Abstract
Telocytes (TCs) represent a new cell type recently described in mammalian skeletal muscle interstitium as well as in other organs. These have a specific morphology and phenotype, both in situ and in vitro. Telocytes are cells with long and slender cell prolongations, in contact with other interstitial cells, nerve fibres, blood capillaries and resident stem cells in niches. Our aim was to investigate the potential contribution of TCs to micro-vascular networks by immunofluorescent labelling of specific angiogenic growth factors and receptors. We found that in human skeletal muscle TCs were constantly located around intermediate and small blood vessels and endomysial capillaries. Epi-fluorescence and laser confocal microscopy showed that TCs express c-kit, platelet-derived growth factor receptor (PDGFR)-β and VEGF, both in situ and in vitro. Telocytes were constantly located in the perivascular or pericapillary space, as confirmed by double staining of c-kit/CD31, PDGFR-β/CD31 and PDGFR-β/α-smooth muscle actin, respectively. Electron microscopy (EM) differentiated between pericytes and other cell types. Laminin labelling showed that TCs are not enclosed or surrounded by a basal lamina in contrast to mural cells. In conclusion, a) PDGFR-β could be used as a marker for TCs and b) TCs are presumably a transitional population in the complex process of mural cell recruitment during angiogenesis and vascular remodelling.
Keywords: angiogenesis, telocyte, PDGFR-β, capillary, growth factors, VEGF, c-kit
Introduction
During the last few years, we identified and characterized a new type of interstitial cell, the TC, in a large variety of mammalian organs [[1],[2],,[3]], including skeletal muscle [4]. Telocytes can be recognized, both in situ and in vitro, by their particular morphology—cells with a small cell body and long cell prolongations, usually very thin, below the resolving power of light microscopy. We named these prolongations as telopodes (Tp). Telocytes represent an ubiquitous population of any interstitium [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. As in the case of the other organs, TCs from skeletal muscle interstitium are preferentially distributed around blood vessels, especially small ones [15].
Numerous pre-clinical studies have shown that pro-angiogenic factors such as VEGF could significantly stimulate neovascularization in ischemic myocardium and skeletal muscles [23,24] by recruiting endothelial cells to form ‘tubes’. Previous studies [6,15,16,17] already demonstrated that TCs express VEGF. Therefore, TCs might function as a cell population involved in the process of mural cell recruitment in angiogenesis during development and tissue remodelling. Telocytes might belong to a hypothetical continuum of phenotypes that starts from extracellular matrix secreting fibroblasts [25] to contractile phenotypes associated with blood vessels, such as pericytes and smooth muscle cells (SMC) [26,27,28,29].
Besides VEGF, other specific intercellular signals are required as mediators of endothelial and mural cell function and of vascular stability. Platelet-derived growth factor B polypeptide (PDGF-B) is secreted by the endothelial cells and drives the formation of the surrounding muscular wall by recruiting nearby mesenchymal cells [30,31]. Its receptor, PDGFR-β is crucial for vascular stability. A recent study proved that VEGF-induced new blood vessels completely lacked detectable signals for both PDGFR-α and PDGFR-β [32]. Hence, even though VEGF can initiate angiogenesis, the newly formed endothelial tubes should be able to further recruit and maintain their mural coat by PDGF/PDGFR-β interaction.
Both cell types that form blood vessel mural coat (SMC and pericytes) are strongly positive for α-SMA and PDGFR-β [32] and are intimately assembled around the endothelial tubes [29,33], enclosed by a basal lamina. Smooth muscle cells have their own basal lamina and are arranged in layers. Pericytes are embedded within the basement membrane of the capillaries, with cytoplasmic processes extending along and encircling the endothelial tube.
Here, we report that TCs are PDGFR-β immunopositive and therefore could be involved in microvessel cell recruitment and angiogenesis.
Materials and methods
Samples
Human skeletal muscle samples were obtained from two patients undergoing quadriceps muscle biopsy for diagnosis, and muscle pathology was ruled out. Mouse skeletal muscle samples were obtained from 4-month-old C57 black mice. Before all procedures, the mice were anaesthetized by giving an injection consisting of a mixture of dormicum and hypnorm in the ratio of 1:1 and were killed with a lethal dose of CO2 at the end of the experiments.
