Abstract
In this study, the underlying mechanisms of the potential anti-inflammatory properties of allyl-isothiocyanate (AITC) were analysed in vitro and in vivo. Murine RAW264.7 macrophages stimulated with lipopolysaccharide (LPS) were supplemented with increasing concentrations of AITC. In addition, C57BL/6 mice (n= 10 per group) were fed a pro-inflammatory high-fat diet and AITC was administered orally via gavage for 7 days. Biomarkers of inflammation were determined both in cultured cells and in mice. AITC significantly decreased tumour necrosis factor α mRNA levels and its secretion in LPS stimulated RAW264.7 macrophages. Furthermore, gene expression of other pro-inflammatory markers including interleukin-1β and inducible nitric oxide synthase were down-regulated following AITC treatment. AITC decreased nuclear p65 protein levels, a subunit of the transcription factor NF-κB. Importantly, our data indicate that AITC significantly attenuated microRNA-155 levels in LPS-stimulated RAW264.7 macrophages in a dose-dependent manner. The anti-inflammatory effects of AITC were accompanied by an increase in Nrf2 nuclear translocation and consequently by an increase of mRNA and protein levels of the Nrf2 target gene heme-oxygenase 1. AITC was slightly less potent than sulforaphane (used as a positive control) in down-regulating inflammation in LPS-stimulated macrophages. A significant increase in nuclear Nrf2 and heme-oxygenase 1 gene expression and only a moderate down-regulation of interleukin-1β and microRNA-155 levels due to AITC was found in mouse liver. Present data suggest that AITC exhibits potent anti-inflammatory activity in cultured macrophages in vitro but has only little anti-inflammatory activity in mice in vivo.
Keywords: inflammation, allyl-isothiocyanate, sulforaphane, Nrf2, NF-κB, microRNA-155
Introduction
AITC is an aliphatic isothiocyanate that is derived from its precursor sinigrin, a widely distributed glucosinolate present in different brassica species including mustard, horseradish and wasabi [1]. Furthermore, AITC is also used as a food additive and flavouring agent [2]. Only few studies have dealt with the cell signalling activity of AITC (for chemical structure: Fig. 1) in cultured cells suggesting potential anti-inflammatory properties [3-5].
Fig 1.
Chemical structure of (A) allyl-isothiocyanate (AITC) and (B) sulforaphane (SFN).
We have previously shown that secondary plant metabolites such as the flavonol quercetin may affect inflammatory microRNA gene expression in macrophages [6]. Furthermore, our data indicate that dietary natural antioxidants, such as α-tocopherol, may affect microRNA levels also in laboratory rodents in vivo[7].
MicroRNAs represent a class of evolutionary conserved small non-coding RNAs of ∼22 nucleotides length that post-transcriptionally modulate the expression of multiple target genes and are thus implicated in a wide array of cellular processes [8-10]. Recently, microRNAs have been identified as potential regulators of the immune response [11]. In studies in microRNA-155 (miR-155) knockout mice, miR-155 was found to be essential for development of intact B- and T-lymphocyte function [12] and T cell differentiation [13]. miR-155 is a component of the macrophage response to different types of inflammatory mediators, such as LPS [9]. However, to the best of our knowledge, the role of miR-155 regarding potential anti-inflammatory effects of AITC has yet not been systematically investigated.
Unlike AITC, the anti-inflammatory properties of sulforaphane (SFN) are well-documented. SFN has been shown to inhibit tumour necrosis factor α (TNF-α)–mediated activation of NF-κB in THP1 human monocytes [14]. In Helicobacter pylori– infected mice, SFN treatment resulted in attenuated gastritis symptoms [15]. Furthermore, SFN down-regulated gene expression of inducible nitric oxide synthase (iNOS) as well as cyclooxygenase 2 (COX-2) and lowered TNF-α secretion in cultured cells, thereby exhibiting anti-inflammatory activity [16, 17]. However, data on potential anti-inflammatory effects of other isothiocyanates is rare. Potential anti-inflammatory properties of isothiocyanates may be mediated by NF-κB and Nrf2 signalling pathways [18].
