Abstract
For clinical application of tissue engineering strategies, the use of animal-derived serum in culture medium is not recommended, because it can evoke immune responses in patients. We previously observed that human platelet-lysate (PL) is favourable for cell expansion, but generates weaker tissue as compared to culture in foetal bovine serum (FBS). We investigated if human serum (HS) is a better human supplement to increase tissue strength. Cells were isolated from venous grafts of 10 patients and expanded in media supplemented with PL or HS, to determine proliferation rates and expression of genes related to collagen production and maturation. Zymography was used to assess protease expression. Collagen contraction assays were used as a two-dimensional (2D) model for matrix contraction. As a prove of principle, 3D tissue culture and tensile testing was performed for two patients, to determine tissue strength. Cell proliferation was lower in HS-supplemented medium than in PL medium. The HS cells produced less active matrix metallo-proteinase 2 (MMP2) and showed increased matrix contraction as indicated by gel contraction assays and 3D-tissue culture. Tensile testing showed increased strength for tissues cultured in HS when compared to PL. This effect was more pronounced if cells were sequentially cultured in PL, followed by tissue culture in HS. These data suggest that sequential use of PL and HS as substitutes for FBS in culture medium for cardiovascular tissue engineering results in improved cell proliferation and tissue mechanical properties, as compared to use of PL or HS apart.
Keywords: tissue engineering, heart valve, human, serum, platelet-lysate, cardiovascular
Introduction
The use of engineered cardiovascular tissues to replace diseased tissues in patients has been proposed as an alternative for current replacement therapies, because of their ability to grow and adapt in vivo [1]. Although this has been demonstrated in long-term animal studies [2], studies in humans have yet to be performed to evaluate the efficacy of this approach. Recent studies by our group have demonstrated differences in cardiovascular tissues engineered from human and ovine cells [3]. This further indicates the relevance of specific, deviating culture protocols for either ovine or human engineered tissues and emphasizes that results obtained with either species cannot be easily compared. To prepare for future clinical studies and to circumvent risks associated with the use of animal-derived culture additives, it is relevant to investigate the outcomes of autologous culture of human engineered tissues.
Engineered (cardiovascular) tissues are generally produced by isolation of cells from patients, expansion of those cells in culture media and seeding on scaffolds of synthetic or biological origin, followed by culture and conditioning in bioreactors to promote tissue formation. Culture media used for cell expansion and tissue culture generally contain FBS as an essential source of proteins and growth factors. Ideally, this animal-derived serum is replaced for preparation of constructs for clinical application, because it may lead to rejection of grafted cells in vivo [4, 5]. Cells are able to take up proteins from culture media and present them on their membranes, thereby activating the immune system and leading to failure of the implanted construct [4-7]. Therefore, our research concentrates on identification of human alternatives for FBS that can be used for cell and tissue culture in (cardiovascular) tissue engineering, as a step in the translation to clinical application.
In previous studies on cardiovascular (heart valve) tissue engineering, we investigated the use of culture media supplemented with human PL, because platelets contain many granules with growth factors and because good results had been reported with PL in other areas of tissue engineering, such as clinical scale expansion of bone marrow cells and bone tissue engineering [8-10]. Several of the identified growth factors are involved in the process of wound repair in vivo, which includes deposition and remodelling of collagen [11-13], or in heart valve development and maturation [14]. The production of collagen and a balance in remodelling of the collagen matrix is also desirable in tissue engineered heart valves, because a strong and well-organized collagen fibre network is extremely important to support the valves’ load-bearing function. We established at first that PL was indeed a suitable human alternative for FBS to stimulate cell proliferation and expression of proteins involved in matrix remodelling, as a result of these differences in growth factor content [15]. However, we subsequently also observed that 3D tissue culture in PL strongly reduced tissue mechanical properties as compared to the culture in serum, supposedly caused by increased cellular expression and activation of MMPs [14, 15]. In our search for clinically favourable medium supplements, the present study investigates if HS can improve tissue mechanical properties, while maintaining high cell proliferation rates.
In cultured cells obtained from 10 patients, we investigated the individual effects of PL and HS on cell proliferation, expression of genes related to collagen production and maturation and MMP expression and activity. Gel contraction assays with cells from all patients were performed to investigate the potential of the cells to contract matrix. As a proof of principle, tensile testing on engineered 3D tissue constructs with cells from two patients was included, to illustrate potential effects in engineered cardiovascular tissue.
Materials and methods
Culture media
PL was produced by obtaining pooled human thrombocytes in serum from five donors with similar blood type and rhesus factor from the hospital bloodbank, buffered with citrate-phosphate dextrose. These thrombocytes were frozen in aliquots at −80°C and thawed and centrifuged (8 min. 900 rcf) to produce PL prior to addition to the culture medium [10, 13]. HS was obtained by collecting blood from five to six healthy controls in coagulation tubes and centrifuging for 15 min. at 1800 × g. The serum was pooled and frozen in aliquots before use as well. Culture was performed in DMEM-advanced (12491015; Gibco, Invitrogen, CA, USA) supplemented with 2 mM GlutaMax (35050-028; Gibco), 1% penicillin/streptomycin and either 5% HS or 5% PL with 10 U/ml heparin (013192-03; LEO-pharma, Breda, The Netherlands).
