, stimulates leukocyte chemotaxis, induces the expression of certain cytokines and causes endothelial apoptosis [26, 40–43].Accumulation of EMAP-II+ microglia/macrophages has been observed in regions of neuronal death at the lesion site and in areas of ongoing secondary damage [10]. Following TBI, EMAP-II is involved in causing edema and hypoxia [44, 45], which are known to alter blood flow rheology leading to microclustering and occlusion of fine microcappilaries resulting in the impairment of CNS parenchymal trophic supply [46]. FTY720 attenuated the accumulation of EMAP-II+ cells at Day 4 following TBI, suggesting FTY720 might have protective effects in early TBI.
In summary, we have studied MHC-II expression pattern in the early phase of weight-drop induced TBI. MHC-II expression is different in several TBI models, indicating that MHC-II defined macrophages sub-population might play different roles in different TBI models. Furthermore, FTY720 attenuates early lesional accumulation of EMAP-II+ reactive macrophages/microglia and of MHC-II+ reactive macrophages in TBI, suggesting that FTY720 could be applied to inhibit brain inflammatory reactions following TBI.
References
- 1.Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–6. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- 2.Engel S, Wehner HD, Meyermann R. Expression of microglial markers in the human CNS after closed head injury. Acta Neurochir Suppl. 1996;66:89–95. [PubMed] [Google Scholar]
- 3.Hausmann R, Kaiser A, Lang C, Bohnert M, Betz P. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int J Legal Med. 1999;112:227–32. doi: 10.1007/s004140050241. [DOI] [PubMed] [Google Scholar]
- 4.Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994;11:499–506. doi: 10.1089/neu.1994.11.499. [DOI] [PubMed] [Google Scholar]
- 5.Soares HD, Hicks RR, Smith D, McIntosh TK. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223–33. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandhir R, Puri V, Klein RM, Berman NE. Differential expression of cytokines and chemokines during secondary neuron death following brain injury in old and young mice. Neurosci Lett. 2004;369:28–32. doi: 10.1016/j.neulet.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Aihara N, Hall JJ, Pitts LH, Fukuda K, Noble LJ. Altered immunoexpression of microglia and macrophages after mild head injury. J Neurotrauma. 1995;12:53–63. doi: 10.1089/neu.1995.12.53. [DOI] [PubMed] [Google Scholar]
- 8.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 9.Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1998;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- 10.Mueller CA, Schluesener HJ, Conrad S, Meyermann R, Schwab JM. Lesional expression of a proinflammatory and antiangiogenic cytokine EMAP II confined to endothelium and microglia/ macrophages during secondary damage following experimental traumatic brain injury. J Neuroimmunol. 2003;135:1–9. doi: 10.1016/s0165-5728(02)00427-7. [DOI] [PubMed] [Google Scholar]
- 11.Mueller CA, Schluesener HJ, Conrad S, Meyermann R, Schwab JM. Spinal cord injury induces lesional expression of the proinflammatory and antiangiogenic cytokine EMAP II. J Neurotrauma. 2003;20:1007–15. doi: 10.1089/089771503770195858. [DOI] [PubMed] [Google Scholar]
- 12.Graeber MB, Bise K, Mehraein P. CR3/43, a marker for activated human microglia: application to diagnostic neuropathology. Neuropathol Appl Neurobiol. 1994;20:406–8. doi: 10.1111/j.1365-2990.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt AB, Buss A, Breuer S, Brook GA, Pech K, Martin D, Schoenen J, Noth J, Love S, Schroder JM, Kreutzberg GW, Nacimiento W. Major histocompatibility complex class II expression by activated microglia caudal to lesions of descending tracts in the human spinal cord is not associated with a T cell response. Acta Neuropathol. 2000;100:528–36. doi: 10.1007/s004010000221. [DOI] [PubMed] [Google Scholar]
- 14.Holmin S, Mathiesen T. Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport. 1999;10:1889–91. doi: 10.1097/00001756-199906230-00017. [DOI] [PubMed] [Google Scholar]
- 15.Adachi K, Kohara T, Nakano N, Arita M, Chiba K, Mishina T, Sasaki S, Fujita T. Design, synthesis, and structure-activity relationships of 2-substituted-2-amino-1,3-propanediols: discovery of a novel immunosuppressant, FTY720. Bioorg Med Chem. 1995;5:853–6. [Google Scholar]
- 16.Fujita T, Yoneta M, Hirose R, Sasaki S, Inoue K, Kiuchi M, Hirase S, Adachi K, Arita M, Chiba K. Simple compounds, 2-alkyl-2-amino-1,3-propanediols, have potent immunosuppressive activity. Bioorg Med Chem. 1995;5:847–52. [Google Scholar]
- 17.Suzuki S, Enosawa S, Kakefuda T, Shinomiya T, Amari M, Naoe S, Hoshino Y, Chiba K. A novel immunosuppressant, FTY720, with a unique mechanism of action, induces long-term graft acceptance in rat and dog allo-transplantation. Transplantation. 1996;61:200–5. doi: 10.1097/00007890-199601270-00006. [DOI] [PubMed] [Google Scholar]
- 18.Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 19.Brinkmann V, Davis MD, Heise CE. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 20.Webb M, Tham CS, Lin FF. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–21. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Fujino M, Funeshima N, Kitazawa Y. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–7. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- 22.Rausch M, Hiestand P, Foster CA, Baumann DR, Cannet C, Rudin M. Predictability of FTY720 efficacy in experimental autoimmune encephalomyelitis by in vivo macrophage tracking: clinical implications for ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2004;20:16–24. doi: 10.1002/jmri.20057. [DOI] [PubMed] [Google Scholar]
