Abstract
Profilin1 (Pfn1) functions as a tumour suppressor against malignant phenotypes of cancer cells. A minimum level of Pfn1 is critical for the differentiation of human epithelial cells, and its lower expression correlates with the tumourigenesis of breast cancer cells and tissues. However, the molecular mechanisms underlying its anti-tumour action remain largely unknown. In this study, we found that stable expression of ectopic Pfn1 sensitized the breast cancer cell line MDA-MB-468 to apoptosis induced by staurosporine, a widely used natural apoptosis-inducing agent. Pfn1 overexpression could also up-regulate the expression of integrin α5β1, which has been shown to inhibit the transformed phenotype of cancer cells. Furthermore, the Pfn1-facilitated apoptosis induced by staurosporine was blocked in cells attached to a supplementary fibronectin substrate, which serves as a ligand of integrin α5β1. These results suggest that the insufficient fibronectin caused by the integrin α5β1 up-regulation might activate a signalling pathway leading to an increase of cellular apoptosis. Moreover, Pfn1 that primarily functions to promote local superstructure formation involving actin filaments and integrin β1 may contribute to its promotion on apoptosis. Our study indicated a previously uncharacterized role of Pfn1 in mediating staurosporine-inducing apoptosis in breast cancer cells via up-regulating integrin α5β1, and suggested a new target for breast cancer therapy.
Keywords: profilin1, integrin, actin, fibronectin, apoptosis
Introduction
Multiple cellular functions such as motility, division and endocytosis involve the dynamic remodelling of the actin cytoskeleton [1]. Stabilization of the actin meshwork is achieved by the cross-linking of the filaments regulated by actin-associated proteins, such as Pfn [2]. Pfn, a ubiquitously expressed actin-binding protein, has been found to be significantly down-regulated in breast, hepatic and pancreatic adenocarcinoma cells and tissues when compared with their normal counterparts [3-5]. It has been reported that overexpression of Pfn1 (the first Pfn protein isolated from the thymus) in cancer cells failed to form tumours when subcutaneously xenografted in nude mice, suggesting that Pfn1 could also be a negative regulator of carcinoma [4-7]. Moreover, the actin-binding site on Pfn1 has been found to be instrumental to its suppressing function, probably due to the alternation of Pfn1 expression which might affect the cellular infrastructure by changing the actin stress fibre, thus altering the balance of growth, death, attachment and migration [4]. However, the mechanism remains to be clarified.
It is well-known that apoptosis involves early detachment from the extracellular matrix (ECM), rearrangements of the actin cytoskeleton, membrane blebs formation and an eventual breakdown into apoptotic bodies [8]. Integrins are transmembrane heterodimers of α and β subunits that provide dynamic, physical links between ECM and intracellular cytoskeleton, allowing cells to sense and respond to environmental stimuli [9]. The integrin family, composed of α and β heterodimers, is classified according to the latter subunits [10]. In the periphery, interactions of integrins with ECM have long been known to regulate viability and apoptosis [11]. Growing evidence indicates that the integrin-mediated signalling pathways, including those that control anoikis, can be specific to an individual integrin [12]. In particular, integrin β1 subfamily is the most ubiquitous and promiscuous integrin distributed throughout epithelial tissues. Many studies have been directed at the aberrant integrin expression found in breast carcinoma, whereas some have demonstrated the diminished level of integrin β1 along with an increasing of de-differentiation and proliferation [13, 14]. Thus, anchorage-independent survival likely results from the uncoupling of cell cycle dependence on signals transduced through attachments to the substratum.
Actin-binding proteins are crucial for the adhesion of cells to extracellular matrices and cell survival as they are involved in the linkage of integrins to the cortical actin cytoskeleton [15]. Our previous studies had shown that Pfn1 as an actin-binding protein could inhibit proliferation and migration [5]. Here, we evaluated the effect of Pfn1 on breast cancer cell apoptosis and its mechanism involving the roles of integrin β1 and the actin cytoskeleton.
Materials and methods
Cell culture and transfections
All cell lines in this study were maintained either in DMEM or in RPMI medium 1640 (Invitrogen, Grand Island, NY, USA) supplemented with 10% foetal bovine serum, 50U of penicillin/ml and 0.1 mg of streptomycin/ml at 37°C in a 5% CO2 humidified atmosphere. Plasmid encoding a full-length Pfn1 gene and its control vector pcDNA3.1 [5] were stably transfected into breast cancer cell line MDA-MB-468, using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer's instructions. The two plasmids expression cells were designated Pfn1–468 and Mock cells. The transient transfection with siRNA-integrin β1 or its nonsense control duplex (Cell Signaling Technology, Danvers, MA, USA) was also performed with Lipofectamine 2000 and then cells were harvested or treated at 48-hr post-transfection.
Cell adhesion assay
Cell adhesion assay was performed as described [16]. Briefly, tissue culture plates were coated twice with poly-HEME (10 mg/ml in ethanol; Sigma-Aldrich, St. Louis, MO, USA) and rinsed extensively with PBS. Cell suspensions were seeded on the poly-HEME plates. At the indicated times, cells were recovered and harvested. For fibronectin (FN)-coated dishes, 15 μg/ml FN (Sigma-Aldrich) in PBS was added to each dish and incubated for 1 hr at 37°C. Excess solution was carefully aspirated and dried, and then washed extensively with PBS. The functional integrin β1–blocking peptide was applied as previously described [17]. Cell suspensions were pre-incubated with the blocking peptide or its nonsense peptide and then incubated for 8 hrs followed by staurosporine (STS) treatment.
Flow cytometry analysis (FACS)
For immunophenotyping applications, 10 μl of a 25–50 μg/ml stock solution of the integrin β1 monoclonal antibody (BD Biosciences, San Jose, CA, USA) was mixed with up to 106 cells in a minimal volume (≤0.2 ml) of buffer [PBS + 0.5% bovine serum albumin (BSA)] and incubated at room temperature for 30 min. Cells were washed twice with the same buffer and centrifuged at 250 χg for 5 min. The cell pellet was resuspended in 0.2 ml of PBS buffer, and 10 μl of a 25 μg/ml secondary FITC-mouse IgG antibody was added to the suspension and incubated for another 30 min. After PBS rinse, cells were resuspended in 0.5 ml of the same PBS buffer for FACS (Becton Dickinson, USA) analysis. Each experiment was repeated twice, with 10,000 events per sample were recorded. Annexin V staining (BD Bioscience Pharmingen, USA) detected by flow cytometry was used to assess apoptosis according to the manufacturer's instructions.