Cell isolation and expansion
Segments of great saphenous vein (±3 cm) were obtained from venous grafts from 10 patients undergoing coronary artery bypass surgery. Individual permission using standard informed consent procedures and prior approval of the medical ethics committee of the University Medical Center Utrecht, according to the world Medical Association Declaration of Helsinki was obtained and tissue was further treated anonymously [16]. Tissue pieces were plated on culture plastic and culture medium supplemented with either PL or HS was added to stimulate outgrowth of myofibroblasts.
Cells were cultured in a humid environment at 37°C and 5% CO2 up to passage 5, which in our hands has proved to be a suitable passage to obtain abundant cells for seeding on scaffolds in future tissue engineering strategies. A number of cells were frozen at each passage for use in subsequent experiments. Passage 1 (P1) was seeded at a density of 3000 cells/cm2, whereas subsequent passages were split in a 1:3 surface ratio. After each passage, the time required to reach 85% confluency was determined per cell isolation and the number of cells was determined using the Countess Automated Cell Counter (Invitrogen). The time in days and the logarithm of the total number of cells that would have been obtained at each passage were fit with a linear model using regression analysis with SPSS 16 software (IBM, Chicago, IL, USA). Slopes of these models were used to compare duplication rates of cells derived from individual patients in each condition, followed by determination of the average slope per condition.
Immunocytochemistry
Immunocytochemistry was performed to identify myofibroblasts on cultured cells at P5, seeded on cover slips and cultured for 3 days. Staining procedures were previously described [16] and included α-smooth muscle actin (α-SMA; A2547, 11.25 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) and vimentin (ab20346, VI-10, 2.5 μg/ml; Abcam, Cambridge, UK) as positive markers and desmin (M0760, Clone D33, 4.7 μg/ml; DAKO, Glostrup, Denmark) as a negative marker. This combination of markers is specific for both venous myofibroblasts and valvular interstitial cells [17], indicating why venous myofibroblasts may serve as a cell source for heart valve tissue engineering [18-20]. Alexa Fluor-labelled goat–anti-mouse (A21428, 5.0 μg/ml; Invitrogen) was used as the secondary antibody. Staining was visualized by fluorescence microscopy (BX60; Olympus, Tokyo, Japan) and CellP-software (Olympus).
RNA isolation and quantification
RNA of cells cultured in HS or PL was extracted with Tripure Isolation Reagent (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Production of cDNA, qRT-PCR and post-run product verification confirmation were performed as previously described [16]. Primers were designed (OligoPerfect; Invitrogen) for collagen types I and III and collagen cross-linking enzymes lysyl-oxidase (LOX) and lysyl-hydroxylase (PLOD2) and annealing temperatures were determined (Table 1). Samples were normalized for glyceraldehyde 3-phosphate dehydrogenase (GAPDH, ΔCt = CtTarget − CtGAPDH). Fold difference of cells cultured in HS medium per patient were compared to cells cultured in PL medium (2ΔΔCt per patient, with ΔΔCt per patient = ΔCtPL − ΔCtHS). The fold differences were averaged per medium. We were unable to obtain cDNA from one of the HS samples, thus all calculations were n = 9 at most. Also, LOX was not detected in one PL patient and PLOD2 in one HS patient.
Table 1.
qRT-PCR-primers and annealing temperatures
| Target | Forward primer | Reverse primer | Annealing temp (8C) |
|---|---|---|---|
| COL1A1 | TGCCATCAAATGCTTCTGC | CATACTCGAACTGGAATCCATC | 56 |
| COL3A1 | CCAGGAGCTAACGGTCTCAG | CAGGGTTTCCATCTCTTCCA | 48 |
| LOX | CCACTATGACCTGCTTGATG | TGTGGTAGCCATAGTCACAG | 60 |
| PLOD2 | AGCTGTGGTCCAATTTCTGG | CTAGCATTTCGGCAAAGAGC | 55 |
| GAPDH | ACAGTCAGCCGCATCTTC | GCCCAATACGACCAAATCC | 56 |
COL: collagen type 1 or 3; LOX: lysyl-oxidase; PLOD2: lysyl-hydroxylase; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
Zymography
Zymography to assess MMP expression and activity was performed as previously described [16]. Conditioned medium was collected and phenol red was removed through dialysis for 2 × 24 hrs using Slide-a-lyzer Mini Dialysis units (Pierce, Thermo Fisher Scientific, Waltham, MA, USA) in 5 L of phosphate-buffered saline. After dialysis, protein quantification was performed with a BCA-assay (Sigma-Aldrich) according to the manufacturer’s protocol. An equal amount of total protein was loaded on the gels. Analysis and quantification of protease secretion was performed with the ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA) and QuantityOne software (Bio-Rad). Quantification was corrected for presence of proteases in non-conditioned medium, that is complete medium that was not exposed to cells.