Therefore, in this study we examined the anti-inflammatory properties of AITC, in comparison to SFN (used as a positive control) in the context of Nrf2, NFκB and miR-155 signalling both in cultured cells and in vivo.
Materials and methods
Cell culture
RAW264.7 macrophages (obtained from the Institute of Applied Cell Culture, Munich, Germany) from murine origin, were cultured in Dulbecco's modified Eagle's medium high glucose (4.5 g/l) containing sodium pyruvate (110 mg/l) supplemented with 10% (v/v) foetal bovine serum (non-heat inactivated), 4 mmol/l L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (all PAA, Coelbe, Germany) and grown in a humidified incubator at 37°C and 5% CO2. For cell culture studies, 100 mmol/l stock solutions of the test compounds in DMSO were prepared and stored at −80°C until further use. AITC and SFN were obtained from Sigma (Deisenhofen, Germany). LPS from Salmonella enterica serotype enteritidis (Sigma) was dissolved in phosphate buffered saline (PBS) to a stock solution of 1 mg/ml and stored at −20°C until further use. For all cell culture assays, vehicle controls have been performed and did not affect any of the parameters measured.
Cytotoxicity
The Neutral Red Assay [19,20] was used to determine the cell viability after incubation with the different test compounds. RAW264.7 macrophages were seeded for 24 hrs and treated with 1, 5 and 10 μmol/l AITC and SFN for 24 hrs, respectively. In brief, the culture medium containing the test substances was replaced with fresh serum-containing medium, including 60 μg/ml of Neutral Red (Carl Roth, Karlsruhe, Germany). After incubation for 2 hrs, the medium was removed and the cells were extracted using a solution comprising 50:49:1 (v/v/v) ethanol, water and glacial acetic acid. The absorbance was measured in a plate reader (Labsystems, Helsinki, Finland) at 540 nm.
RNA isolation and real time PCR
RAW264.7 macrophages were seeded in a 12-well plate for 24 hrs. Subsequently, cells were co-treated with 10 ng/ml LPS and 1, 5 and 10 μmol/l AITC and SFN for 1 or 6 hrs, respectively. Liver samples from AITC-treated and control mice were stored in RNAlater (Qiagen, Hilden, Germany) at −20°C until analysis. RNA was isolated using TRIsure (Bioline, Luckenwalde, Germany) according to manufacturer's instructions. Primers for murine genes (TNF-α, IL-1β, iNOS, HO-1) were designed by Primer3 software and ordered from Eurofins MWG Operon, Ebersberg, Germany (Table 1). Real time PCR was performed with a SensiMix one-step kit (Quantace, Berlin, Germany). In RAW264.7 cells, murine β-actin served as housekeeping gene. In animals three housekeepers were measured (18SRNA, β—actin, GAPDH).
Table 1.
Primer sequences of murine tumour necrosis factor α (TNF-α), interleukin 1β (IL-1β), inducible nitric oxide synthase (iNOS), heme-oxygenase 1 (HO-1), β-actin, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18SRNA for real-time PCR measurements
| Gene | Sequence (5′–3′) |
|---|---|
| TNF-α | F CATCTTCTCAAAATTCGAGTGACAA |
| R TGGGAGTAGACAAGGTACAACCC | |
| IL-1β | F CAGGCAGGCAGTATCACTCA |
| R AGCTCATATGGGTCCGACAG | |
| iNOS | F GGCAGCCTGTGAGACCTTTG |
| R GCATTGGAAGTGAAGCGTTTC | |
| HO-1 | F GAGCCTGAATCGAGCAGAAC |
| R AGCCTTCTCTGGACACCTGA | |
| β–actin | F GACAGGATGCAGAAGGAGATTACT |
| R TGATCCACATCTGCTGGAAGGT | |
| GAPDH | F CCGCATCTTCTTGTGCAGT |
| R GGCAACAATCTCCACTTTGC | |
| 18SRNA | F CCTGCGGCTTAATTTGACTC |
| R AACTAAGAACGGCCATGCAC |
miR-155 measurement
miR-155 levels were determined by two-step real time-PCR. Reverse transcription reaction was performed with specific microRNA primers. Real time PCR amplification was carried out with a Rotorgene 3000 machine (Corbett) as described previously [6, 7] Relative microRNA concentrations are given as the ratios between the amount of the target gene and the endogenous control snoRNA-202 (mouse).