Real-time PCR
Total cellular RNA was extracted using Trizol reagent (Invitrogen). Quantitative real-time PCR was performed with PCR Mastermix containing Sybgreen I and hotstart Taq DNA polymerase (Toyobo, Osaka, Japan). The primers of integrin β1 and GAPDH used in this study have been described previously [17]. The oligonucleotides of Pfn1 used in PCR amplification were designed according to the reference [3]. Real-time detection of the emission intensity of SYBR Green bound to double-stranded DNAs was performed with the Icycler instrument (Bio-Rad, Hercules, CA, USA). PCR reactions were performed in triplicate for each sample-primer set, and the mean of the three experiments was used as the relative quantification value. The level of mRNA was expressed as a ratio relative to the GAPDH mRNA in each sample.
Immunostaining and confocal microscopy
The cells, seeded on Chamber Slides, were washed with cold PBS (pH 7.4) and fixed with 4% paraformaldehyde for 30 min. on ice, rinsed with cold PBS and permeabilized with 0.1% Triton X-100 for 30 min. on ice. After blocking with 3% BSA/PBS, the primary antibodies anti-integrin β1 and anti-Pfn1 (BD Biosciences) were added at 1:100 dilutions with 3% BSA/PBS. The cells were incubated at 4°C overnight followed by incubation with the secondary antibodies IgG-Rhodamine IgG-Cy5, or F-actin specific dye phalloidin (1:500 dilution with 3% BSA/PBS; Sigma-Aldrich) for 1 hr before being washed with cold PBS and mounted. Fluorescence images were recorded with the confocal microscope Olympus EX51 and processed with analysis software (Leica LAS AF Lite).
Western blotting (WB) and immunoprecipitation (IP)
Equal amounts of proteins were separated by SDS-PAGE, and then transferred onto polyvinylidene (PVDF) membranes (Millipore, Saint-Quentin en Yvelines, Belgium) by electrotransfer. Membranes were blocked with 5% skim milk in PBS-T containing 0.1% Tween-20, and proteins of interest were visualized using specific Pfn1, poly (ADP-ribose) polymerase (PARP), integrin β1, integrin α5, actin (BD Bioscience), caspase9 (Cell Signaling Technology), p27, caspase-3 (Santa Cruz, CA, USA), and tubulin (Upstate, Charlottesville, VA, USA) antibodies, followed by HRP-conjugated secondary antibodies. The proteins were visualized using an enhanced chemiluminescence system (Pierce, Rockford, IL, USA). For IP, cells were lysed in buffer containing 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP40, 5 mM EDTA, 5 mM EGTA, 15 mM MgCl2, 60 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 0.1 mM NaF, 0.1 mM benzamide, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM PMSF. Twenty microlitres of protein A/G agarose beads (BD Bioscience Pharmingen) were added to the lysates for proper periods of incubation. The beads were then washed and subjected to SDS-PAGE and immunoblotting.
Protein extraction
Harvested cells were lysed in buffer containing 50 μM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, phosphatase inhibitors (100 mM Na3VO4, 10 mM NaF) and protease inhibitor (1 mM phenyl methylsulphonyl fluoride, PMSF) to obtain the whole cell lysates. The purified membrane protein extractions were carried out with the membrane bound protein kit (DBI). To acquire cytoskeleton-based Triton-insoluble fractions, cells were washed with PBS before treated with lysis buffer A containing 10 mM Tris-HCl, 0.15M NaCl, 1 mM EDTA, 0.25% NP40, 1% Triton X-100, 1 mM PMSF and 1 mM NaVO3, which lasted 30 min. on ice. Lysates were centrifuged and washed three times with buffer A to remove the Triton X-100 soluble fractions. The remaining Triton-insoluble fractions were suspended in extraction buffer containing 10 mM Tris-HCl, 2 mM EGTA, 0.15 M NaCl, 1% SDS, 1 mM leupeptin and 1 mM PMSF and boiled for 10 min. followed by centrifugation. Triton-insoluble cytoskeletal fractions were extracted in the supernatants.
Cell viability assay
Cells were cultured in a 96-well plate and pre-treated with Latrunculin B (Sigma-Aldrich) for 1 hr. Cells were treated with STS (Roche, Basel, Switzerland) from a DMSO stock (2.5 mM) or left untreated, washed and then administered to cell viability detection by Cell Counting Kit-8 (CCK-8, Beyotime) according to the manufacturer's instructions. CCK-8 is a more highly sensitive non-radioactive colorimetric assay than the traditional MTT assay. The kit uses the unique water-soluble tetrazolium salt-8 (WST-8) in measuring NADH production resulting from dehydrogenase activity. Absorbance was measured at 450 nm using an ELISA reader (Bio-Tek, Houston, TX, USA).
Statistical analysis
Values were expressed as the mean ± S.E. where appropriate. Comparisons between groups were made using one-way ANOVA or the two-sided Student's t-test, with statistical significance assumed when P < 0.05 was obtained.
Results
Pfn1 positively regulates integrin β1 protein and its mature form in MDA-MB-468 cells
Based on the fact that consistently lower Pfn1 levels were shown in different breast cancer cells in comparison with normal mammary ones [7], we assessed the expression of Pfn1 in several cell lines (MDA-MB-468, MDA-MB-231, T47D, MCF-7, BT-474, SKBR-3) and selected MDA-MB-468 owing to its lower Pfn1 endogenous content (data not shown). We found that Mock cells with empty vector-transfection presented a very low level of integrin β1. These cells contained two forms of immunoreactive β1, which SDS-PAGE revealed as a 125-kD band of mature β1 with a less intense above, and a 105-kD precursor below. In Pfn1–468 cells with ectopic Pfn1 overexpression, the intensities of both integrin β1 bands detected by WB increased significantly, approximately 3.6-fold in the total amount (Fig. 1A and B), as verified by the up-regulation of p27kip1, a downstream effector of integrin β1 [18,19]. We also performed the transient transfection of Pfn1 plasmid in MDA-MB-231 and MCF-7 cells, and detected the expression of integrin β1 by WB (Fig. S1A). As in the case of MDA-MB-468, integrin β1 levels were increased in Pfn1-transfected cells compared to controls. However, because it is the mature integrin β1 but not the immature form that translocates from the Golgi complex to the cell membrane and plays an important role in cell adhesion or cell signalling [20,21], we sought to determine its surface exposure by flow cytometry assays. We found that the staining of integrin β1, in contrast to Mock cells, exhibited a progressive increase with 4.6-fold greater abundance on the surface of Pfn1–468 cells (Fig. 1C and D).
Fig 1.