Collagen gel contraction assay
To evaluate the potential of cells cultured in different media to contract the extracellular matrix, we used a collagen gel contraction assay as described by Merryman et al. [21], with small modifications. In brief, the collagen gels (PureCol, Advanced BioMatrix, San Diego, CA, USA) were made and seeded as has been described according to their protocol and the instructions of the collagen manufacturer. The gels were cast in Teflon rings with a 13-mm inner diameter and a 2-mm thickness. After polymerization of the gel, cells were seeded on the gel in 200 μl medium at a concentration of 1.5 × 105 cells/ml and left to attach for 30 min., before additional medium was added and the Teflon ring was removed. Cells were seeded in the same medium as was used for expansion and the gels were subsequently kept in this medium as well. Pictures were taken 2, 16 and 40 hrs after removal of the ring. The area covered by the gel was measured using CellP-software (Olympus) and noted as the percentage of the initial area of the gel, that is the inner surface area of the Teflon-rings. The difference was calculated by subtracting the surface area of HS gels from the surface area of the PL gels per patient.
Preparation and testing of engineered tissue constructs
As a proof of principle for tissue-forming capacities in both media, we set-up a small experiment with 3D tissue constructs (n = 2 patients and two to three strips per patient). For this purpose, cells were transferred to the Eindhoven University of Technology, where rectangular constructs (20 × 6 mm strips) were cultured, tested and stained according to previously published protocols [14]. Briefly, 1.88 × 106 cells were seeded onto rapidly degrading scaffolds using fibrin as a cell carrier, as previously described by Mol et al. [22]. The constructs were cultured under constrained conditions (i.e. attached at both ends) for 4 weeks and medium was changed every 2–3 days. During the tissue culture experiments, medium was supplemented with L-ascorbic acid 2-phosphate (0.25 mg/ml; Sigma-Aldrich) [23].
Mechanical properties were determined after 4 weeks of culture by uniaxial tensile testing in longitudinal direction of the engineered constructs [14]. The ultimate tensile strength (UTS in MPa) was defined as the maximum force at break divided by the cross-sectional area.
Collagen organization in the tissue constructs was visualized with the CNA35-probe directly labelled with Oregon-Green, as designed by Krahn et al. [24] and used as described for cardiovascular constructs by Boerboom et al. [25]. The probe has been shown to bind to several types of collagen (types I–VI), but with the highest affinities for collagen types I and III [24]. It has previously been used in combination with other vital stains such as cell tracker blue [25-28] and compared with other histological staining such as picrosirius red and masson trichrome [29-31].
Statistics
All results were compared between PL and HS groups, using paired samples T-tests (two-tailed) and a P < 0.05 was considered significant. Results are expressed as means ± S.D. SPSS 16 software (Microsoft corporation, Redmond, WA, USA) was used for all statistical analyses.
Results
Proliferation rates
Successful cell outgrowth and isolations were obtained from all 10 patients in both culture media, including seven male and three female patients (71 ± 6 years old). Overall, cell viability was similar for both medium groups and was on average 94% ± 8 in PL medium and 93% ± 7 in HS medium. Analysis of proliferation rates showed an approximate twofold increase when cells were cultured in PL medium (Fig. 1, slopes: 0.173 ± 0.054 and 0.092 ± 0.034 for PL and HS cells, respectively, P < 0.01). Thus, when starting with 2.25 × 105 cells, PL cells reached 108 cells in 17 ± 5 days, whereas HS cells on average required 32 ± 12 days. This number of cells is considered sufficient for seeding onto scaffolds for heart valve tissue engineering purposes [32].
Fig 1.
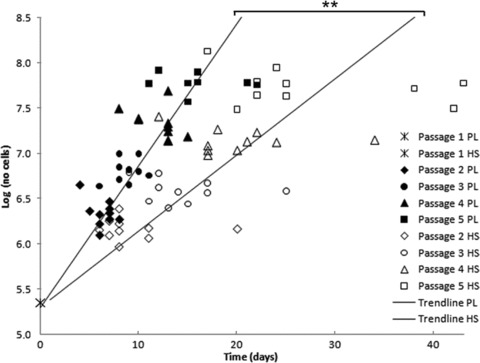
Duplication rates, starting at 225,000 cells, expressed as log(number of cells) for cells cultured in PL and HS for 10 patients. The time in days and number of cells per cell isolation are indicated. The PL cells are depicted by the filled symbols and the HS cells with the clear symbols, including a different symbol per passage. The regression lines with the averaged slope are also shown in the figure. The averaged slope of PL cells was significantly steeper than for the HS cells (P < 0.01).
Immunohistochemistry
At passage 5, all cells in both media were positively stained for α-SMA (Fig. 2A and B) and vimentin (Fig. 2C and D), while being negative for desmin (Fig. 2E and F). This indicates that after repetitive divisions, cells in both media display phenotypes that resemble myofibroblasts.
Fig 2.