This study was approved by the Bioethics Committee of the “Victor Babe” National Institute of Pathology, Bucharest, according to generally accepted international standards.
In situ immunostaining and confocal analysis
Frozen skeletal muscle cryosections (10-μm thick) were fixed in 4% formaldehyde for 15 min., washed for 30 min. in PBS, pH 7.4, and blocked with 2% bovine serum albumin (BSA). The samples were then incubated for 30 min. with 2% normal goat serum (Sigma-Aldrich, St. Louis, MO, USA) overnight at 4°C in PBS either with rabbit anti c-Kit (1:50; DAKO, Glostrup, Denmark), anti-PDGFR and anti-VEGF antibodies (1:75 or 1:150, respectively; both from Abcam, Cambridge, UK) or mouse anti-α-SMA, CD31 or laminin (all from DAKO) or combinations for double immunostaining labelling. After washing in PBS with 0.1% (vol/vol) Triton X-100, the sections were incubated with Alexa Fluor-conjugated secondary goat anti-rabbit or goat anti-mouse antibodies (Invitrogen, Molecular Probes, Eugene, OR, USA) for another 2 hrs, at room temperature. Following an extensive washing step, the nuclei were stained with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich).
Negative controls were obtained by omitting the primary antibody, in an otherwise similar protocol. Three to five immunolabelled sections from each case were examined by laser scanning microscopy, with Nikon A1 laser microscope on ECLIPSE Ti-E inverted microscope (Nikon, Tokyo, Japan). The confocal images were collected using Plan Fluor 60× oil objective, 1.25-NA water (z-axis step 0.16 μm).
The following lasers and emission filters were used: Ar laser at 488 nm (used for the excitation of Alexa Fluor 488); emission filter 500–550 nm; 561.2 nm G-HeNe laser (for Alexa Fluor 546); emission filter 570–620 nm, and 405 nm laser diode and 425–475 nm emission filter for DAPI.
To improve image quality, the original laser scanning microscopy data were subjected to digital deconvolution and three-dimensional reconstruction using Imaris ×64 (version 6.3.1.) from Bitplane AG (Zürich, Switzerland).
Cell cultures and immunostaining
Adult C57 black mice were first treated with 1000 U/kg heparin and subsequently killed by cervical dislocation. Mouse thigh was dissected under the stereomicroscope and the entire medial package was transferred in transport medium and processed for cell cultures.
To obtain cell cultures highly enriched in interstitial cells, the samples were mechanically minced into small pieces of about 1 mm3. Tissue fragments were first incubated in 0.05% trypsin/0.02% EDTA (Biochrom AG, Berlin, Germany) at 37°C for 5 min. and then placed in 35-cm2 Petri dishes and left to adhere. After 5 min., the explants were covered with DMEM/F12 culture medium supplemented with 10% foetal calf serum and 100 U/ml penicillin–100 mg/ml streptomycin (all from Sigma-Aldrich). After 10 days, the migrated cells were detached from the culture vessel and re-plated on glass cover slips for immunolabelling.
Cells grown on cover slips were fixed in 2% paraformaldehyde for 10 min., washed in PBS, then incubated in PBS containing 2% BSA for another 10 min. Afterwards, the cells were permeabilized with 0.075% saponin for 10 min. (all reagents were from Sigma-Aldrich). Incubation with the primary antibodies was performed overnight, at 4°C, with rat anti-c-kit/ACK45 (1:25; BD Biosciences, San Jose, CA, USA), rabbit anti-PDGFR-β or VEGF (1:50 or 1:200, respectively; both from Abcam) and mouse anti-α-SMA (1:100; Neo Markers). After three serial rinses, the primary antibodies were detected with secondary anti-rabbit or anti-mouse conjugated to Alexa Fluor fluorophores (Molecular Probes). Finally, the nuclei were counterstained with 1 mg/ml DAPI (Sigma-Aldrich). Samples were examined under a Nikon TE300 microscope equipped with a Nikon DX1 camera, Nikon PlanApo 100× objectives and the appropriate fluorescence filters.