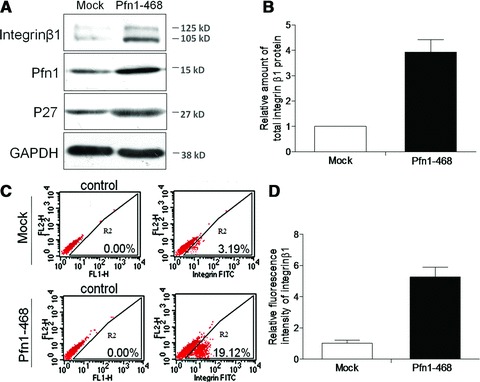
Pfn1 positively regulated integrin β1 level. (A) Western blot of total lysates from Mock and Pfn1–468 cells using specific antibodies as indicated. Representative clone we chose to show here. The labelled were the molecular weights of pre-stained protein ladders used in electrophoresis. (B) Quantitative analysis of total integrin β1 protein amount performed with Total-Lab software. (C) Flow cytometric analysis of integrin β1 levels on the cell surface; the negative control showing the background fluorescence. (D) Relative statistical analysis of fluorescent intensity from three independent experiments.
Pfn1 facilitates MDA-MB-468 to STS-induced apoptosis by up-regulated–integrin β1 engagement
It has been reported that disruptions of the actin-bundling protein α-actinin and integrin interactions render osteoblasts susceptible to apoptosis [22,23]. To explore the potential role of Pfn1 that is involved in the cytoskeleton network containing actin and integrin β1 in the behaviour of cellular apoptosis, we set up a stress environment with STS as a kind of actin-modifying drug extensively utilized for the induction of apoptosis [24]. It was found that STS treatment was capable of efficiently decreasing cell viability in a time- and dose-dependent manner, resulting in more serious growth inhibition in Pfn1–468 than that in Mock cells (Fig. 2A and B). Furthermore, the promotion of Pfn1 on apoptosis was confirmed by the detection of the index of apoptotic endpoints, and Pfn1 facilitated the cleavage of its substrate Poly (ADP-ribose) polymerase (PARP), reflecting the activation of the effector protease caspase 9 in response to intrinsic apoptotic stimuli (Fig. 2C). It was apparent that the early staged apoptosis determined by AnnexinV-PE-7-AAD staining showed a population with the Annexin positive and 7-AAD negative immunophenotype, and the staining percentage in Pfn1–468 cells was significantly higher than that in Mock cells (Fig. 2D and E).
Fig 2.
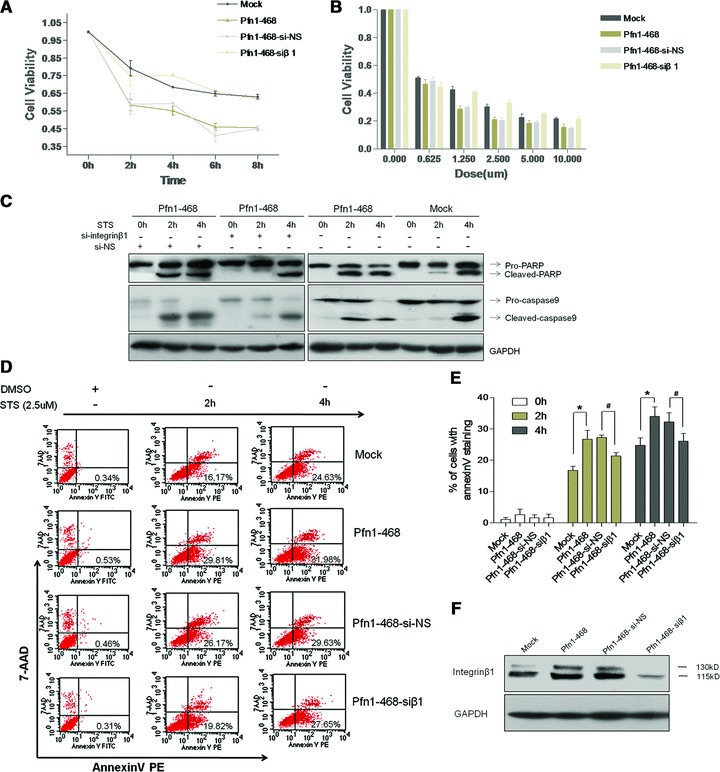
Integrin β1 as a critical response factor to Pfn1 sensitized apoptosis in cells exposed to STS. (A) Quantitative analysis of cells viabilities in the absence or presence of si-integrin β1 transient transfection exposed to 2.5 μM STS in time course as determined by CCK-8 assay. Mock versus Pfn1–468, *P < 0.01; #Pfn1–468-si-NS versus Pfn1–468-siβ1, P < 0.01. (B) Dose-response bar of STS-induced apoptosis in cells with or without si-integrin β1 transfection. The cells treated with DMSO or STS for eight hrs in different dose points. Mock versus Pfn1–468, *P < 0.05; #Pfn1–468-si-NS versus Pfn1–468-siβ1, P < 0.05. (C) The cleavages of caspase 9 and PARP as apoptotic markers examined by Western blot with total cell lysates. (D, E) Comparisons of STS-induced apoptosis manifested by AnnexinV-7AAD staining at indicated time intervals. Mock versus Pfn1–468, *P < 0.05; #Pfn1–468-si-NS versus Pfn1–468-siβ1, P < 0.05. (F) Efficiency of integrin β1 knocked down determined by WB with total cell lysates.
To further assess the role of integrin β1 in Pfn1-facilitated apoptosis, interfering RNA duplexes of integrin β1 (siβ1) were used to knock down the level of integrin β1. It was found that the down-regulation of integrin β1 in Pfn1–468 cells resulted in a significant restoration of cell viability, whereas no significant difference was observed in Pfn1–468 cells treated with siβ1 oligonucleotide compared to Mock cells without ectopic Pfn1 expression. However, transfection with the si-NS control in Pfn1–468 cells failed to have a significant effect on cell viability (Fig. 2A and B). Other index changes including the decreased cleavages of PARP and caspase 9 (Fig. 2C) and the decreased percentage of Annexin V positive/7-AAD negative cell populations in Pfn1–468 cells owing to siβ1 indicated that siβ1 rescued the Pfn1-enhanced apoptotic susceptivity of Pfn1–468 cells exposed to STS (Fig. 2D and E). The efficiency of siβ1 was observed (Fig. 2F). To further confirm the inhibition of Pfn1 in cancer cell survival, we subjected MDA-MB-231 cells with a relative higher Pfn1 expression to RNA interference of Pfn1 (Fig. S1B and C). The data showed that Pfn1 knockdown in MDA-MB-231 resulted in increased cell survival under STS conditions and the down-regulation of integrin β1 level.