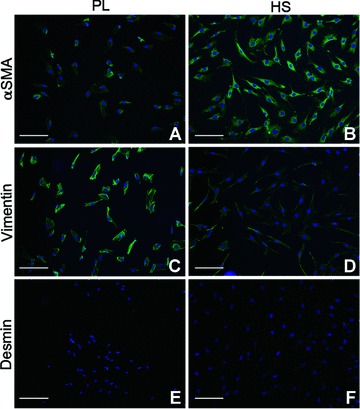
Immunofluorescent staining of MF cultured in PL and HS. Results for phenotyping of one patient are displayed, but results were comparable for all patients. (A, B) α-SMA, (C, D) vimentin and (E, F) desmin. Nuclei are stained with Hoechst. Original magnification was 10×. Bars represent 200 μm.
mRNA quantification
We studied mRNA expression of collagen types I and III, which are considered most relevant for heart valve tissue engineering, and collagen cross-linking enzymes LOX and PLOD2. Significant differences in expression of these mRNAs could be an indication for a difference in the ability to form strong and mature collagen fibres. Although we did not observe a statistically significant difference between the two groups, we did see a two-fold increase in average production of collagen types I and III and PLOD2 by HS cells as compared to PL (Fig. 3: 2.30-, 3.16- and 1.96-fold increase, respectively). LOX expression was comparable in both media (1.10 ± 0.81 fold increase in HS relative to PL).
Fig 3.
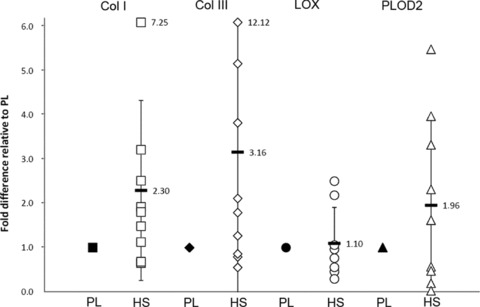
Fold-change of target genes in cells cultured in presence of HS (mean ± S.D.), when normalized for cells cultured in presence of PL. LOX expression is comparable between groups, but collagen types 1 and 3 and PLOD2 expression tend to be higher in cells cultured in HS, although differences are not significant due to large interpatient variations.
Zymography
Figure 4A shows zymographic analysis of gelatinolytic activity corrected for their presence in non-conditioned media, and displays release of active and inactive forms of MMP2 (72 and 64 kD) and active MMP9 (82 kD) by the cells in both PL- and HS-conditioned cells, but not of inactive MMP9 (92 kD). When expression in PL was compared to HS, no significant differences were found for inactive forms of MMP2 and MMP9 and active MMP9 [averages PL: 27718 ± 6803, 0 ± 4479 and 4306 ± 1701 and HS: 23299 ± 5299, 91 ± 3151 and 6105 ± 1640 arbitrary units (a.u.) respectively], but quantification of active MMP2 revealed a significant twofold increase in PL-cultured cells (PL: 6215 ± 1278 versus HS 3608 ± 1375 a.u.). On the collagen-substituted gels (Fig. 4B), we did not find significant differences in MMP-activity between cells cultured in PL or HS. Taken together, zymography analysis revealed a reduced matrix-degradation capacity through a significant decrease of active MMP2 levels when cells are cultured in HS-supplemented medium.
Fig 4.
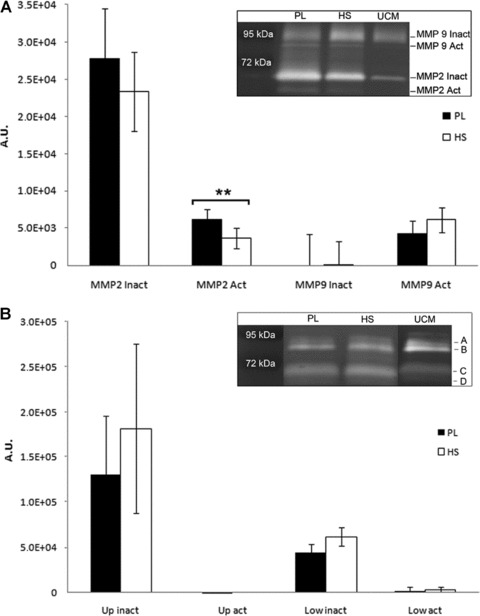
(A) Quantification of MMP2 and MMP9 expression by cells in culture (mean ± S.D.), corrected for unconditioned medium (UCM). Cells cultured in both media produce and activate MMP2, whereas MMP9 did not show a difference compared to the unconditioned control. Activated MMP2 expression was significantly lower in the cells cultured with HS (**P < 0.01). The inlay shows an example of expression patterns by one patient and the size of the signals that were quantified. A.U.: arbitrary units as a measure for number of counted pixels in a pre-determined surface. (B) Quantification of collagenases by cells in culture (mean ± S.D.). Expression patterns are presented in the inlay. We did not find significant differences in collagenase expression between cells cultured in PL or HS. Up: MMPs expressed at marks A and B; Low: MMPs expressed at marks C and D. Active forms (Act, B and D), are slightly smaller in size when compared to inactive forms (Inact, A and C) of these MMPs.