- 23.Kaneider NC, Lindner J, Feistritzer C, Sturn DH, Mosheimer BA, Djanani AM, Wiedermann CJ. The immune modulator FTY720 targets sphingosine-kinase-dependent migration of human monocytes in response to amyloid beta-protein and its precursor. FASEB J. 2004;18:1309–11. doi: 10.1096/fj.03-1050fje. [DOI] [PubMed] [Google Scholar]
- 24.Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 25.Golarai G, Greenwood AC, Feeney DM, Connor JA. Physiological and structural evidence for hippocampal involvement in persistent seizure susceptibility after traumatic brain injury. J Neurosci. 2001;21:8523–37. doi: 10.1523/JNEUROSCI.21-21-08523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schluesener HJ, Seid K, Zhao Y, Meyermann R. Localization of endothelial-monocyte-activating polypeptide II (EMAP II), a novel proinflammatory cytokine, to lesions of experimental autoimmune encephalomyelitis, neuritis and uveitis: expression by monocytes and activated microglial cells. Glia. 1997;20:365–72. doi: 10.1002/(sici)1098-1136(199708)20:4<365::aid-glia8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Csuka E, Hans VH, Ammann E, Trentz O, Kossmann T, Morganti-Kossmann MC. Cell activation and inflammatory response following traumatic axonal injury in the rat. Neuroreport. 2000;11:2587–90. doi: 10.1097/00001756-200008030-00047. [DOI] [PubMed] [Google Scholar]
- 28.DeSimone R, Giampaolo A, Giometto B, Gallo P, Levi G, Peschle C, Aloisi F. The costimulatory molecule B7 is expressed on human microglia in culture and in multiple sclerosis lesions. J Neuropathol Exp Neurol. 1995;54:175–87. doi: 10.1097/00005072-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 30.Mattiace LA, Davies P, Dickson WD. Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors. Am J Pathol. 1990;136:1101–14. [PMC free article] [PubMed] [Google Scholar]
- 31.McGeer PL, Itagaki S, McGeer EG. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76:550–7. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 32.Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–7. [Google Scholar]
- 33.Weinstein DL, Walker DG, Akiyama H, McGeer PL. Herpes simplex virus type infection of the CNS induces major histocompatibility complex antigen expression on rat microglia. J Neurosci Res. 1990;26:55–65. doi: 10.1002/jnr.490260107. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt AB, Brook GA, Buss A, Nacimiento W, Noth J, Kreutzberg GW. Dynamics of microglial activation in the spinal cord after cerebral infarction is revealed by expression of MHC class II antigen. Neuropathol Appl Neurobiol. 1998;24:167–76. doi: 10.1046/j.1365-2990.1998.00103.x. [DOI] [PubMed] [Google Scholar]
- 35.Streit WJ, Graeber MB, Kreutzberg GW. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989;105:115–26. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gehrmann J, Gold R, Linington C, Lannes-Vieira J, Wekerle H, Kreutzberg GW. Spinal cord microglia in experimental allergic neuritis. Evidence for fast and remote activation. Lab Invest. 1992;67:100–13. [PubMed] [Google Scholar]
- 37.Cho BP, Song DY, Sugama S, Shin DH, Shimizu Y, Kim SS, Kim YS, Joh TH. Pathological dynamics of activated microglia following medial forebrain bundle transaction, Glia. 2006;53:92–102. doi: 10.1002/glia.20265. [DOI] [PubMed] [Google Scholar]
- 38.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–25. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 39.Matloubian M, Lo CG, Cinamon G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–60. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 40.Kao J, Ryan J, Brett G, Chen J, Shen H, Fan YG, Godman G, Familletti PC, Wang F, Pan YC. Endothelial monocyte-activating polypeptide II. A novel tumor-derived polypeptide that activates host-response mechanisms. J Biol Chem. 1992;267:20239–47. [PubMed] [Google Scholar]
- 41.Knies UE, Behrensdorf HA, Mitchell CA, Deutsch U, Risau W, Drexler HC, Clauss M. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proc Natl Acad Sci USA. 1998;95:12322–7. doi: 10.1073/pnas.95.21.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tas MP, Murray JC. Endothelial monocytes activating polypeptide II. Int J Biochem Cell Biol. 1996;28:837–41. doi: 10.1016/1357-2725(96)00038-6. [DOI] [PubMed] [Google Scholar]
- 43.Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999;284:147–51. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- 44.Adelson PD, Whalen MJ, Kochanek PM, Robichaud P, Carlos TM. Blood brain barrier permeability and acute inflammation in two models of traumatic brain injury in the immature rat: a preliminary report. Acta Neurochir Suppl. 1998;71:104–6. doi: 10.1007/978-3-7091-6475-4_31. [DOI] [PubMed] [Google Scholar]
- 45.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- 46.Mori N, Fukatsu T. Anticonvulsant effect of DN-1417, a derivative of thyrotropin-releasing hormone, and lipo-some-entrapped DN-1417, on amygdaloid-kindled rats. Epilepsia. 1992;33:994–1000. doi: 10.1111/j.1528-1157.1992.tb01749.x. [DOI] [PubMed] [Google Scholar]


