Abstract
Specific classes of interstitial cells exist in visceral organs and have been implicated in several physiological functions including pacemaking and mediators in neurotransmission. In the bladder, Kit+ interstitial cells have been reported to exist and have been suggested to be neuromodulators. More recently a second interstitial cell, which is identified using antibodies against platelet-derived growth factor receptor-α (PDGFR-α) has been described in the gastrointestinal (GI) tract and has been implicated in enteric motor neurotransmission. In this study, we examined the distribution of PDGFR-α+ cells in the murine urinary bladder and the relation that these cells may have with nerve fibres and smooth muscle cells. Platelet-derived growth factor receptor-α+ cells had a spindle shape or stellate morphology and often possessed multiple processes that contacted one another forming a loose network. These cells were distributed throughout the bladder wall, being present in the lamina propria as well as throughout the muscularis of the detrusor. These cells surrounded and were located between smooth muscle bundles and often came into close morphological association with intramural nerve fibres. These data describe a new class of interstitial cells that express a specific receptor within the bladder wall and provide morphological evidence for a possible neuromodulatory role in bladder function.
Keywords: platelet-derived growth factor receptor-α, interstitial cells, interstitial cells of Cajal, urinary bladder, telocyte
Introduction
The bladder is a compliant storage vessel that adapts to increasing volume without concomitant increase in pressure as urine enters via the ureters. The mechanisms for adaptive compliance in response to filling are not completely understood, but recent studies suggest a role for stretch-dependent K+ channels that tend to maintain a low level of detrusor excitability as volume increases [1]. Upon filling, pressure gradually rises and a threshold is reached at which voiding contractions involving an autonomic reflex are initiated. The bladder is innervated by cholinergic and purinergic motor neurons that regulate the contractions of the detrusor smooth muscle cells. Normal bladder voiding contractions are generally attributed to cholinergic neuromuscular transmission, while purinergic motor neurons are thought to play an increasing role under pathological conditions [2,3]. Thus, proper voiding responses depend upon a complex interplay between detrusor smooth muscle cells, urothelial cells and sensory and motor neurons [4].
Evidence has been emerging that additional cell types (interstitial cells) may also contribute to normal bladder function. Cells labelled with vimentin [5], an intermediate filament protein, have been linked to the interstitial cells of Cajal (ICC) in the GI tract [6], however, antibodies to the receptor tyrosine kinase, c-Kit, a gold standard for labelling interstitial cells in the gut, have not been as reliable as that in the bladder. Kit immunoreactivity has been demonstrated in mouse urinary bladder [7], but others have not succeeded in labelling interstitial cells via c-Kit immunohistochemistry [[8,9]; independent observations by Koh et al. and Sergeant et al.]. A transgenic mouse, engineered to express a bright green fluorescent protein in cells that normally express c-Kit, displayed intensely labelled interstitial cells in the GI tract [10,11], but failed to label interstitial cells in the bladder (S. D. Koh and S. M. Ward, unpublished observations). Furthermore, techniques to induce loss of interstitial cells in the gut (e.g. blocking of c-Kit, loss-of-function mutations in key genes in the stem cell factor/c-Kit signalling pathway and pharmacological manipulation of signalling molecules downstream from Kit; [12,13,,14]) have either not been tested or have failed to affect bladder function. The relatively faint expression or absence of c-Kit in bladder interstitial cells may explain the insensitivity of the bladder to experimental manipulation of c-Kit signalling.
Rather than being analogous to ICC, bladder interstitial cells may be myofibroblasts (as identified by expression of α-actin in cells of the lamina propria) [15]; or fibroblast-like cells with a specialized function in the tunica muscularis of the GI tract [16,17]. Another interstitial cell, which is distinct from the ICC and was originally called interstitial Cajal-like cell (ICLC), but more recently termed telocyte has been described in a variety of tissues including heart, lung, placenta and skeletal muscle [18,19,20,21,22]. Telocyte was used due to ultrastructural differences that exist between ICLC and ICC [23], and have been implicated in a variety of physiological processes including angiogenesis and skeletal muscle repair [22].
Recently, it was shown that a sub-population of interstitial cells in the GI tract express PDGFR-α and can be labelled robustly with antibodies against this receptor in a highly specific manner [17,24]. Here, we have investigated the distribution of PDGFR-α immunopositive cells in the murine bladder. We found these cells to be widely distributed in the muscularis and lamina propria, many to be positive for vimentin, some closely associated with nerve fibres. Although the function of PDGFR-α+ cells in the urinary bladder is unknown, numerous PDGFR-α+ cells were present in the bladder wall thought to be niches for myofibroblasts or ICLC. Transgenic mice with PDGFR-α+ cells constitutively labelled with enhanced green fluorescent protein (eGFP) were shown to provide a novel means to identify these cells in mixed-cell enzymatic dispersions of bladder tissues so that the phenotype and functions of these cells can be evaluated in future studies with molecular or physiological assays.
Materials and methods
Animal tissue
Three types of mice were used for this study. C57BL/6 (Jackson Laboratory, Bar Harbor, ME, USA) were purchased; smooth muscle myosin heavy chain/Cre/eGFP (smMHC/Cre/eGFP) mice were donated by Dr. Michael Kotlikoff (Cornell University); and Pdgfratm11(EGFP)Sor/J mice were purchased (Jackson Laboratory). Mice were exposed to isofluorane (Baxter, Deerfield, IL, USA) inhalation immediately followed by cervical dislocation. The abdominal wall was cut open and urinary bladder was exposed and severed at the neck. The protocol for euthanizing mice was approved by the Institutional Animal Care and Use Committee (IACUC) and assures compliance with the United States Department of Agriculture (USDA).