Collagen gel contraction assay
Collagen gel contraction assays were performed to assess cell potential to contract matrix. Collagen gels seeded with cells cultured in PL and HS were shown to contract over time (Fig. 5A). After 16 hrs, HS gels were significantly smaller than PL gels (Fig. 5B, P < 0.01). After 40 hrs, a maximal contraction was observed in all gels. This suggests that cells in HS medium are more active in contracting collagen matrices.
Fig 5.
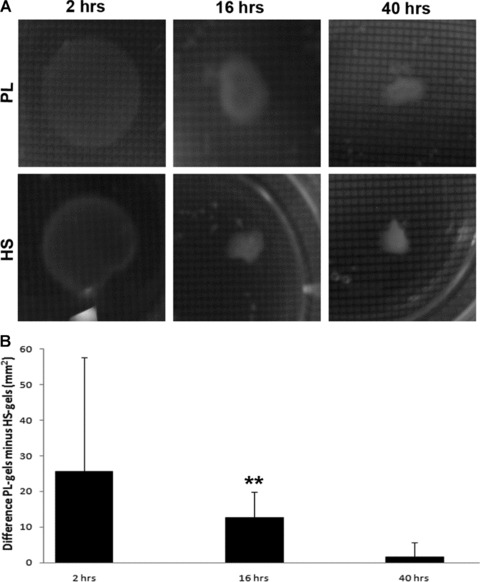
Results of the collagen gel contraction assay (mean ± S.D.). The picture series (A) show shrinking of the gels over time and clearly shows the difference after 16 hrs between PL and HS conditions. The 1 mm2 grids that were used to determine the actual size of the gels are also visible. After quantification the difference of the gel areas between PL and HS over time is depicted in the graph (B) and shows significant differences after 16 hrs (**P < 0.01).
Tissue studies: contraction, matrix and tensile testing
Similar to the collagen gels, the tissue constructs revealed increased contraction when cultured in HS as compared to PL (Fig. 6A and C). This was accompanied by enhanced alignment of collagen structures in HS (Fig. 6B and D). Finally, tensile testing was performed to test the load-bearing capacities of the constructs cultured in either PL- or HS-supplemented medium. The first two columns in the bar plot in Figure 6 show a difference in load-bearing capacity between tissue cultured in PL (0.27 MPa ± 0.21) and HS (0.81 MPa ± 0.16), although obviously this cannot be tested.
Fig 6.
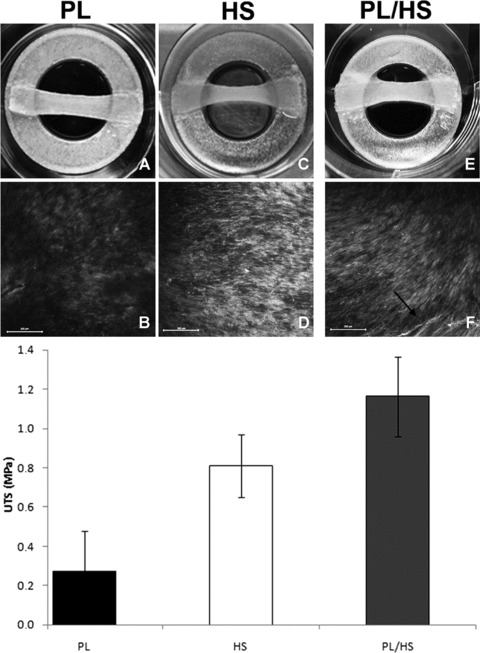
Results of the tissue-formation experiments (mean ± S.D.). The pictures in (A) and (C) show tissue contraction four weeks of tissue culture in both PL-supplemented medium and HS medium, respectively. (B, D) Fluorescent images obtained with a collagen-staining probe showed differences in matrix structure in PL and HS. The pictures (10× magnification), taken in the middle of the constructs at an approximate depth of 7–11 μm, reveal differences in fibre density. Collagen fibres in HS medium also seem thicker and more aligned. Scale bars represent 200 μm. Tensile testing (first two bars of bar plot) suggests differences in ultimate tensile strength (UTS) between constructs cultured in PL and HS medium. (E) Tissue contraction when cells expanded in PL medium are transferred to HS medium for tissue culture (PL/HS). The collagen-fibre staining reveals abundant aligned collagen bundles in this condition, emphasized with an arrow (F). The third column of the bar plot shows an additional increase in UTS in PL/HS as compared to the conditions in which cells were solely cultured in PL or HS.
Discussion
In this study, we aimed to test if HS is a better human replacement for FBS than PL in culture media for (cardiovascular) tissue engineering strategies and future clinical applications [4, 5], We have shown here and in previous studies that the use of human PL resulted in an increased cell proliferation rate, but weaker tissue [14, 16]. The use of HS resulted in lower MMP2 activity levels, enhanced tissue remodelling capacity, and higher load-bearing properties.