ELISA
TNF-α was determined in cell culture supernatants (24 hrs) after appropriate dilution by the mouse TNF-α Duo Set ELISA development kit (R&D Systems, Wiesbaden, Germany).
Western blotting
For HO-1 detection, RAW264.7 macrophages were co-treated with 10 ng/ml LPS and test compounds (10 μmol/l) for 24 hrs. For p65, Nrf2 and Bach1 measurements, the cells were co-incubated with 10 ng/ml LPS and 10 μmol/l of the test compounds for six hrs. Whole cell and nucleic extracts were prepared as previously described [21]. For preparation of nuclear extracts from mouse liver, ∼ 100 mg liver tissue was homogenized in 500 μl buffer A [10 mmol/l HEPES (pH 7.9), 10 mmol/l KCl, 1.5 mmol/l MgCl2, 0.5 mmol/l dithiothreitol (DTT), 0.1 % Nonidet-P40, complete protease inhibitor cocktail (Sigma, Deisenhofen, Germany)], incubated on ice for 15 min. Following centrifugation (1 min, 4 °C, 4000X g), the supernatant (cytosolic extract) was removed and the remaining pellet was resuspended in 300 μl of ice-cold buffer B [40 mmol/l HEPES (pH 7.9), 400 mmol/l KCl, 1 mmol/l DTT, 312.5 mmol/l NaCl, 10 % glycerol and protease inhibitors], left on ice for 30 min. and centrifuged at 18,000 χg at 4°C for 30 min. Protein concentrations of cell and tissue extracts were determined with the BCA assay (Pierce, Bonn, Germany) according to manufacturer's instructions. Forty micrograms of protein of each sample were separated on either a 10% or 12% SDS-PAGE. Subsequently, the samples were transferred onto a PVDF membrane and blocked with 5% (w/v) skim milk dissolved in TBS+0.05% (v/v) Tween-20 (TBST) for at least 1 hr and probed with a HO-1 (1:1000; Stressgen, MI, USA), Bach1, Nrf2 or p65 (1:500; Santa Cruz, Heidelberg, Germany) at 4°C over night. Following, the membranes were incubated with a secondary antibody (1:4000 anti-rabbit) for 45 min. and the bands were visualized by using ECL reagent (Pierce) in a ChemiDoc XRS system (BioRad, Munich, Germany). The calculated molecular weight of Nrf2 is 68 kD, but it runs at single or double bands slightly above 100 kD, due to posttranslational modification [22,23].
Feeding study
Twenty female C57BL/6 mice (Janvier, St Berthevin Cedex, France) with an initial body weight of 17.2 ± 0.8 g were divided into two groups of 10 animals and fed a high-fat diet (modified TD88137 western diet; ssniff Special Diets, Soest, Germany) based on corn starch (14.5%), casein (17.1%), sucrose (32.8%) and butter fat (21.2%), containing 1.25% cholesterol and 0.5% sodium cholate per kg for 8 weeks. The diet was supplemented with 7.6 g calcium, 4.6 g phosphorus, 3.7 g sodium, 0.8 g magnesium, 5.4 g potassium, 48 mg iron, 23 mg manganese, 39 mg zinc, 11 mg copper, 0.28 mg iodine, 0.14 mg selenium, 0.02 mg cobalt, 15,000 IU vitamin A, 1500 IU vitamin D3, 20 mg vitamin E, 20 mg vitamin K (menadione), 1.03 g vitamin C, 16 mg thiamin (B1), 16 mg riboflavin (B2), 18 mg pyridoxin (B6), 30 μg cobalamin (B12), 45 mg nicotinic acid, 55 mg pantothenic acid, 19 mg folic acid, 305 μg biotin, 2.04 g choline chloride, 80 mg inositol per kg. After seven weeks either 15 mg/kg body weight AITC or PBS as solvent control was orally administered via gavage for seven days. The animals were housed under conventional conditions with a room temperature of 23 ± 3°C and a 12-hrs day/night cycle. Diets and water were provided ad libitum and live weight was recorded weekly. Mice were kept according to the German Regulations for Animal Welfare approved by the Ministerium für Landwirtschaft, Umwelt und ländliche Räume [No. V312-72241.121-33(88-7/09); MLUR, Kiel, Germany]. After 8 weeks, animals were anaesthetized with carbon dioxide and killed by cervical dislocation.