Results and discussion
We show here, based on in situ double immunolabelling, the presence of c-kit-positive TCs in skeletal muscle, surrounding small blood vessels marked by the endothelial marker CD31 (Fig. 1A), or located in the vicinity of endomysial capillaries (Fig. 1A and B), results confirmed by electron microscopy (Fig. 1C). As shown previously, perivascular TCs synthesize VEGF (Fig. 2).
Fig 1.
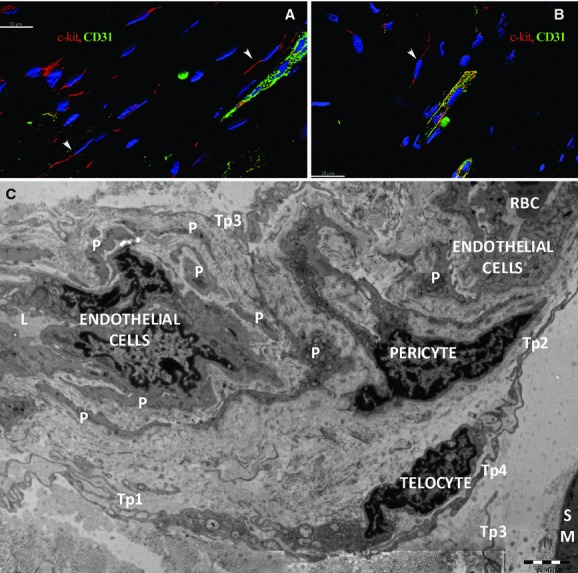
Human skeletal muscle; laser scanning confocal microscopy; three-dimensional shadow projection image. Double immunofluorescence labelling shows CD117/c-kit-positive cells (red) distributed around small blood vessels (arrowheads) (A) or capillaries (B) visualized by CD31 endothelial marker (green) in the perimysial and endomysial interstitial spaces. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue). Original magnification: 600×. (C) Electron microscopy. A telocyte with two telopodes (Tp1, Tp2) is located next to a pericyte which is visible in a twist of a capillary. Small fragments from pericytes (P) border the capillary. The basal lamina edges the pericytes. Telopodes (Tp3, Tp4, Tp5) belong to other telocytes.
Fig 2.
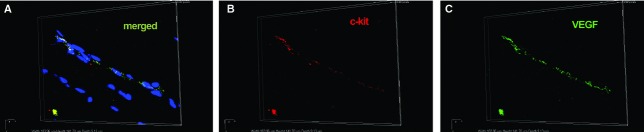
Human skeletal muscle; laser scanning confocal microscopy; volume reconstruction. Double immunofluorescence labelling shows co-localization (A) for CD117/c-kit (B, red) and VEGF (C, green) in endomysial interstitial cells nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue). Original magnification: 600×.
All mural cells (pericytes and vascular SMCs) exhibited strong positive signals for PDGFR-β (Fig. 3A). In addition, we constantly detected PDGFR-β expression on perivascular cells with TC appearance, showing long and extremely thin cell processes, which correspond to TC morphology, in the connective tissue surrounding skeletal muscle blood vessels (Fig. 3A and C). These cells also co-expressed c-kit (Fig. 3B and C) as demonstrated by PDGFR-β, α-SMA and c-kit simultaneous labelling. Such cells were also detected in the thin layer of endomysial connective tissue, not related to the blood vessel wall (Fig. 4).
Fig 3.
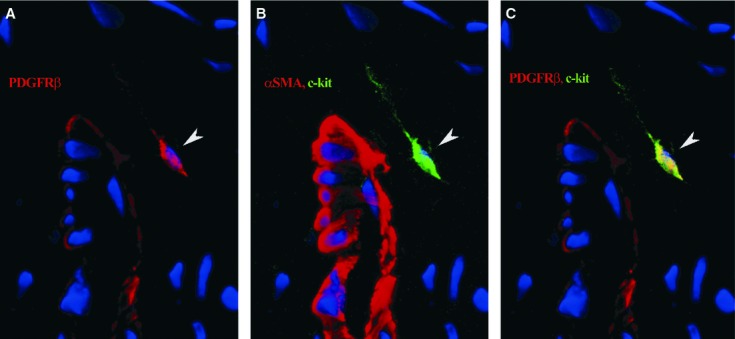
Human skeletal muscle; laser scanning confocal microscopy; three-dimensional shadow projection image. Immunofluorescence labelling shows platelet-derived growth factor receptor (PDGFR)-β expression (red) in mural cells and a perivascular interstitial cell (arrowhead), especially along the long, thin cell prolongation (A). Same perivascular cell (arrowhead) is positive for c-kit (green); mural cells are marked by α-SMA (red) (B); co-localization for PDGFR-β and c-kit appears as yellow areas (arrowhead) (C). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue). Original magnification: 600×.