Integrin β1 protein is stabilized by overexpression of Pfn1
Real-time RT-PCR of integrin β1 mRNA was performed in Mock and Pfn1–468 cells to further evaluate the role of Pfn1 on integrin β1 levels by increasing the mRNA amount or maintaining the protein stabilization. Pfn1 overexpression produced no obvious effect on the mRNA level of integrin β1, but led to the up-regulation of integrin β1 protein level (Fig. 3A). Translation inhibition with cycloheximide (CHX) was employed to determine the stability of integrin β1, and the results indicated that the half-life of integrin β1 in Pfn1–468 cells was significantly longer than that in Mock cells, demonstrating that Pfn1 overexpression alleviated the degradation of integrin β1 (Fig. 3B). It has been reported that excess integrin β1 could be degraded via the proteasome-dependent pathway [25]. However, whether Pfn1 can regulate proteasome-dependent proteolysis of integrin β1 is still unknown. To further examine the possibility, a proteasome inhibitor, MG132, was applied to both cells. Consequently, the degradation of mature integrin β1 was efficiently rescued in the presence of MG-132 as observed in Mock cells, whereas no change was observed in Pfn1–468 (Fig. 3C). We also employed the lysosome inhibitor chloroquine to rule out unspecific effects of MG132 in the lysosome, finding that protein levels of integrin β1 did not change in the presence of chloroquine in both cells (Fig. 3D). These results suggested that Pfn1 overexpression prohibited integrin β1 from going into the proteasome pathway leading to degradation.
Fig 3.
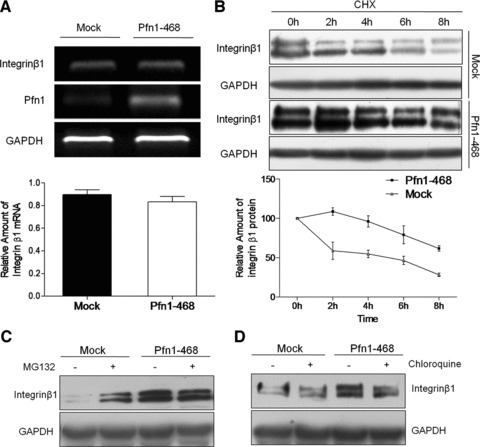
Pfn1 overexpression increased integrin β1 protein stability. (A) The expression level of integrin β1 mRNA determined by real-time PCR analysis; Agarose gel electrophoresis data showing relative level of integrin β1, Pfn1 and GAPDH (loading control) mRNA. (B) The stable transfected cells harvested at the indicated time after treatment with cycloheximide (100 μM) and the total protein extracts prepared for immunoblotting and GAPDH as a control; three independent experiments performed. (C, D) The cells treated with proteasome inhibitor MG132 (50 μM) or lysosome inhibitor chloroquine (20 μM), and the amount of integrin β1 assessed by Western blot.
A strengthened formation in the ternary complex among Pfn1, integrin β1 and actin in response to Pfn1 overexpression
There is a wealth of data revealing the importance of Pfn1 for actin polymerization and dynamics by the affinity of Pfn1–actin complexes for actin filament ends [26, 27]. Integrins also are linked to a multitude of structural and signalling molecules as well as the actin cytoskeleton via their internal, cytoplamic domains [28]. To better define the linkage among Pfn1, integrin and actin, and to illustrate the underlining mechanism of how Pfn1 up-regulates integrin β1, we focused on the investigation of the network at the hub of Pfn1 overexpression. Confocal microscopy showed that Pfn1 bound to the actin cytoskeleton and also linked to integrin β1. Both Pfn1 and integrin β1 were distributed throughout the cytoplasm, especially the plasma membrane where anchored with F-actin (Fig. 4A). We therefore asked whether the up-regulation of integrin β1 mediated by overexpressing Pfn1 could have any effects on the network involving Pfn1, actin skeleton and integrin β1, as suggested from our microscopy experiments. To address this question, we performed reciprocal IP assays on them, finding that Pfn1 immunoprecipitated with mature integrin β1 at the basal level, and overexpression of Pfn1 resulted in a significant increase in their association in Pfn1–468 cells (Fig. 4B). To further examine whether integrin β1 anchored to the cytoskeleton could be altered due to ectopic Pfn1 expression, we measured the integrin β1 linkage with actin. The mature and immature forms of integrin β1 in Pfn1–468 cells were found to be fixed in high amounts with actin. In line with the previous findings that the stabilization of actin filaments may be caused by the promoted actin polymerization activity due to Pfn1 [29, 30], a stronger immunoactive band of Pfn1 precipitated by actin antibody was observed upon ectopic Pfn1expression. Furthermore, we used the Triton X-100 protein extraction experiment to detect integrin β1 associated with the detergent insoluble fraction. The higher occupancy of integrin β1 at the cytoskeleton was observed in Pfn1–468 cells by WB (Fig. 4C). These results suggested that Pfn1 could contribute to the quantity of integrin β1 linked to the cytoskeleton on the cell surface.
Fig 4.
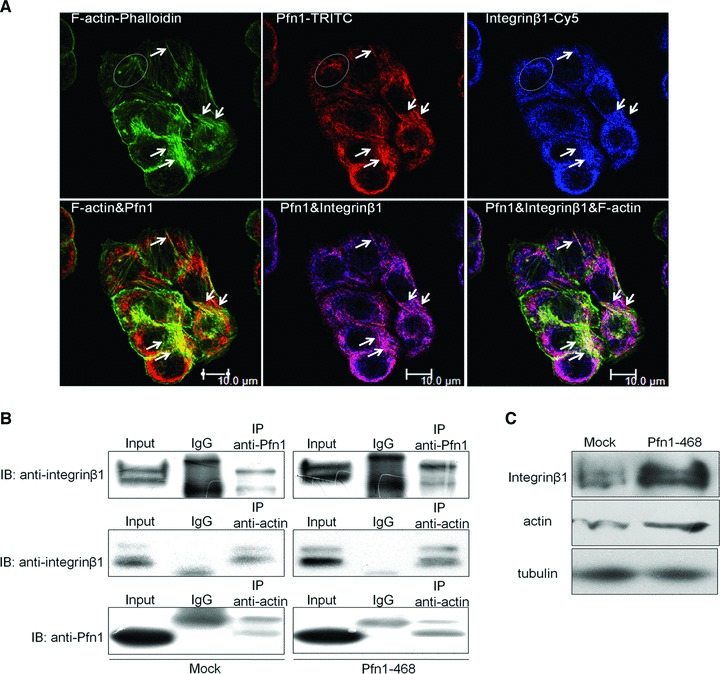
Pfn1 reinforced the complex formation among Pfn1, actin and integrin β1 converging into cytoplasm membrane. (A) Immune-confocal detection with FITC-Phalloidin (green) specifically staining filament actin, Rhodamine (red dye) and Cy5 (blue dye) for Pfn1 and integrin β1, respectively. (B) Total Pfn1–468 and Mock cells extracts immunoprecipitated (IP) with Pfn1 or actin or IgG antibodies and then immunoblotted with Pfn1 or integrin β1 antibodies. (C) Lysates prepared as Materials and Methods with Triton X-100 to isolate insoluble proteins linked to cytoskeleton structure and then subjected to Western blot; tubulin used as a control.