Immunohistochemistry
Whole mount immunohistochemistry
C57BL/6, smMHC/Cre/eGFP and Pdgfratm11(EGFP)Sor/J bladders were cut open from the neck up to the dome. Tissues were dissected in Krebs ringer solution containing 118.5 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 23.8 mM NaHCO3, 1.2 mM KH2PO4 and 11 mM dextrose, then pinned down on Sylgard dish and stretched 150% from the resting state. We used two preparations with and without urothelium. For urothelial denudation, urothelium was removed and surface of the muscle was scraped to remove any residual sub-urothelial cells. Tissues were fixed in 3% paraformaldehyde or acetone for 15 min., blocked with 10% normal donkey serum (Sigma-Aldrich, St. Louis, MO, USA) and incubated overnight in primary antibody diluted in 0.5% Triton-X (Sigma-Aldrich) at 4°C. Tissue was then incubated in appropriate Alexa Fluor (Invitrogen, Grand Island, NY, USA) secondary antibody diluted 1:1000 in PBS for 1 hr. For double labelling studies, tissues were re-blocked for 1 hr in 10% normal donkey serum (Sigma-Aldrich) and incubated overnight in antibody of choice, diluted in 0.5% Triton-X (Sigma-Aldrich) and incubated in the appropriate Alexa Fluor (Invitrogen) antibody diluted 1:1000 in PBS for 1 hr. Processed tissues were mounted with Aqua mount mounting media (Lerner Laboratories, Pittsburgh, PA, USA) on glass slides and cover slipped and imaged. The primary antibodies used can be found in Table. Primary antibodies were omitted in the procedure for negative controls.
Table 1.
Primary antibodies used
| Primary antibodies | Species | Catalogue no. | Dilution | Source |
|---|---|---|---|---|
| PDGFR-α (acetone fixative) | Rat | 16-1401-82 | 1:200 | eBioscience, San Diego, CA, USA |
| PDGFR-α | Goat | AF1062 | 1:200 | R&D Systems, Minneapolis, MN, USA |
| PGP9.5 | Rabbit | RA-95101 | 1:2000 | UltraClone Ltd, Isle of Wight, UK |
| Vimentin | Rabbit | 3932 | 1:100 | Cell Signaling, Danvers, MA, USA |
| vAchT | Goat | AB1588 | 1:500 | Millipore, Billerica, MA, USA |
| ACK-2 | Rat | N/A | 1:500 | Produced in house |
| mSCFR | Goat | AF1356 | 1:500 | R&D Systems |
| cKit | Goat | SC-1493 | 1:100 | Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA |
| cKit | Rabbit | NBP1-49582 | 1:200 | Novus Biologicals, Littleton, CO, USA |
| cKit | Rat | NB110-93600 | 1:100 | Novus Biologicals |
| Vimentin | Goat | V4930 | 1:100 | Sigma-Aldrich, St. Louis, MO, USA |
PDGFR: platelet-derived growth factor receptor; vAchT: vesicular acetylcholine transferase; PGP9.5: protein gene product 9.5; ACK-2: monoclonal anti-c-kit antibody; mSCFR: mouse stem cell factor receptor.
Cryostat sections
Bladders were fixed in 3% paraformaldehyde or acetone for 15 min., washed in PBS, dehydrated successively in 5%, 10% and 15% sucrose/PBS solution for 1 hr each, and overnight in 20% solution. Tissues were bisected laterally and frozen in Cryomold (Sakura Finetek, Torrence, CA, USA) containing 1:1 20% sucrose/PBS and Tissue-Tek O.C.T. (Sakura Finetek) and snap frozen in liquid nitrogen-cooled isopentane. Ten micrometre sections were cut on a Leica 3050S cryostat (Leica Microsystems, Wetzlar, Germany) on Vectabond (Vector Laboratories, Burlingame, CA, USA)-coated slides. Tissues were blocked with 10% normal donkey serum for 1 hr and then incubated in with the primary antibody overnight diluted in 0.5% Triton-X at 4°C. Slides were incubated with secondary antibody for 1 hr (1:1000), and mounted with Aqua mount mounting medium (Lerner Laboratories). For double labelling studies, tissues were re-blocked in 10% normal donkey serum and incubated overnight with the primary antibody at 4°C and then with the secondary antibody for 1 hr. Primary antibodies were omitted for the negative control experiments. Specimens were imaged with the Olympus FV1000 (Olympus America Inc., Center Valley, PA, USA) and Carl Zeiss LSM 510 confocal microscope (Carl Zeiss Microimaging, LLC, Thornwood, NY, USA). Images were processed and arranged in Adobe Photoshop CS5 (Adobe Systems Incorporated, San Jose, CA, USA).