Fig 4.
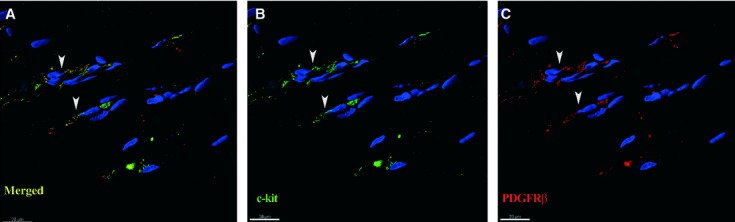
Human skeletal muscle; laser scanning confocal microscopy; three-dimensional shadow projection images. Double immunofluorescence labelling shows co-localization (A) for CD117/c-kit (B, green) and platelet-derived growth factor receptor-β (C, red) in endomysial interstitial cells (arrowheads). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue).
Telocytes located outside the blood vessel wall expressing c-kit (Fig. 5A) or PDGFR-β (Fig. 5B), were not covered by a basal lamina, compared with SMC and pericytes, as proven by double immunostaining for either c-kit/PDGFR-β and laminin, an universal component of basal laminae.
Fig 5.
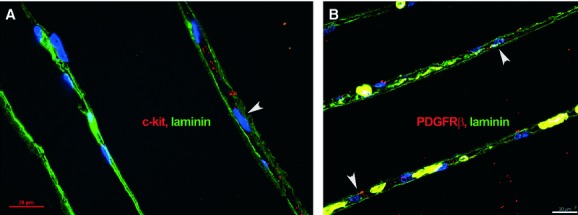
Human skeletal muscle; laser scanning confocal microscopy; basal laminae are visualized by laminin expression (green). c-kit-positive cells (A) and pericapillary platelet-derived growth factor receptor-β-positive cells (B) are clearly located in the interstitial space (arrowheads), between two adjacent skeletal muscle fibres enclosed by basal laminae. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue).
Immunofluorescent labelling showed that PDGFR-β-positive cells represented the major population in cell cultures obtained from explants of mouse skeletal muscle tissue. Some of the PDGFR-β-positive cells exhibited a typical TC morphology (Fig. 6A and B) and expressed c-kit, especially along their long, thin cell processes (Fig. 7A). Consistent with in situ findings such cells also expressed VEGF (Fig. 7B). We also tested samples for α-SMA expression. Even though TCs were weakly positive, α-SMA did not form stress fibres in their cytoplasm as in SMC (Fig. 8).
Fig 6.
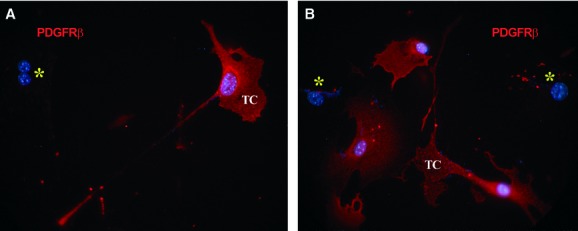
Mouse skeletal muscle explant culture. Epi-fluorescence microscopy shows platelet-derived growth factor receptor (PDGFR)-β-positive cells (red) with a typical aspect of telocytes, with very long and thin, moniliform prolongations. Nuclei are counterstained blue with 4′,6-diamidino-2-phenylindole. Note the PDGFR-β-negative cells (asterisks). Original magnification: 600×.
Fig 7.