We found that the proliferation rate of cells expanded in HS was lower than when PL was used, requiring 28 days to reach 100 million cells instead of 15 days. However, earlier studies showed that cells cultured in FBS required 60 days to obtain a similar number of cells, meaning that the use of HS would still reduce time by 50% as compared to the use of FBS [16]. These results with PL and HS were consistent with the results others obtained when used for expansion of other human cells [33, 34]. The duplication rate of cells is of considerable importance to obtain high a number of cells for clinical use of tissue engineering strategies, as it will reduce the time patients have to wait for autologous constructs to be generated. In this perspective, PL would be preferred as medium supplement for cell expansion over HS and FBS.
In contrast to other groups, who have primarily investigated the use of PL and HS in combination with mesenchymal stem cells and bone tissue engineering [9, 33–42], we focus on soft tissue engineering. One of the most important determinants for cardiovascular, and in particular heart valve, tissue engineering is the load-bearing capacity of the tissue, which is highly related to the quality and architecture of the extracellular matrix, in which collagen types I and III predominate [43]. Indicative for the importance of matrix maturation and architecture are our previously reported results, in which a significantly reduced tissue strength was presented in tissue constructs prepared in PL as compared to FBS, although no differences in collagen content were found [14]. Similarly, this study does not show significant differences in expression of collagen mRNA when cells are cultured in HS or PL, but indicates that tissue strength is higher when HS is used. We have previously reasoned that matrix remodelling and formation regulated by proteins present in PL can negatively influence the load-bearing capacity of tissue engineered constructs [14, 16]. In this study, we did observe that the activation of MMP2 was reduced when cells are cultured in HS as compared to PL. Interestingly, the load-bearing capacities of tissue constructs cultured in HS-supplemented medium were slightly higher than the ultimate tensile strength of tissue constructs previously produced in FBS-supplemented medium (0.81 and 0.73 MPa, respectively). Constructs cultured in PL did not reach this strength in both the present and previous studies (0.27 and 0.25 MPa, respectively) [14]. The strength of tissue constructs cultured in HS is also closer to the UTS of 2.0 MPa previously measured in circumferential direction of native adult aortic valve leaflets [14]. This emphasizes our earlier reasoning that tissue culture in serum is preferable over tissue culture in PL because it generates stronger tissue as a result of lower protease activity levels.
To combine the benefits of both PL and HS, we performed additional experiments to test if cell proliferation with PL-supplemented medium can be followed by tissue formation in HS-supplemented medium. For this purpose, the 2D collagen gel assays and culture of 3D tissue constructs were also performed in HS-containing medium after cell expansion in PL (a condition named: ‘PL/HS’). The collagen gel assays revealed that average gel areas became smaller than PL gels and larger than HS gels after 2 hrs and slightly larger than PL gels after 16 and 40 hrs (13, −1 and −1 mm2, respectively, when PL/HS was subtracted from PL, similar to Fig. 5B), but not significantly different when compared to either of the other groups. The 3D-tissue constructs also showed moderate contraction, an organized collagen architecture and an increased ultimate tensile strength (1.16 MPa ± 0.20, Fig. 6E and F and bar plot, respectively) as compared to strips culture in only PL or HS. This UTS is higher than the values previously reported for constructs exclusively cultured in FBS, PL or HS (0.73, 0.27 and 0.81 MPa, respectively) [14]. Thus, these results indicate that high proliferation caused by PL-supplemented medium can be followed by sequential tissue formation in HS-supplemented medium, resulting in an improved protocol for tissue engineering with HS substitutes.
It also underlines the relevance of designing specialized or ‘chemically defined media’, optimized for every step of tissue engineering strategies [15, 44]. Our studies with FBS, PL and HS show that growth factors and cytokines in the medium supplement have a distinct and large influence on cellular proliferation and formation and remodelling of a collagen network, which is difficult to predict from early expression markers, such as obtained from cell studies alone. Future studies should systematically investigate the effects of the different growth factors abundantly present, or absent, in HS and PL, to determine which growth factors could be specifically responsible for either proliferation, increased MMP levels or formation and maturation of collagen fibres.
We included a 2D-collagen contraction assay to how the cells would contract the matrix under different culture conditions, to see if this assay has predictive value for tissue formation in 3D constructs. The assay predicted the ability of cells cultured in different media to cause tissue contraction in the 3D-tissue constructs. Recent reports pointed out that there was ongoing compaction of heart valve constructs after implantation leading to failure of the valve constructs in vivo, making it more important to test contractive activity of cells in vitro [45]. Methods to stop excessive tissue compaction in vivo will be important in heart valve tissue engineering and the collagen gels can be a fast and simple in vitro model for future experiments.
In conclusion, we have shown that the use of HS during tissue culture results in stronger tissue, whereas PL is a more potent stimulator of cellular proliferation during expansion. More importantly, sequential use of human PL and HS can be used as a substitute protocol for the use of FBS in tissue engineering strategies. This means another step towards optimal autologous tissue engineering protocols and future clinical applications of cardiovascular tissue engineering.