Statistical analysis
Statistical analysis was conducted using PASW Statistics 18 (IBM; Chicago, IL, USA). Data were analysed for normality of distribution (Kolmogorov–Smirnov); ln-transformation was performed if data were not normally distributed. ANOVA with an LSD (homogeneous variances) or Games-Howell (heterogeneous variances) post-hoc test was performed. Animal data were tested for normality of distribution (Kolmogorov–Smirnov) and ln-transformation was performed if data were not normally distributed. Normally distributed data were compared by t-test, not normally distributed data were compared by the non-parametric Mann–Whitney U-test. Data are expressed as mean ± S.E.M. Significance was accepted at P < 0.05.
Results
Cell viability
AITC and SFN were not cytotoxic up to a concentration of 10 μmol/l (data not shown).
AITC and SFN inhibited the NF-κB target genes TNF-α, IL-1β and iNOS
AITC and SFN significantly decreased TNF-α mRNA levels (Fig. 2A) as well as TNF-α secretion in LPS stimulated RAW264.7 macrophages (Fig. 2B). Furthermore, both IL-1β (Fig. 2C) and iNOS (Fig. 2D) gene expression were down-regulated by AITC and SFN treatments.
Fig 2.
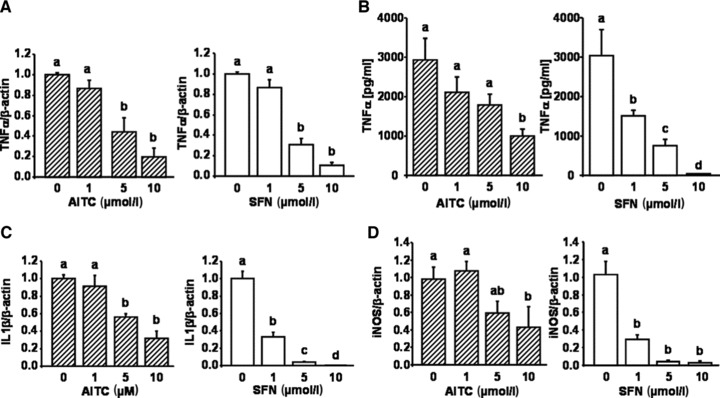
Effect of allyl-isothiocyanate (AITC) and sulforaphane (SFN) on biomarkers of inflammation in murine macrophages. RAW264.7 murine macrophages were incubated with increasing concentrations (1, 5 and 10 μmol/l) of AITC and SFN and stimulated with 10 ng/ml LPS for one hr. Following RNA isolation TNF-α mRNA levels were measured by real time PCR (A). In addition, TNF-α secretion into the cell culture medium was measured by ELISA in RAW264.7 cells treated with 10 ng/ml LPS and increasing concentrations (1, 5 and 10 μmol/l) of AITC and SFN for 24 hrs (B). mRNA levels of IL-1β (C) and iNOS (D) were examined with real-time PCR in LPS-induced murine macrophages incubated with 1, 5 and 10 μmol/l AITC and SFN for six hrs, respectively. Each bar represents the mean (±S.E.M.) of at least two independent experiments measured in duplicate. Means without a common letter differ significantly (P < 0.05).