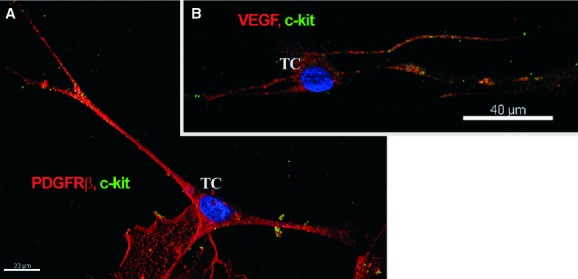
Mouse skeletal muscle explant culture; laser scanning confocal microscopy; three-dimensional shadow projection images. Double immunofluorescence labelling shows co-localization (A) for CD117/c-kit (green) and platelet-derived growth factor receptor-β (red) and (B) CD117/c-kit (green) and VEGF (red) in cells with long, thin cell prolongations that correspond to telocyte morphology. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue). Original magnification: 600×.
Fig 8.
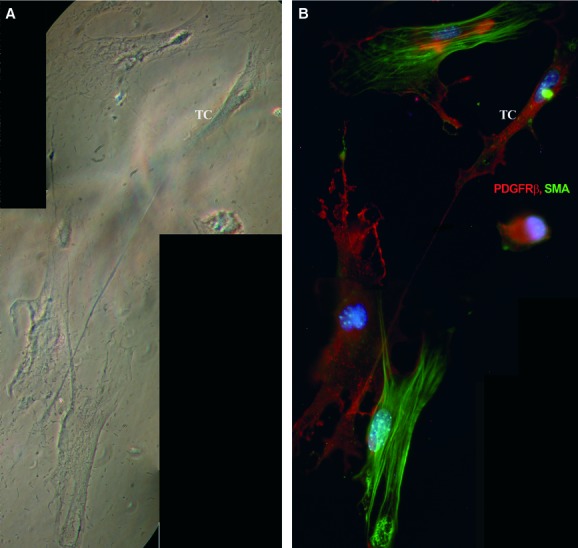
Mouse skeletal muscle explant culture. Phase-contrast microscopy (A) telocyte (TC) in primary culture (day 8). The same microscopic field was analysed by epi-fluorescence microscopy (B). Telocyte is weakly positive for SMA (green); the actin filaments are homogenously distributed and do not form stress fibres as in SMC, but they are strongly positive for platelet-derived growth factor receptor-β (red). Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (blue). Photographic reconstruction; original magnification: 1000×.
Most probably, the cells identified so far as pericytes by their immunophenotype might also include other types of interstitial cells, such as TCs. Furthermore, TCs might represent a heterogeneous and versatile cell population capable of acquiring different fates depending on location and tissue distribution, including microvessel recruitment and pericyte differentiation. Interestingly, very recently Ardeleanu and Bussolatti 2011 suggested that TCs could be the origin of gastrointestinal stromal tumour and perivascular epithelioid cell tumours as TCs and pericytes share phenotypic characteristics. An apparently opposite scenario of phenotype change was recently documented by Göritz et al. 2011, who showed that a peculiar pericytes population can migrate from the vessel walls and differentiate into cells involved in scar formation in the spinal cord. However, in our view these data taken together suggest that interstitial cells form a reservoir used for cell replacement depending on intercellular signalling.
Conclusions
In conclusion, our results indicate that TCs might represent important players in the complex process of angiogenesis and vascular development. Clinical trial designs have not always taken into consideration the basic mechanisms of angiogenesis, arteriogenesis and blood vessel stability [35]. The formation of a mature, well-organized, stable vasculature is a key goal in tissue engineering, regenerative medicine, therapeutic angiogenesis and in the treatment of vascular diseases.
Acknowledgments
The authors thank Dr. Mihaela Gherghiceanu for kindly providing the electron microscopic figure. This study was partially supported by the Sectoral Operational Programme, Human Resources Development, financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/89/1.5/S/64109. This study was also partially supported by the Romanian National Research Council (CNCS) grant no. 32/27.10.2011 (PN-II-RU-TE-2011-3-0206). Prof. L. M. Popescu thanks Cord Blood Center (Cluj) for financial assistance.