Freshly dispersed cells
Pdgfratm11(EGFP)Sor/J urinary bladders were obtained and cut open from neck to the dome and pinned out on a Sylgard dish. Urothelium was removed and the muscle layer was scraped to remove any residual sub-urothelial cells. The tissue was incubated at 37°C for 30 min. in Hank's Ca2+ Free solution (containing 125 mM NaCl, 5.36 mM KCl, 15.5 mM NaHCO3, 0.336 mM Na2HPO4, 0.44 mM K2HPO4, 10 mM dextrose, 2.9 mM sucrose and 11 mM HEPES), 4 mg/ml of bovine serum albumin (Sigma-Aldrich), 4 mg/ml of trypsin inhibitor II (Sigma-Aldrich) and 2 mg/ml of collagenase type II (Worthington Biochemical, Lakewood, NJ, USA). After three to five washes with Hank's solution to remove residual enzyme, the tissue was triturated through blunt pipettes. Cells were then put on to microscope slides, cover slipped and imaged on a Carl Zeiss LSM 510 microscope. Images were then processed and arranged in Adobe Photoshop CS5.
Results
Antibodies against PDGFR-α label cells known as ‘fibroblast-like’ in the GI tract [17,24,,25], and physiological functions, such as mediating inhibitory purinergic neurotransmission, have recently been ascribed to these cells [17]. The distribution of PDGFR-α immunopositive cells (PDGFR-α+) was examined via immunohistochemistry cryostat sections and in whole mounts of murine bladder muscles using confocal microscopy. Platelet-derived growth factor receptor-α+ cells were widely distributed and possessed spindle- and stellate-shaped morphologies. These cells were often observed as an interconnecting network with multiple cell processes branching towards and making apparent contact with neighbouring cells (Fig. 1A and B). Labelling of muscles from smMHC/Cre/eGFP mice (in which smooth muscle cells express eGFP) with antibodies against PDGFR-α showed that these cells lie along the borders of smooth muscle bundles within the detrusor muscle (Fig. 2A). Platelet-derived growth factor receptor-α+ cells were also found between individual smooth muscle cells in smaller bundles of smooth muscle (Fig. 2B). A dense population of PDGFR-α+ cells was also found within the lamina propria of the bladder with the cellular network closely packed in the sub-urothelium region (Fig. 2C). Whole mount preparation on smMHC/Cre/eGFP labelled with PDGFR-α further displayed the location of PDGFR-α+ in between smooth muscle bundles (Fig. 2D). Platelet-derived growth factor receptor-α expression was not resolved within the urothelium per se.
Fig 1.
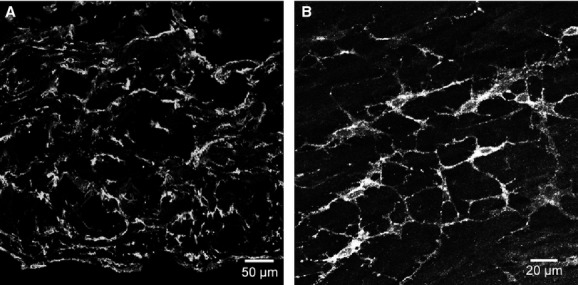
Platelet-derived growth factor receptor (PDGFR)-α cells in the bladder detrusor. (A) Cryostat section of detrusor muscle showing PDGFR-α immunoreactivity throughout the entire mucularis. (B) Whole mount of the bladder muscularis. PDGFR-α+ cells run parallel in the direction of the smooth muscle cells in the detrusor. PDGFR-α+ cells possess spindle- and stellate-shaped morphology with multiple processes that formed a discrete network. Scale bars are indicated in each panel.
Fig 2.
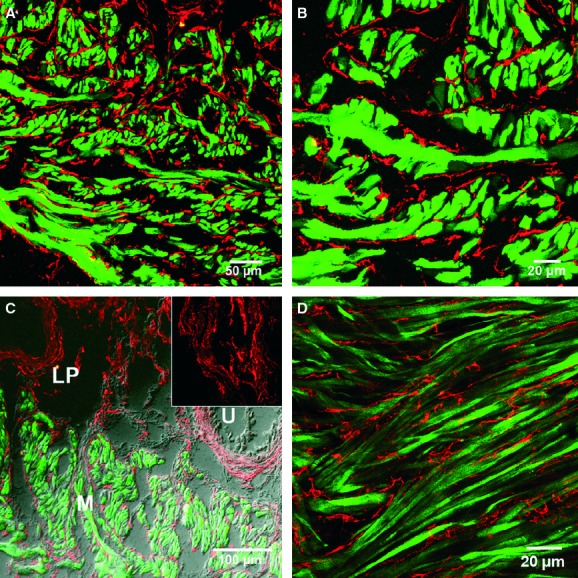
Double labelling of platelet-derived growth factor receptor (PDGFR)-α cells and smooth muscle cells in the detrusor. (A) Cryostat sections of PDGFR-α (red) and smMHC/Cre/eGFP (green) labelling within the detrusor muscle. (B) At high power, PDGFR-α+ cells were observed to lie interstitially along and in between smMHC/Cre/eGFP+ muscle bundles. (C) PDGFR-α+ cells were found in the muscularis (M) and in the lamina propria (LP) but absent in the urothelium (U). A high-power image of PDGFR-α+ cells in the lamina propria is shown in the inset. (D) Confocal image of a single slice (0.5 μm) of a whole mount revealing the complicated arrangement of PDGFR-α+ cells (red) and smMHC/Cre/eGFP (green) immunohistochemistry within the detrusor muscularis. Scale bars are indicated in each panel.