FN decreases the apoptosis susceptibility facilitated by Pfn1
Elevated expression of integrin β1 chain precursors bound to certain α subunits upon their arrival at the membrane exerted an inhibiting effect on cell malignant phenotypes. Integrin α5β1, a widely expressed FN receptor, is one of the best-characterized integrins that recognizes the tripeptide sequence, Arg-Gly-Asp. The association between FN and integrin α5β1 is implicated in regulating not only cell adhesion and migration, but also cell differentiation and proliferation [23, 31]. Intriguingly, we found that Pfn1 overexpression could also induce an increase in the protein level of endogenous integrin α5 subunit (Fig. 5A), but not its mRNA level (data not shown) concurrently with up-regulation of integrin β1. Integrin α5β1 is known to mediate strong survival signalling in response to attachment to FN [32]. Microscopic examination of the target cells plated in the wells coated with FN revealed striking morphological differences between Pfn1–468 cells and Mock cells in the STS-free culture conditions (Fig. 5B).
Fig 5.
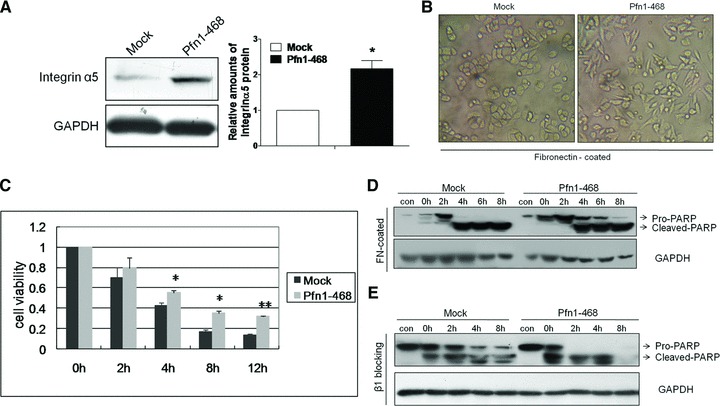
Apoptosis enhanced by Pfn1 was reversed by Pfn1–468 cells attachment to FN. (A) Corresponding integrin α5 immunoblot with total cells proteins showed stronger expression in the case of Pfn1 overexpression and GAPDH blot served as the loading control. (B) The cells seeded on FN-coated plates in basal medium and taken photos under light microscope after adherence. (C) 96-well plates coated with FN at 5 μg/ml, and cells allowed to attach for 8 hrs in basal medium before treatment with 2.5 μM STS at indicated time points; significant difference between groups indicated at *P < 0.05 or **P < 0.01 level. (D) The acquired lysates of cells grown on FN-coated plates under 2.5 μM STS condition immunoblotted by PARP and GAPDH antibodies; extracts from cells plated in no FN-coated surfaces performed as control samples (con). (E) Aliquots of cells suspension pre-incubated with the β peptide or nonsense peptide (100 μM) as control and incubated for 12 hrs before STS treatment at diverse time points; the total protein lysates probed with PARP and GAPDH antibodies.
A large number of Pfn1–468 cells exhibited a narrow, elongated morphology, with a tendency to have protrusive extensions on the FN-coated plates. This was likely due to an active process depending on culture conditions and adhesive interactions between cells and their substrates in vitro[33]. In contrast, Mock cells typically displayed a round shape, with small clumps similar to their originating MDA-MB-468 cells. Because of this phenomenon, we further examined cell viability on the cells grown on the FN-coated medium under STS conditions. It was shown that adhesion to a FN-coated substratum reversed the apoptosis facilitation displayed by Pfn1-overexpression in Pfn1–468 cells (Fig. 5C). Furthermore, immunoblotting of the processed cell lysates under STS stimuli revealed that, in addition to the appearance of PARP cleavage for Pfn1–468 cells at 4 hrs, but not at 2 hrs shown in Figure 2C, the ratio of cleaved-PARP to pro-PARP was lower than that in Mock cells due to FN supply (Fig. 5D). Taken together, these data suggested that integrin α5β1-specific adhesion to FN blocked Pfn1-sensitized apoptosis in Pfn1–468 cells. We observed that the suppression of functional integrin β1 with blocking peptide could also promote apoptosis, as indicated by the PARP cleavages in Pfn1–468 and Mock cells, with a stronger apoptotic effect in the former other than in the latter (Fig. 5E).
F-actin structure required in Pfn1-enhanced apoptosis
The spontaneous organization of actin superstructures is reported to be driven by ensembles of actin-binding proteins, but at different times and places and in response to different stimuli [34]. We found that Pfn1 overexpression up-regulated the membrane-linked F-actin level in breast cancer cells MDA-MB-468 (Fig. 6A) and MDA-MB-231 (data not shown), which signified that Pfn1 promoted actin polymerization beneath the plasma membrane. To further clarify whether the regulation of Pfn1 on actin assembly engages Pfn1-sensitized apoptosis in MDA-MB-468 cells, we treated the cells with latrunculin B (LatB), a drug that is capable of rapidly, reversibly and specifically disrupting the actin cytoskeleton [35]. LatB associates only with actin monomers, thereby preventing them from repolymerizing into filaments, without inhibiting binding by Pfn [36, 37]. The administration of LatB not only abolished the facilitated effect of Pfn1 in STS-induced apoptosis, but reversely enhanced the survival ability of Pfn1–468 cells when compared with Mock cells, especially at the interval of 4 hrs (Fig. 6B). In addition, no conspicuous difference in PARP activation was detected by WB between the two kinds of cells (Fig. 6C and D). These findings suggested that the maintenance of the actin cytoskeleton architecture in cells was necessary for the apoptotic susceptivity exerted by Pfn1.
Fig 6.
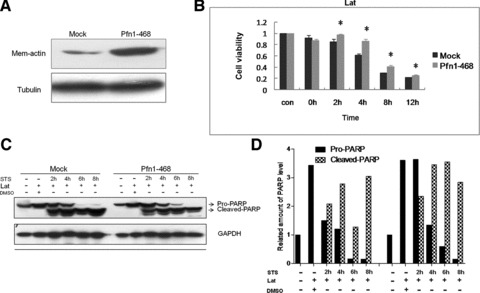
Actin superstructure maintenance was requisite of Pfn1-enhanced apoptosis under STS action. (A) Increased actin location in the plasma membrane determined by Western blot in Pfn1–468 compared to Mock cells; tubulin used as a control. (B) LatruculinB (4 μM) added before STS action and apoptosis measured by the estimation of cell viability with the CCK-8 assay; the units representing mean ± S.D. from three independent experiments; *differences significant at P < 0.05. (C) Equal amounts of total lysates analysed by immunoblot with antibodies against PARP and GAPDH; analysis of the band greyscales determined by Total Lab software shown in (D).