References
- Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Popescu LM. Telocytes. BioMol Concepts. 2011 doi: 10.1515/BMC.2011.039. ; doi: 10.1515/BMC.2011.039. [DOI] [PubMed] [Google Scholar]
- Popescu L. The tandem: telocytes – stem cells. Int J Biol Biomed Eng. 2011;2:83–92. [Google Scholar]
- Gherghiceanu M, Manole CG, Popescu LM. Telocytes in endocardium: electron microscope evidence. J Cell Mol Med. 2010;14:2330–4. doi: 10.1111/j.1582-4934.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010;14:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang Y, Wen X, et al. Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment. J Cell Mol Med. 2010;14:2641–5. doi: 10.1111/j.1582-4934.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu MC, Jianu AM, Mirancea N, et al. Tracheal telocytes. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01465.x. ; in press: doi: 10.1111/j.1582-4934.2011.01465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolescu MI, Bucur A, Dinca O, et al. Telocytes in parotid glands. Anat Rec. 2011 doi: 10.1002/ar.21540. ; in press: doi: 10.1002/ar.21540. [DOI] [PubMed] [Google Scholar]
- Nicolescu MI, Popescu LM. Telocytes in the interstitium of human exocrine pancreas: ultrastructural evidence. Pancreas. 2011 doi: 10.1097/MPA.0b013e31823fbded. ; in press: doi: 10.1097/MPA.0b013e31823fbded. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Bai C, Wang X. Telocyte morphologies and potential roles in diseases. J Cell Physiol. 2011 doi: 10.1002/jcp.23022. ; doi: 10.1002/jcp.23022. [DOI] [PubMed] [Google Scholar]
- Cantarero Carmona I, Luesma Bartolome MJ, Junquera Escribano C. Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy. J Cell Mol Med. 2011;15:26–30. doi: 10.1111/j.1582-4934.2010.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero I, Luesma MJ, Junquera C. The primary cilium of telocytes in the vasculature: electron microscope imaging. J Cell Mol Med. 2011;15:2594–600. doi: 10.1111/j.1582-4934.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Manole E, Serboiu CS, et al. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15:1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu LM, Gherghiceanu M, Suciu LC, et al. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011;345:391–403. doi: 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manole CG, Cismasiu V, Gherghiceanu M, et al. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011;15:2284–96. doi: 10.1111/j.1582-4934.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Li H, Manole CG, et al. Telocytes in trachea and lungs. J Cell Mol Med. 2011;15:2262–8. doi: 10.1111/j.1582-4934.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretoiu D, Cretoiu SM, Simionescu AA, et al. Telocytes: a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol. 2012 doi: 10.14670/HH-27.1067. ; in press. [DOI] [PubMed] [Google Scholar]
- Gevaert T, De Vos R, Van Der Aa F, et al. Identification of telocytes in the upper lamina propria of the human urinary tract. J Cell Mol Med. 2012 doi: 10.1111/j.1582-4934.2011.01504.x. ; in press: doi: 10.1111/j.1582-4934.2011.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusu MC, Pop F, Hostiuc S, et al. Telocytes form networks in normal cardiac tissues. Histol Histopathol. 2012 doi: 10.14670/HH-27.807. ; in press. [DOI] [PubMed] [Google Scholar]
- Toyota E, Warltier DC, Brock T, et al. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112:2108–13. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2003;2:863–71. doi: 10.1038/nrd1226. [DOI] [PubMed] [Google Scholar]
- Göritz CDD, Tomilin N, Barbacid M, et al. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–42. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- Chambers RC, Leoni P, Kaminski N, et al. Global expression profiling of fibroblast responses to transforming growth factor-beta1 reveals the induction of inhibitor of differentiation-1 and provides evidence of smooth muscle cell phenotypic switching. Am J Pathol. 2003;162:533–46. doi: 10.1016/s0002-9440(10)63847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-beta, and heterotypic cell–cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–14. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Crosby JR, Seifert RA, Soriano P, et al. Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet. 1998;18:385–8. doi: 10.1038/ng0498-385. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Pericyte-specific expression of PDGF beta receptor in mouse models with normal and deficient PDGF beta receptor signaling. Mol Neurodegener. 2010;5:32. doi: 10.1186/1750-1326-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cao R, Zhang Y, et al. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 2009;23:153–63. doi: 10.1096/fj.08-113860. [DOI] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–9. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Ardeleanu C, Bussolati G. Telocytes are the common cell of origin of both PEComas and GISTs. An evidence-supported hypothesis. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01461.x. ; in press: doi: 10.1111/j.1582-4934.2011.01461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M. Angiogenesis: where do we stand now? Circulation. 2005;111:1556–66. doi: 10.1161/01.CIR.0000159345.00591.8F. [DOI] [PubMed] [Google Scholar]