Vimentin, an intermediate filament marker, has been used to label cells thought to be Kit+ interstitial cells in the urinary bladder [26,27,,28]. Thus, we performed double labelling immunohistochemistry to examine whether vimentin and PDGFR-α were co-localized in the same population of cells. Many of the PDGFR-α+ cells in both the lamina propria as well as the detrusor muscle were co-labelled with vimentin antibodies (Fig. 3A), however, a few vimentin+ cells were not labelled with PDGFR-α+ (Fig. 3B and D). Whole mount immunohistochemistry revealed that vimentin and PDGFR-α were co-localized within the same cell (Fig. 3C and D). In the detrusor, 100% of the PDGFR-α+ cells were co-labelled with vimentin, however, PDGFR-α was not resolved in 18% of vimentin+ cells. Stellate c-Kit+ cells, running along the periphery of smooth muscle bundles, have been reported in the bladder of guinea pig [26], and this appears to be in a similar anatomical location as PDGFR-α+ cells. We attempted double labelling of PDGFR-α+ cells and c-Kit+ cells to determine whether the cells expressing c-Kit are PDGFR-α+ cells. These proteins are expressed by distinct populations of cells in the GI tract [17,24,,25]. We were unable to resolve Kit immunoreactivity in the murine bladder using multiple attempts and five antibodies (Fig. 4A). As a positive control, we also processed tunica muscularis of the murine colon using the same protocols as studies on bladder. Robust Kit immunoreactivity was observed routinely in ICC in the colon (Fig. 4B). These data suggest that the antibodies and techniques used in the present study were suitable for detection of c-Kit immunoreactivity, but as we could not confirm c-Kit immunoreactivity, we cannot make a conclusion about whether PDGFR-α+ cells are the same population of cells described previously [4].
Fig 3.
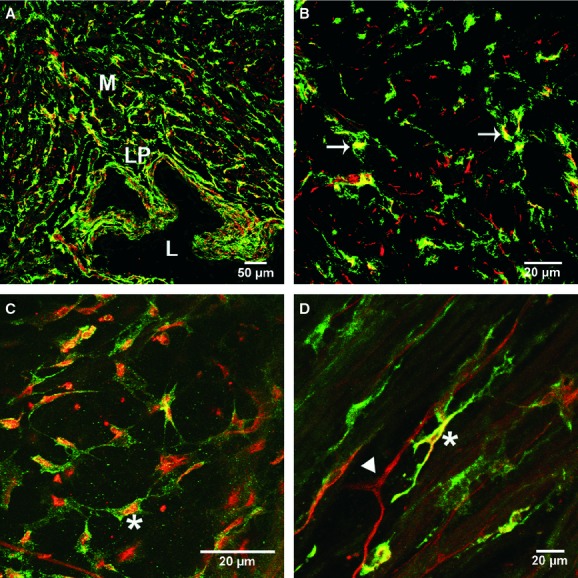
(A) Platelet-derived growth factor receptor (PDGFR)-α and vimentin immunoreactivity in the bladder wall. Cryostat section of PDGFR-α (green) double-labelled with vimentin (red). (B) Higher magnification of a double-labelled image of a cryostat section revealing the distribution of both PDGFR-α and vimentin immunoreactivity in the bladder wall. Note only partial cellular overlap of PDGFR-α and vimentin immunoreactivity (arrows; yellow). (C, D) Confocal reconstructions of whole mounts labelled with vimentin (red) showing only partial cellular localization with PDGFR-α (green) in a discrete population of cells (yellow, *). Other cells were either PDGFR-α+ (arrow) or vimentin+ (arrowhead). Scale bars are indicated in each panel. Lamina propria (LP), lumen (L) and muscularis (M) are shown in (A).
Fig 4.
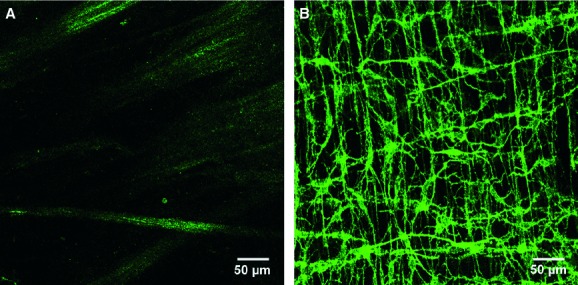
Lack of Kit immunoreactivity in the bladder detrusor. (A) The absence of Kit immunoreactivity within the muscularis of the urinary bladder. Only minimal autofluorescense was observed with the muscularis. (B) As a positive control, a whole mount preparation in the mouse colon revealing Kit immunoreactivity using the same antibody as in (A). These tissues were processed at the same time in an identical manner. Images were also collected using similar laser and detector settings. Scale bars are indicated in each panel.
Platelet-derived growth factor receptor-α+ cells in the GI tract are closely associated with enteric nerve fibres [17,24,,25] and possess receptors that respond to purines. We investigated whether similar anatomical association occurs between PDGFR-α+ cells and terminals of autonomic neurons in bladder muscles. Double labelling experiments were performed using PDGFR-α+ antibodies and the pan neuronal marker, protein gene product 9.5 (PGP9.5) to determine the proximity of neurons to PDGFR-α+ cells. A second series of experiments were performed using antibodies raised against vesicular acetylcholine transporter (vAChT) and PDGFR-α to specifically examine the anatomical location of PDGFR-α+ cells and cholinergic neurons.
Neural processes (PGP9.5+) were found in close proximity to some PDGFR-α+ cells (Fig. 5A and B). However, the majority of PDGFR-α+ cells as shown in cross-sectional preparations did not appear to be closely associated with neurons (Fig. 5C). The PDGFR-α+ cells that made close contacts were usually those surrounding large nerve trunks (Fig. 5D–F). There were occasional close associations between PDGFR-α+ cells and vAChT+ processes (Fig. 6A and B), but most vAChT+ varicose processes were not closely associated with these cells. These data suggest that PDGFR-α+ cells may be innervated by cholinergic neurons and contribute to cholinergic regulation, but much of the input in bladder appears to be via cells, other than PDGFR-α+ cells, most likely direct innervation of bladder smooth muscle cells as suggested previously by electron microscopy [29].