Discussion
The regulation of cellular apoptosis, a complex phenomenon, requires a rigorous and timely interaction involving multiple signalling and transcription processes. Understanding the signal molecules that contribute to chemotherapeutic agent-induced apoptosis in tumours may lead to better strategies for novel drug designs in treating cancer. Among the numerous researches on the point, the roles of anchorage to the substratum in the regulation of apoptosis have been the subjects of much scrutiny [38, 39]. Although many previous reports have examined the functions of integrin in the migration, proliferation and apoptosis of various cell types, the role of the Pfn1 as one kind of actin-binding protein involved in integrin regulation has not been reported. This is the first time that Pfn1 has been linked with integrin and extracellular substances in the apoptotic process.
The importance of Pfn1 as an actin cytoskeleton regulator for human tissue differentiation has been demonstrated by the findings that human breast cancer cells express conspicuously low Pfn1 levels and adopt a non-tumourigenic phenotype upon raising their Pfn1 level [7]. Pfn1 overexpression could increase the sensitivity of breast cancer cells MDA-MB-231 to camptothecin-induced apoptosis [40], which is similar to the effect of Pfn1 overexpression on staurosporine toxicity. And, it has been found that Pfn1 inhibits the proliferation of MDA-MB-231 partly through p27kip1 up-regulation [40]. In our study we found that Pfn1 overexpression increased integrin β1 and p27kip1 levels in Pfn1 stable transfected breast cancer cells MDA-MB-468, and the change of integrin β1 protein occurred in the post-translation stage by proteolysis regulation but not in transcriptional control. Along with our previous findings that overexpression of integrin β1 increased the protein stability and overall cellular level of p27kip1, which is involved in the proliferation-inhibition induced by integrin β1 [19], we suggest that Pfn1 was likely to elevate p27kip1 levels through up-regulating endogenous integrin β1 in its tumour suppressive action. However, the further mechanism of Pfn1's apoptosis enhancement remains to be uncovered.
Integrin adhesion receptors and the actin cytoskeleton mediate bidirectional transmission of force and biochemical signals across the plasma membrane [41], which is essential for the development and function of normal cells and various physiological processes including cancer. Although the connection between integrin β1 and actin has been demonstrated by integrin-associated actin-binding proteins such as talin [41], the specific role of Pfn1, including its function as an actin-binding protein and the hierarchy of its assembly in adhesion complexes, has not been revealed. As reported, integrin β1 is initially expressed as the most prevalent premature form (105 kD) in the endoplasmic reticulum (ER) and Golgi apparatus. The immature integrin β1 matures into the 125 kD form by complete glycosylation in the Golgi apparatus, and the mature β1 is mainly translocated to the plasma membrane [22]. This work revealed for the first time that the actin-binding protein Pfn1 played a pivotal role in apoptosis via the connection in integrin signalling with cytoskeleton and extracellular substances. Pfn1 overexpression was found to have significantly reinforced the associations in the complex of Pfn1, actin and integrin β1 beneath the plasma membrane, as reflected by the increased binding among the three proteins. Furthermore, the linkage between Pfn1 and integrin β1 could tether and tarry these integrins to the cortical actin skeleton, which may be the reason for the increased stabilization of integrin β1 protein in Pfn1–468 cells. However, our observation was that their binding just existed between Pfn1 and the mature and functional integrin β1. Triton X-100, a non-ionic surfactant, could dissolve proteins in the cytomembrane and cytoplasm but not proteins anchored to the actin cytoskeleton [42]. The research on the integrin β1 in the Triton X-100-insoluble F-actin fractions supported the fact that Pfn1 could function as a promoting factor of actin polymerization and the stabilization of integrin β1 with linkage to the F-actin. It is conceivable that the immature β1 might also need the actin cytoskeleton dynamic in the process of intracellular translocation and glycosylated maturation. Tumourigenicity is reportedly suppressed in β1 overexpressors and enhanced in β1-negative cells [43]. Therefore, it is reasonable that integrin β1 up-regulation on the cytosolic membrane could induce the cells harbouring ectopic Pfn1 to be more susceptive to STS stimuli.
Integrin as a cell adherent receptor represents an intermediary in the physical link between the ECM and the actin cytoskeleton [31]. However, it is pre-requisite for integrin β1 to initially dimerize with variant α subunits, which confers the binding specificity for ECM proteins such as FN, collagen (CoG), laminin (LM), etc. Among α subunits, α5 is distinguished from others that could couple with various β subunits because it predominantly couples with β1 and only interacts with FN. It has been reported that high levels of α5 expression are negatively correlated with transformation and tumour progression in cancer [44]. Our results add new supporting information demonstrating that Pfn1 overexpression could also up-regulate the α5 integrin subunit, directly or indirectly, with the increased integrin β1 effect. It is well accepted that FN and the integrin α5β1 play a complex role in malignancy. Although normal cells usually deposit an FN matrix around themselves, malignant cell lines often fail to do so perhaps due to the low expression of α5β1 heterodimers [43]. The most striking effect of the ectopic FN supplement is that increased α5β1 by Pfn1-mediated cell attachment to FN-coated medium solidly, thereby inhibiting the role of Pfn1 in STS-induced chemotherapeutic apoptosis. The data allowed us to propose a speculative framework. The low expression of integrin α5β1 could lead to deficient matrix deposition of FN for breast cancer cells MDA-MB-468. However, integrin α5β1 up-regulation triggered by Pfn1 overexpression further aggravated the relative deficiency of FN and the binding loss of the ECM to the receptor, which elicited apoptosis or death signals to be transmitted into the cells. Thus, the mechanism of insufficient ligands may be the best clarification that Pfn1 can improve the apoptotic sensitivity of breast cancer cells MDA-MB-468 through integrin β1 up-regulation, which was also proved by the experiment of attachment deprivation by plating targeted cells on poly-HEME–coated petri dishes. The anchorage-dependent cells prevented from attaching to an ECM substrate stopped proliferating and underwent apoptosis. In our study, we used polyHEMA (2-Hydroxyethylmethacrylate) to prevent adhesion and trigger aggregation. As a result of the presence of polyHEMA, Pfn1–468 cells were more sensitive to apoptosis, as indicated by a significant decrease in pro-PARP and pro-caspase3. In Mock cells cultured on polyHEMA, there was no significant apoptotic evidence (Fig. S1D).