Acknowledgments
The authors thank P.K. Wagemakers, BSc, for his technical contributions.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Schoen FJ, Levy RJ. Founder’s Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28–May 2, 1999. Tissue heart valves: current challenges and future research perspectives. J Biomed Mater Res. 1999;47:439–65. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Hoerstrup SP, Cummings Mrcs I, Lachat M, et al. Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation. 2006;114:I159–66. doi: 10.1161/CIRCULATIONAHA.105.001172. [DOI] [PubMed] [Google Scholar]
- 3.van Geemen D, Driessen-Mol A, Grootzwagers LGM, et al. Variation in ovine and human engineered cardiovascular constructs to predict the outcome of heart valve tissue engineering. Regenerative Medicine. In press. [DOI] [PubMed]
- 4.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to foetal calf serum with arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89:776–9. [PubMed] [Google Scholar]
- 6.Schmidt D, Joyce EJ, Kao WJ. Foetal bovine serum xenoproteins modulate human monocyte adhesion and protein release on biomaterials in vitro. Acta Biomater. 2011;7:515–25. doi: 10.1016/j.actbio.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin MJ, Muotri A, Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–32. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 8.Capelli C, Domenghini M, Borleri G, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant. 2007;40:785–91. doi: 10.1038/sj.bmt.1705798. [DOI] [PubMed] [Google Scholar]
- 9.Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol. 2005;205:228–36. doi: 10.1002/jcp.20391. [DOI] [PubMed] [Google Scholar]
- 10.Prins HJ, Rozemuller H, Vonk-Griffioen S, et al. Bone-forming capacity of mesenchymal stromal cells when cultured in the presence of human platelet lysate as substitute for foetal bovine serum. Tissue Eng Part A. 2009;15:3741–51. doi: 10.1089/ten.TEA.2008.0666. [DOI] [PubMed] [Google Scholar]
- 11.Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–59e. doi: 10.1097/01.prs.0000239606.92676.cf. [DOI] [PubMed] [Google Scholar]
- 12.Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:156–65. [PubMed] [Google Scholar]
- 13.Weibrich G, Kleis WK, Hafner G, et al. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 14.van Geemen D, Riem Vis P, Soekhradj-Soechit S, et al. Decreased mechanical properties of heart valve tissue constructs cultured in platelet lysate as compared to foetal bovine serum. Tissue Eng Part C Methods. 2011. doi: 10.1089/ten.TEC. 2010.0556. [DOI] [PubMed]
- 15.Riem Vis PW, Kluin J, Sluijter JP, et al. Environmental regulation of valvulogenesis: implications for tissue engineering. Eur J Cardiothorac Surg. 2011;39:8–17. doi: 10.1016/j.ejcts.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Riem Vis PW, Bouten CV, Sluijter JP, et al. Platelet-lysate as an autologous alternative for foetal bovine serum in cardiovascular tissue engineering. Tissue Eng Part A. 2010;16:1317–27. doi: 10.1089/ten.TEA.2009.0331. [DOI] [PubMed] [Google Scholar]
- 17.Aikawa E, Whittaker P, Farber M, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–52. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman-Kim D, Maish MS, Krueger PM, et al. Comparison of three myofibroblast cell sources for the tissue engineering of cardiac valves. Tissue Eng. 2005;11:288–301. doi: 10.1089/ten.2005.11.288. [DOI] [PubMed] [Google Scholar]
- 19.Mol A, Rutten MC, Driessen NJ, et al. Autologous human tissue-engineered heart valves: prospects for systemic application. Circulation. 2006;114:I152–8. doi: 10.1161/CIRCULATIONAHA.105.001123. [DOI] [PubMed] [Google Scholar]
- 20.Schopka S, Schmid FX, Hirt S, et al. Recellularization of biological heart valves with human vascular cells: in vitro hemocompatibility assessment. J Biomed Mater Res B Appl Biomater. 2009;88:130–8. doi: 10.1002/jbm.b.31159. [DOI] [PubMed] [Google Scholar]
- 21.Merryman WD, Liao J, Parekh A, et al. Differences in tissue-remodeling potential of aortic and pulmonary heart valve interstitial cells. Tissue Eng. 2007;13:2281–9. doi: 10.1089/ten.2006.0324. [DOI] [PubMed] [Google Scholar]
- 22.Mol A, van Lieshout MI, Dam-de VeenCG, et al. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials. 2005;26:3113–21. doi: 10.1016/j.biomaterials.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Hoerstrup SP, Zund G, Schnell AM, et al. Optimized growth conditions for tissue engineering of human cardiovascular structures. Int J Artif Organs. 2000;23:817–23. [PubMed] [Google Scholar]