Fig 5.
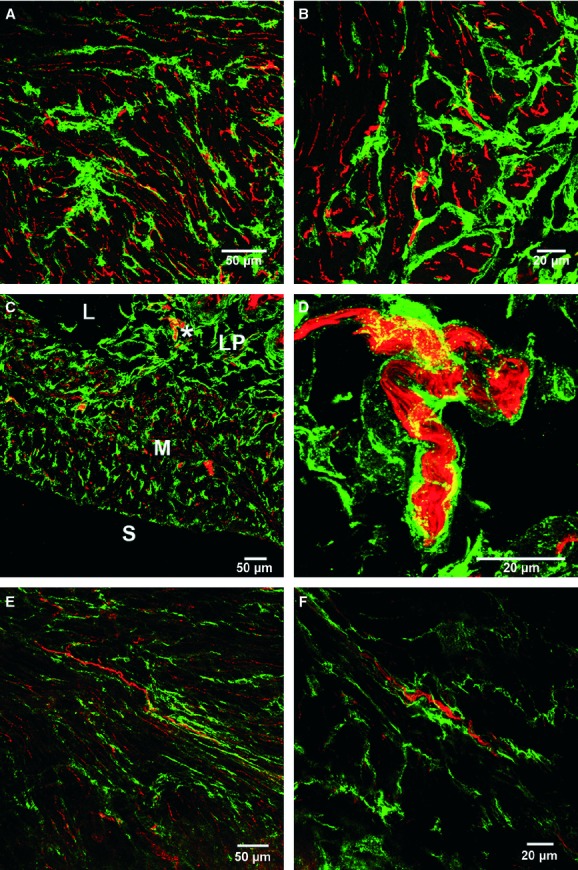
Close anatomical apposition between nerve fibres and platelet-derived growth factor receptor (PDGFR)-α+ cells within the bladder wall. (A–D) Confocal reconstructions of cryostat sections double labelled with a pan-neuronal marker, PGP9.5 (red) and PDGFR-α+ cells (green). (A, C) Low-power image through the bladder wall revealing the distribution of neural elements and PDGFR-α+ cells. (B) At higher power, some nerve fibres can be observed to be closely associated with PDGFR-α+ cells while others are not in close apposition. (D) Larger nerve trucks [denoted by * in (C)] can be seen surrounded by PDGFR-α+ cells. (E, F) Whole mounts double labelled with PDGFR-α+ (green) and PGP9.5 (red) revealing the close proximity of some nerve fibres with PDGFR-α+ cells. (F) Many but not all nerve fibres are seen running parallel to the long axis of PDGFR-α+ cells. Scale bars are indicated in each panel. Lamina propria (LP), lumen (L), muscularis (M) and serosa (S) are shown in (C).
Fig 6.
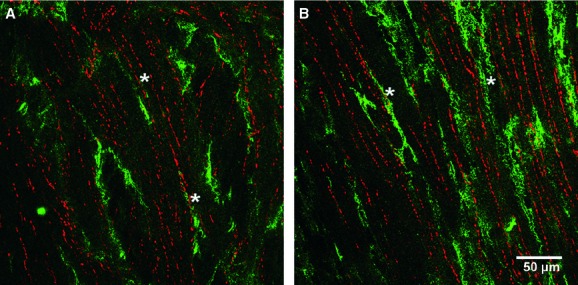
Double labelling of excitatory nerve fibres and platelet-derived growth factor receptor (PDGFR)-α+cells in the bladder detrusor. (A, B) Whole mount labelled with vesicular acetylcholine transferase (vAChT, red) to identify cholinergic nerves and PDGFR-α+ cells (green). The majority of vAChT+ nerves were not intimately associated with PDGFR-α+ cells but some fibres were in a similar anatomical location (*). Scale bars are indicated in each panel.
A mouse engineered to express a histone 2B-eGFP fusion protein [30] in the nuclei of cells that express PDGFR-α, was used to validate the specificity of antibodies used in this study and to provide a new tool for identification of these cells in enzymatically dispersed cell preparations. Immunohistochemistry using antibodies against PDGFR-α in bladders of mice that express the histone 2B-eGFP fusion protein demonstrated the specific cellular co-localization of histone 2B-eGFP fusion protein in PDGFR-α+ cells (Fig. 7A–D). Enzymatic dispersions of bladder detrusor muscle resulted in a mixed population of cells but many containing eGFP could easily be distinguished. The morphology of the cells with eGFP was neither spindle nor stellate shaped, as in situ, but ovoid in appearance (Fig. 8A–C). This change in morphology suggests that cell processes were retracted during the dispersion process. eGFP+ was localized to the nucleus of the dispersed cells, analogous to the cells in situ. The eGFP+ cells were distinct from smooth muscle cells and other unidentified cells present in the mixed cell population (Fig. 8D–F).
Fig 7.