It has long been known that the actin cytoskeleton is substantially modified in transformed cells, and this occurs in concert with changes in a host of actin filament–associated regulatory proteins. For instance, a moderate decrease in cellular polymerized actin level is apparent in the transformed cells compared with non-transformed ones [37]. Interestingly, Pfn1 as an actin-severing protein exerted a powerful effect on actin filament organization and stabilization within membrane regulated system in our study. Thus, it is conceivable that by increasing the F-actin content in MDA-MB-468 cells, Pfn1 overexpression may create a similar cytoskeletal background and favour cell apoptosis. A noteworthy point here is that LatB, which functioned to disturb actin assembly before STS, unexpectedly resulted in the loss of Pfn1-facilitated apoptosis and even the enhancement of cell resistance to STS. Given the fact that an increased concentration of the sequestered G-actin correlated with increased aggressiveness of carcinoma cells [37], LatB not only eliminated the distinction of actin superstructure triggered by Pfn1 overexpression between Pfn1–468 and Mock cells, but also caused an increased accumulation of dissociative G-actin from F-actin in the former cells. This suggests that the significance of a steady F-actin framework for consequences in Pfn1 can facilitate apoptosis, although further mechanistic studies are needed for elucidation. Moreover, the cytoskeleton may play a key role in the physical anchorage of integrin signal and apoptosis.
In conclusion, this study have clearly demonstrated how Pfn1 facilitates the STS-induced apoptosis by integrin and actin complex (Fig. 7) and provided new insight into the still ill-defined role of Pfn1 in the control of breast cancer cells responsiveness to chemotherapy. The use of Pfn1 and STS may be an important clinical consideration in treating breast cancer harbouring low integrin expression. The combination of Pfn1 and STS with clinically relevant medications deserves further investigations in vitro and in vivo, in view of the fact that there is still ongoing debate concerning the relevant mechanisms.
Fig 7.
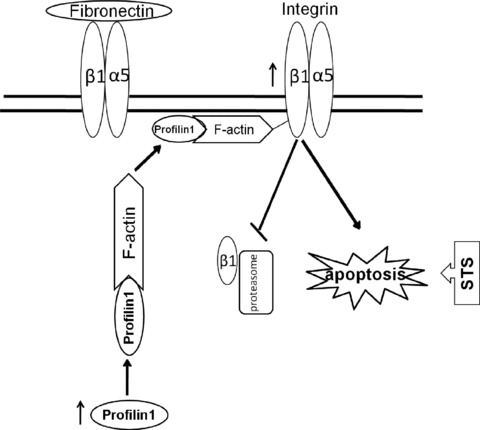
Schematic diagram for the role of Pfn1 under STS condition. Pfn1 overexpression increases actin filament assembly and then enhances the binding among Pfn1, actin and integrin β1, which form a more stable complex on the plasma membrane. The co-administration of them in such cells leads to retention of integrin β1 on the membrane, thus inhibiting its degradation by proteosome. The up-regulated integrin β1 involves in apoptotic susceptivity facilitated by Pfn1 overexpression. The increased integrin α5β1 receptor by Pfn1 overexpression is excess to fibronectin as an extracellular ligand. Accordingly, the ligation state of the integrin α5β1 molecule with FN influenced by ectopic Pfn1 accounts for the cell apoptotic sensitivity under STS treatment.
Acknowledgments
We thank X. Sun and Y. Huang for confocal microsopy manipulation. This work was supported by the fund of National Key Sci-Tech Special Project of China (2008ZX10002-018 and 2008ZX10002-019).
Conflict of interest
No potential conflicts of interest were disclosed.
Supporting Information
(A) Breast cancer cell lines MDA-MB-231and MCF-7 transient transfected with Pfn1 or its control plasmidand the total protein lysates subjected to WB. (B)MDA-MB-231 transient transfected with Pfn1 siRNA and its nonsense(NS) control and the efficiency of siRNA determined by WB. Cellviability under STS stimuli detected and quantified with the CCK-8assay. (C) Immunoblot assays showing PARP cleavage inMDA-MB-231 with or without Pfn1 knockdown under STS treatment.(D) Pfn1–468 and Mock Cells grown on polyHEME-coatedsurfaces and harvested at the appointed time; WB analysis ofpro-PARP and pro-caspase3 with these cell extracts shown.
References
- 1.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–65. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 2.Kasina S, Rizwani W, Radhika KV, et al. Nitration of profilin effects its interaction with poly (L-proline) and actin. J Biochem. 2005;138:687–95. doi: 10.1093/jb/mvi163. [DOI] [PubMed] [Google Scholar]
- 3.Janke J, Schluter K, Jandrig B, et al. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191:1675–86. doi: 10.1084/jem.191.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wittenmayer N, Jandrig B, Rothkegel M, et al. Tumour suppressor activity of profilin requires a functional actin binding site. Mol Biol Cell. 2004;15:1600–8. doi: 10.1091/mbc.E03-12-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu N, Zhang W, Yang Y, et al. Profilin 1 obtained by proteomic analysis in all-trans retinoic acid-treated hepatocarcinorna cell lines is involved in inhibition of cell proliferation and migration. Proteomics. 2006;6:6095–106. doi: 10.1002/pmic.200500321. [DOI] [PubMed] [Google Scholar]
- 6.Roy P, Jacobson K. Overexpression of profilin reduces the migration of invasive breast cancer cells. Cell Motil Cytoskeleton. 2004;57:84–95. doi: 10.1002/cm.10160. [DOI] [PubMed] [Google Scholar]
- 7.Janke J, Schluter K, Jandrig B, et al. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191:1675–85. doi: 10.1084/jem.191.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002;9:493–504. doi: 10.1038/sj.cdd.4400987. [DOI] [PubMed] [Google Scholar]
- 9.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 10.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damsky CH, Ilic D. Integrin signaling: it's where the action is. Curr Opin Cell Biol. 2002;14:594–602. doi: 10.1016/s0955-0674(02)00368-x. [DOI] [PubMed] [Google Scholar]
- 12.Giancotti FG. Complexity and specificity of integrin signalling. Nat Cell Biol. 2000;2:E13–4. doi: 10.1038/71397. [DOI] [PubMed] [Google Scholar]
- 13.Newham P, Humphries MJ. Integrin adhesion receptors: structure, function and implications for biomedicine. Mol Med Today. 1996;2:304–13. doi: 10.1016/1357-4310(96)10021-6. [DOI] [PubMed] [Google Scholar]