- 24.Krahn KN, Bouten CV, van TuijlS, et al. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal Biochem. 2006;350:177–85. doi: 10.1016/j.ab.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Boerboom RA, Krahn KN, Megens RT, et al. High resolution imaging of collagen organisation and synthesis using a versatile collagen specific probe. J Struct Biol. 2007;159:392–9. doi: 10.1016/j.jsb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Boerboom RA, Rubbens MP, Driessen NJ, et al. Effect of strain magnitude on the tissue properties of engineered cardiovascular constructs. Ann Biomed Eng. 2008;36:244–53. doi: 10.1007/s10439-007-9413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foolen J, van Donkelaar C, Nowlan N, et al. Collagen orientation in periosteum and perichondrium is aligned with preferential directions of tissue growth. J Orthop Res. 2008;26:1263–8. doi: 10.1002/jor.20586. [DOI] [PubMed] [Google Scholar]
- 28.Rubbens MP, Driessen-Mol A, Boerboom RA, et al. Quantification of the temporal evolution of collagen orientation in mechanically conditioned engineered cardiovascular tissues. Ann Biomed Eng. 2009;37:1263–72. doi: 10.1007/s10439-009-9698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Lee SK, Abd-Elgaliel WR, et al. Assessment of cardiovascular fibrosis using novel fluorescent probes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019097. : e19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Megens RT, Oude Egbrink MG, Cleutjens JP, et al. Imaging collagen in intact viable healthy and atherosclerotic arteries using fluorescently labeled CNA35 and two-photon laser scanning microscopy. Mol Imaging. 2007;6:247–60. [PubMed] [Google Scholar]
- 31.Megens RTA, oude Egbrink MGA, Merkx M, et al. Two-photon microscopy on vital carotid arteries: imaging the relationship between collagen and inflammatory cells in atherosclerotic plaques. J Biomed Opt. 2008;13 doi: 10.1117/1.2965542. : 044022; doi: 10.1117/1.2965542. [DOI] [PubMed] [Google Scholar]
- 32.Mol A. Functional tissue engineering of human heart valve leaflets. Eindhoven: Technical University Eindhoven; 2005.
- 33.Mazlyzam AL, Aminuddin BS, Saim L, et al. Human serum is an advantageous supplement for human dermal fibroblast expansion: clinical implications for tissue engineering of skin. Arch Med Res. 2008;39:743–52. doi: 10.1016/j.arcmed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Kurita M, Aiba-Kojima E, Shigeura T, et al. Differential effects of three preparations of human serum on expansion of various types of human cells. Plast Reconstr Surg. 2008;122:438–48. doi: 10.1097/PRS.0b013e31817d618d. [DOI] [PubMed] [Google Scholar]
- 35.Alexander TH, Sage AB, Chen AC, et al. Insulin-like growth factor-I and growth differentiation factor-5 promote the formation of tissue-engineered human nasal septal cartilage. Tissue Eng Part C Methods. 2010;16:1213–21. doi: 10.1089/ten.tec.2009.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ang LP, Tan DT, Seah CJ, et al. The use of human serum in supporting the in vitro and in vivo proliferation of human conjunctival epithelial cells. Br J Ophthalmol. 2005;89:748–52. doi: 10.1136/bjo.2004.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crowley ST, Dempsey EC, Horwitz KB, et al. Platelet-induced vascular smooth muscle cell proliferation is modulated by the growth amplification factors serotonin and adenosine diphosphate. Circulation. 1994;90:1908–18. doi: 10.1161/01.cir.90.4.1908. [DOI] [PubMed] [Google Scholar]
- 38.Felka T, Schafer R, De Zwart P, et al. Animal serum-free expansion and differentiation of human mesenchymal stromal cells. Cytotherapy. 2010;12:143–53. doi: 10.3109/14653240903470647. [DOI] [PubMed] [Google Scholar]
- 39.Kuznetsov SA, Mankani MH, Robey PG. Effect of serum on human bone marrow stromal cells: ex vivo expansion and in vivo bone formation. Transplantation. 2000;70:1780–7. doi: 10.1097/00007890-200012270-00018. [DOI] [PubMed] [Google Scholar]
- 40.Poloni A, Maurizi G, Rosini V, et al. Selection of CD271(+) cells and human AB serum allows a large expansion of mesenchymal stromal cells from human bone marrow. Cytotherapy. 2009;11:153–62. doi: 10.1080/14653240802582125. [DOI] [PubMed] [Google Scholar]
- 41.Pytlik R, Stehlik D, Soukup T, et al. The cultivation of human multipotent mesenchymal stromal cells in clinical grade medium for bone tissue engineering. Biomaterials. 2009;30:3415–27. doi: 10.1016/j.biomaterials.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Schallmoser K, Bartmann C, Rohde E, et al. Human platelet lysate can replace foetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436–46. doi: 10.1111/j.1537-2995.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 43.Taylor PM. Biological matrices and bionanotechnology. Philos Trans R Soc Lond B Biol Sci. 2007;362:1313–20. doi: 10.1098/rstb.2007.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141–51. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gottlieb D, Kunal T, Emani S, et al. In vivo monitoring of function of autologous engineered pulmonary valve. J Thorac Cardiovasc Surg. 2010;139:723–31. doi: 10.1016/j.jtcvs.2009.11.006. [DOI] [PubMed] [Google Scholar]