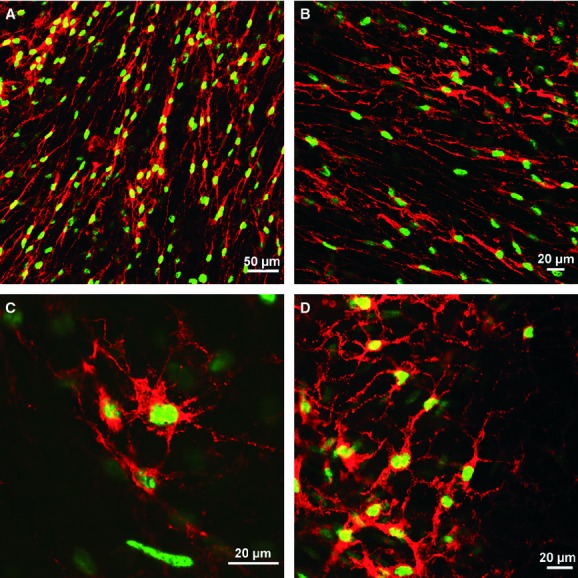
Histone 2B-eGFP fusion protein expression in the nuclei of cells that express platelet-derived growth factor receptor (PDGFR)-α. (A, B) Low-power images revealing eGFP expression in all the nuclei of PDGFR-α+ cells. (C, D) High-power images showing the exact location of eGFP fluorescent nuclei within PDGFR-α+ cells. Scale bars are indicated in each panel.
Fig 8.
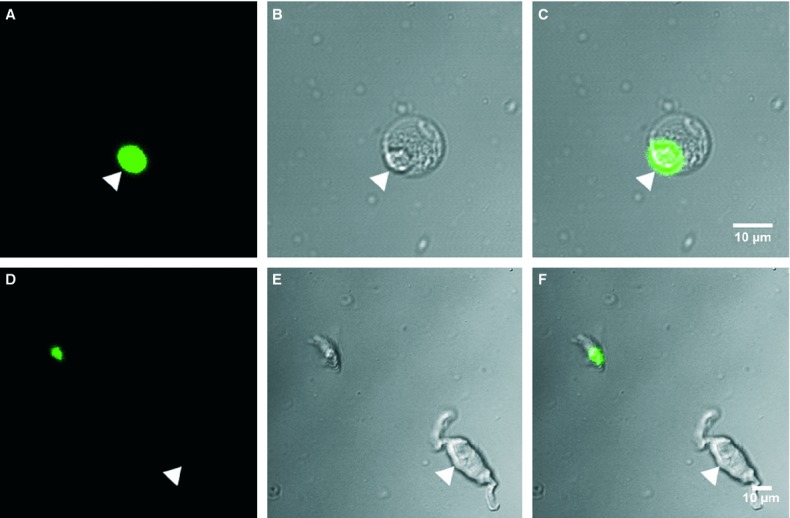
Enzymatically dispersed cells from the bladder detrusor of mice with histone 2B-eGFP fusion protein expression in platelet-derived growth factor receptor (PDGFR)-α+ cells. (A–C) Fluorescent (A) and differential interference contrast (B) images revealing typical morphology of freshly dispersed GFP+ cells. eGFP was localized to the nuclei of small rounded cells [arrowheads (C)]. (D–F) PDGFR-α+ cells were found to be smaller in diameter and ovoid in shape compared with typical smooth muscle cells that were not fluorescent (arrowhead). Scale bars are indicated in each panel.
Discussion
In this study, we have shown that antibodies against PDGFR-α provide robust labelling of a specific class of interstitial cell in both the sub-urothelium and in detrusor muscle bundles in the urinary bladder. Platelet-derived growth factor receptor-α+ cells were distributed as a densely packed network in the lamina propria, and these cells were also distributed throughout the detrusor muscularis, being located within and around the periphery of smooth muscle bundles. In previous reports, vimentin has been used as an immunolabel to identify ICC in the urinary bladder, where some populations of Kit+ cells have been reported to express vimentin [26,28]. Vimentin is an intermediate filament [31] and cannot be considered specific for ICC. A significant percentage of PDGFR-α+ cells were co-labelled with antibodies for vimentin. The observation that some vimentin+ cells were also PDGFR-α+ suggests that caution must be taken in studies in which vimentin labelling has been used to characterize the distribution or loss of Kit+ interstitial cells in the bladder. We also verified that mice with a histone 2B-eGFP fusion protein engineered to express this reporter in cells with PDGFR-α are a powerful new tool for identifying this class of interstitial cell in situ or in mixed cell dispersions. The latter approach will facilitate more extensive studies of phenotype and physiology of PDGFR-α+ cells in the bladder. We also characterized the proximity of nerve terminals to PDGFR-α+ cells. There were close associations between nerve terminals and PDGFR-α+ cells, however, there were also many varicose neural processes that did not appear to be associated with PDGFR-α+ cells. Thus, it is possible that PDGFR-α+ cells may receive input from autonomic nerves and contribute to tissue responses to nerve stimulation, but direct innervation of smooth muscle cells, as demonstrated by ultrastructural techniques [29], is likely to mediate neural control of bladder contraction.