- 14.Pignatelli M, Hanby AM, Stamp GW. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991;165:25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Sengupta A, Glogauer M, et al. Filamin A regulates cell spreading and survival via beta1 integrins. Exp Cell Res. 2008;314:834–46. doi: 10.1016/j.yexcr.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Liang YL, Fu Y, Chen SG, et al. Integrin beta1 subunit overexpressed in the SMMC-7721 cells regulates the promoter activity of p21(CIP1) and enhances its transcription. FEBS Lett. 2004;558:107–13. doi: 10.1016/S0014-5793(03)01469-8. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Liang Y, Li Z, et al. Increase in beta1-6 GlcNAc branching caused by N-acetylglucosaminyltransferase V directs integrin beta1 stability in human hepatocellular carcinoma cell line SMMC-7721. J Cell Biochem. 2007;100:230–41. doi: 10.1002/jcb.21071. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Ozaki I, Mizuta T, et al. Mechanism of beta 1-integrin-mediated hepatoma cell growth involves p27 and S-phase kinase-associated protein 2. Hepatology. 2003;38:305–13. doi: 10.1016/S0270-9139(03)80353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y, Fang Z, Liang Y, et al. Overexpression of integrin beta1 inhibits proliferation of hepatocellular carcinoma cell SMMC-7721 through preventing Skp2-dependent degradation of p27 via PI3K pathway. J Cell Biochem. 2007;102:704–18. doi: 10.1002/jcb.21323. [DOI] [PubMed] [Google Scholar]
- 20.Argraves WS, Suzuki S, Arai H, et al. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987;105:1183–90. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama SK, Yamada KM. Biosynthesis and acquisition of biological activity of the fibronectin receptor. J Biol Chem. 1987;262:17536–42. [PubMed] [Google Scholar]
- 22.Triplett JW, Pavalko FM. Disruption of alpha-actinin-integrin interactions at focal adhesions renders osteoblasts susceptible to apoptosis. Am J Physiol Cell Physiol. 2006;291:C909–21. doi: 10.1152/ajpcell.00113.2006. [DOI] [PubMed] [Google Scholar]
- 23.Kuwada SK, Kuang J, Li X. Integrin alpha5/beta1 expression mediates HER-2 down-regulation in colon cancer cells. J Biol Chem. 2005;280:19027–35. doi: 10.1074/jbc.M410540200. [DOI] [PubMed] [Google Scholar]
- 24.Nakano H, Omura S. Chemical biology of natural indolocarbazole products: 30 years since the discovery of staurosporine. J Antibiot (Tokyo) 2009;62:17–26. doi: 10.1038/ja.2008.4. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida Y, Chiba T, Tokunaga F, et al. E3 ubiquitin ligase that recognizes sugar chains. Nature. 2002;418:438–42. doi: 10.1038/nature00890. [DOI] [PubMed] [Google Scholar]
- 26.Pollard TD, Blanchoin L, Mullins RD. Actin dynamics. J Cell Sci. 2001;114:3–4. doi: 10.1242/jcs.114.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Neidt EM, Scott BJ, Kovar DR. Formin differentially utilizes profilin isoforms to rapidly assemble actin filaments. J Biol Chem. 2009;284:673–84. doi: 10.1074/jbc.M804201200. [DOI] [PubMed] [Google Scholar]
- 28.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, et al. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothkegel M, Mayboroda O, Rohde M, et al. Plant and animal profilins are functionally equivalent and stabilize microfilaments in living animal cells. J Cell Sci. 1996;109:83–90. doi: 10.1242/jcs.109.1.83. [DOI] [PubMed] [Google Scholar]
- 30.Finkel T, Theriot JA, Dise KR, et al. Dynamic actin structures stabilized by profilin. Proc Natl Acad Sci U S A. 1994;91:1510–4. doi: 10.1073/pnas.91.4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varner JA, Cheresh DA. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–30. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien V, Frisch SM, Juliano RL. Expression of the integrin alpha 5 subunit in HT29 colon carcinoma cells suppresses apoptosis triggered by serum deprivation. Exp Cell Res. 1996;224:208–13. doi: 10.1006/excr.1996.0130. [DOI] [PubMed] [Google Scholar]
- 33.Vitale M, Illario M, Di Matola T, et al. Integrin binding to immobilized collagen and fibronectin stimulates the proliferation of human thyroid cells in culture. Endocrinology. 1997;138:1642–8. doi: 10.1210/endo.138.4.5052. [DOI] [PubMed] [Google Scholar]
- 34.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–21. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 35.Spector I, Shochet NR, Kashman Y, et al. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–5. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- 36.Coue M, Brenner SL, Spector I, et al. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–8. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 37.Yarmola EG, Somasundaram T, Boring TA, et al. Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J Biol Chem. 2000;275:28120–7. doi: 10.1074/jbc.M004253200. [DOI] [PubMed] [Google Scholar]
- 38.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene. 2001;20:4995–5004. doi: 10.1038/sj.onc.1204554. [DOI] [PubMed] [Google Scholar]
- 39.Tsagaraki I, Tsilibary EC, Tzinia AK. TIMP-1 interaction with alphavbeta3 integrin confers resistance to human osteosarcoma cell line MG-63 against TNF-alpha-induced apoptosis. Cell Tissue Res. 2010;342:87–96. doi: 10.1007/s00441-010-1025-1. [DOI] [PubMed] [Google Scholar]
- 40.Zou L, Ding Z, Roy P. Profilin-1 overexpression inhibits proliferation of MDA-MB-231 breast cancer cells partly through p27kip1 upregulation. J Cell Physiol. 223:623–9. doi: 10.1002/jcp.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calderwood DA, Ginsberg MH. Talin forges the links between integrins and actin. Nat Cell Biol. 2003;5:694–7. doi: 10.1038/ncb0803-694. [DOI] [PubMed] [Google Scholar]
- 42.Kim DY, Ingano LA, Kovacs DM. Nectin-1alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem. 2002;277:49976–81. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 43.Ruoslahti E. Fibronectin and its alpha 5 beta 1 integrin receptor in malignancy. Invas Metast. 1994;14:87–97. [PubMed] [Google Scholar]
- 44.Qian F, Zhang ZC, Wu XF, et al. Interaction between integrin alpha(5) and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem Biophys Res Commun. 2005;333:1269–75. doi: 10.1016/j.bbrc.2005.06.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Breast cancer cell lines MDA-MB-231and MCF-7 transient transfected with Pfn1 or its control plasmidand the total protein lysates subjected to WB. (B)MDA-MB-231 transient transfected with Pfn1 siRNA and its nonsense(NS) control and the efficiency of siRNA determined by WB. Cellviability under STS stimuli detected and quantified with the CCK-8assay. (C) Immunoblot assays showing PARP cleavage inMDA-MB-231 with or without Pfn1 knockdown under STS treatment.(D) Pfn1–468 and Mock Cells grown on polyHEME-coatedsurfaces and harvested at the appointed time; WB analysis ofpro-PARP and pro-caspase3 with these cell extracts shown.