The existence of specific classes of interstitial cells within the bladder wall has received considerable attention in recent years. The immunohistochemical demonstration that a population of Kit+ interstitial cells existed in the urinary bladder raised the possibility that these cells may play functional roles analogous to the GI tract [14,32,,33], that is, (i) pacemakers and (ii) mediators of neurotransmission in regulation of motor function. However, to date there is no clear demonstration of the functional role of these presumed ICC in the bladder [26,27,,34]. W/Wv (c-Kit mutants) mice, with reduced receptor tyrosine kinase function and greatly reduced numbers of ICC, have been used to demonstrate the importance of ICC in the GI tract [14,35]. However, physiological studies did not find any significant differences between urinary bladders of W/Wv and wild-type mice [7]. In this study, we failed to demonstrate immunohistochemical labelling of Kit+ interstitial cells in the bladder (independent observations by Koh et al. and Sergeant et al.) and several others studies have reported poor success in replicating immunohistochemical labelling of c-Kit in the murine bladder [[9],28]. In human bladder, CD117/cKit+ cells have been shown to be immunoreactive for mast cell tryptase and very few tryptase−, CD117/cKit+ cells were found. In the same study, another population of interstitial cells that were CD34+ was also described. These cells were located around muscle fascicles with bipolar and stellate morphology but appeared to have no relation to c-Kit+ cells [36].
If Kit+ interstitial cells exist in the urinary bladder, there is no strong indication that they have functions similar to this class of cells in the GI tract. Microelectrode experiments performed simultaneously with Ca2+ imaging of Fluo-4-loaded guinea pig bladders concluded that there was no evidence that pacemaker activity is generated by ICC [37]. Gleevec, a Kit tyrosine kinase inhibitor, was reported to inhibit spontaneous and evoked contractions in the detrusor of the urinary bladder [38]. Gleevec has also been shown to reduce spontaneous rise in pressure in whole bladder experiments as well as abolish action potentials in bladder smooth muscle [39]. However, there is no clear evidence that Gleevec worked exclusively on ICC-type cells. Gleevec is also an inhibitor of PDGFR-α and -β subtypes [40], for example, and therefore the cells we described in this study could possibly have mediated the effects of this compound. The rapid actions of this drug, however, suggest non-specific effects, possibly on L-type calcium channels of smooth muscle cells, as has been reported in the gastric antrum [41]. Further studies are needed to determine whether there are also effects of Gleevec on PDGFR-α+ cells in the bladder. More recently, Gleevec has been shown to inhibit spontaneous contractions in human non-pregnant myometrium but the site of action of this compound remains to be determined [42].
Others have hypothesized that ICC could play a role in neuromodulation of smooth muscle activity in the urinary bladder [34]. This hypothesis was based primarily on morphological studies that found close proximity between nerve fibres and ICC and cells with morphological features consistent with ICC responded to exogenous application of muscarinic agonists by generating Ca2+ waves [34]. However, to date there is no direct functional data supporting a role for ICC in neuromodulation of bladder contractility. For example, if electrically evoked neural responses were observed in Kit+ ICC, this would be direct evidence for a possible role of ICC in neuromodulation of bladder activity. Here, we found that PDGFR-α+ cells also form close anatomical associations with intramural nerve fibres in the bladder. These cells may therefore be innervated and contribute to post-junctional responses to neural inputs. In the GI tract, PDGFR-α+ cells are closely aligned with enteric nerve terminals [25], possess P2Y1 receptors and apamin-sensitive small-conductance Ca2+-activated K+ channels (SK3 channels) and respond to purine transmitter candidates β-NAD and ATP [17]. Thus, it is possible that a signalling pathway, linked to contraction rather than relaxation, could exist in the urinary bladder. For example, in the bladder of guinea pig, exogenous ATP generates intracellular Ca2+ transients via binding of P2Y receptors and this activates Ca2+-activated Cl− current in cells identified as myofibroblasts in the lamina propria [43]. It should also be noted that the myofibroblasts that responded to ATP in the bladder of guinea pig were vimentin and P2Y6 immunopositive, suggesting that some of the PDGFR-α+ cells may respond to purinergic stimulation [36]. Unfortunately, we have failed to demonstrate reliable labelling with antibodies against P2YR or P2XR. Molecular studies using cells that can be isolated using the model with constitutive expression of eGFP in PDGFR-α+ cells will allow more careful phenotyping of these cells and the ability to assess their responses to neurotransmitters.
In conclusion, this is the first report to describe the presence and distribution of PDGFR-α+ cells that form a discrete network in the mouse urinary bladder. We believe that this immunolabel and the transgenic mouse model we characterized will provide powerful new tools to investigate the role of this class of interstitial cells in normal bladder function and in pathophysiological models.
Acknowledgments
The authors like to thank Yulia Bayguinov for her technical expertise.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- Baker SA, Hatton WJ, Han J, et al. Role of TREK-1 potassium channel in bladder overactivity after partial bladder outlet obstruction in mouse. J Urol. 2010;183:793–800. doi: 10.1016/j.juro.2009.09.079. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Kaiho Y, Miyazato M, et al. Therapeutic receptor targets for lower urinary tract dysfunction. NaunynSchmiedebergs Arch Pharmacol. 2008;377:437–48. doi: 10.1007/s00210-007-0209-z. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Andersson K, Persson K. Nitric oxide-induced cGMP accumulation in the mouse bladder is not related to smooth muscle relaxation. Eur J Pharmacol. 2000;401:241–50. doi: 10.1016/s0014-2999(00)00457-x. [DOI] [PubMed] [Google Scholar]
- McCloskey KD. Interstitial cells of Cajal in the urinary tract. Handb Exp Pharmacol. 2011;202:233–54. doi: 10.1007/978-3-642-16499-6_11. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–56. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- McCloskey KD, Anderson UA, Davidson RA, et al. Comparison of mechanical and electrical activity and interstitial cells of Cajal in urinary bladders from wild-type and W/Wv mice. Br J Pharmacol. 2009;156:273–83. doi: 10.1111/j.1476-5381.2008.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JI, Markerink-van Ittersum M, de Vente J. cGMP-generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int. 2004;94:1114–24. doi: 10.1111/j.1464-410X.2004.05186.x. [DOI] [PubMed] [Google Scholar]
- Pezzone MA, Watkins SC, Alber SM, et al. Identification of c-kit-positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am J Physiol Renal Physiol. 2003;284:F925–9. doi: 10.1152/ajprenal.00138.2002. [DOI] [PubMed] [Google Scholar]
- Zhu MH, Kim TW, Ro S, et al. A Ca(2+)-activated Cl(−) conductance in interstitial cells of Cajal linked to slow wave currents and pacemaker activity. J Physiol. 2009;587:4905–18. doi: 10.1113/jphysiol.2009.176206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Park C, Jin J, et al. A model to study the phenotypic changes of interstitial cells of Cajal in gastrointestinal diseases. Gastroenterology. 2010;138:1068–78. doi: 10.1053/j.gastro.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikawa S, et al. c-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Ward SM, Brennan MF, Jackson VM, et al. Role of PI3-kinase in the development of interstitial cells and pacemaking in murine gastrointestinal smooth muscle. J Physiol. 1999;516:835–46. doi: 10.1111/j.1469-7793.1999.0835u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, et al. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MJ, Fry CH, Eyden B. Structural characterization of myofibroblasts in the bladder. BJU Int. 2006;97:29–32. doi: 10.1111/j.1464-410X.2006.05818.x. [DOI] [PubMed] [Google Scholar]
- Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/W(nu) mouse small intestine. J Auton Nerv Syst. 2000;80:142–7. doi: 10.1016/s0165-1838(00)00089-8. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, et al. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Gherghiceanu M, Suciu LC, et al. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345:391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- Popescu LM, Manole E, Serboiu CS, et al. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15:1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S, Horiguchi K, Horiguchi S, et al. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- Cobine CA, Hennig GW, Kurahashi M, et al. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011;344:17–30. doi: 10.1007/s00441-011-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RA, McCloskey KD. Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J Urol. 2005;173:1385–90. doi: 10.1097/01.ju.0000146272.80848.37. [DOI] [PubMed] [Google Scholar]
- Johnston L, Woolsey S, Cunningham RM, et al. Morphological expression of KIT positive interstitial cells of Cajal in human bladder. J Urol. 2010;184:370–7. doi: 10.1016/j.juro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smet PJ, Jonavicius J, Marshall VR, et al. Distribution of nitric oxide synthase-immunoreactive nerves and identification of the cellular targets of nitric oxide in guinea-pig and human urinary bladder by cGMP immunohistochemistry. Neuroscience. 1996;71:337–48. doi: 10.1016/0306-4522(95)00453-x. [DOI] [PubMed] [Google Scholar]
- Gabella G. The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. J Neurocytol. 1995;24:159–87. doi: 10.1007/BF01181533. [DOI] [PubMed] [Google Scholar]
- Hamilton TG, Klinghoffer RA, Corrin PD, et al. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–25. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang DD. Intermediate filaments in smooth muscle. Am J Physiol Cell Physiol. 2008;294:C869–78. doi: 10.1152/ajpcell.00154.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, et al. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci USA. 1996;93:12008–13. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey KD, Gurney AM. Kit positive cells in the guinea pig bladder. J Urol. 2002;168:832–6. [PubMed] [Google Scholar]
- Ordog T, Baldo M, Danko R, et al. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/W mutant mice. Gastroenterology. 2002;123:2028–40. doi: 10.1053/gast.2002.37056. [DOI] [PubMed] [Google Scholar]
- Rasmussen H, Hansen A, Smedts F, et al. CD34-positive interstitial cells of the human detrusor. APMIS. 2007;115:1260–6. doi: 10.1111/j.1600-0643.2007.00759.x. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol. 2004;559:567–81. doi: 10.1113/jphysiol.2004.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biers SM, Reynard JM, Doore T, et al. The functional effects of a c-kit tyrosine inhibitor on guinea-pig and human detrusor. BJU Int. 2006;97:612–6. doi: 10.1111/j.1464-410X.2005.05988.x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Biers SM, Kohri K, et al. Effects of imatinib mesylate (Glivec) as a c-kit tyrosine kinase inhibitor in the guinea-pig urinary bladder. Neurourol Urodyn. 2006;25:205–10. doi: 10.1002/nau.20085. [DOI] [PubMed] [Google Scholar]
- Buchdunger E, Cioffi CL, Law N, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–45. [PubMed] [Google Scholar]
- Hashitani H, Hayase M, Suzuki H. Effects of imatinib mesylate on spontaneous electrical and mechanical activity in smooth muscle of the guinea-pig stomach. Br J Pharmacol. 2008;154:451–9. doi: 10.1038/bjp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretoiu SM, Simionescu AA, Caravia L, et al. Complex effects of imatinib on spontaneous and oxytocin-induced contractions in human non-pregnant myometrium. Acta Physiol Hung. 2011;98:329–38. doi: 10.1556/APhysiol.98.2011.3.10. [DOI] [PubMed] [Google Scholar]
- Sui GP, Wu C, Roosen A, et al. Modulation of bladder myofibroblast activity: implications for bladder function. Am J Physiol Renal Physiol. 2008;295:F688–97. doi: 10.1152/ajprenal.00133.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