AITC and SFN inhibited p65 translocation
A 6-hrs treatment of RAW264.7 cells with 10 ng/ml LPS caused an increase of p65-protein in the nucleus. This increase was lowered following incubation with 10 μmol/l AITC and SFN, respectively (Fig. 3).
Fig 3.
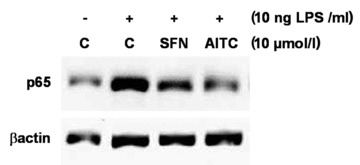
Effect of allyl-isothiocyanate (AITC) and sulforaphane (SFN) on NF-κB nuclear translocation. Protein levels of p65, a subunit of the transcription factor NF-κB, were measured by Western blotting in nucleic extracts from RAW264.7 macrophages incubated with the test compounds (10 μmol/l) and stimulated with LPS (10 ng/ml) for six hrs. One representative Western blot is shown.
Effects of AITC and SFN on miR-155
miR-155 levels were significantly induced through treatment of RAW264.7 cells with LPS (10 ng/ml). As presented in Figure 4, LPS-induced miR-155 levels were significantly down-regulated by both AITC and SFN in a dose-dependent manner.
Fig 4.
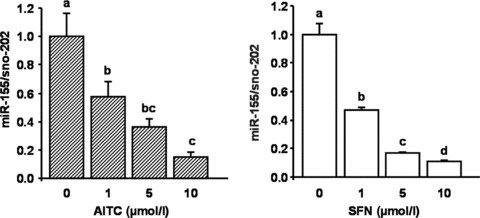
Effect of allyl-isothiocyanate (AITC) and sulforaphane (SFN) on LPS-induced miR-155 levels in murine RAW264.7 macrophages. Cells were incubated with 10 ng/ml LPS and increasing concentrations (1, 5 and 10 μmol/l) of AITC and SFN for six hrs. Each bar represents the mean (±S.E.M.) of at least two independent experiments measured in duplicate. Means without a common letter differ significantly (P < 0.05).
AITC and SFN increase nuclear Nrf2 levels and induce HO-1 gene expression
AITC and SFN treatments of RAW264.7 macrophages resulted in an increase of nuclear Nrf2 protein levels. The induction of Nrf2 nuclear translocation by AITC and SFN (Fig. 5) was accompanied by an increase in HO-1 gene expression (Fig. 6A). The induction of HO-1 by AITC and SFN was also reflected on its protein level as shown in Figure 6B. Under the conditions investigated, SFN was more potent than AITC in inducing HO-1 gene expression.
Fig 5.
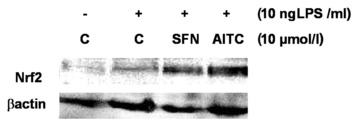
Effect of allyl-isothiocyanate (AITC) and sulforaphane (SFN) on Nrf2 nuclear translocation. Protein levels of Nrf2 were measured by Western blotting in nucleic extracts from RAW264.7 macrophages incubated with AITC and SFN (10 μmol/l) stimulated with LPS (10 ng/ml) for 6 hrs. One representative Western blot is shown.
Fig 6.
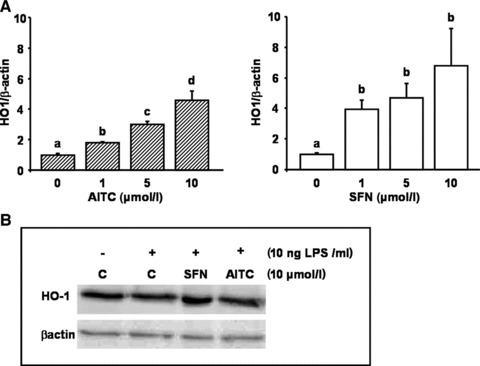
Effect of allyl-isothiocyanate (AITC) and sulforaphane (SFN) on heme-oxygenase 1 (HO-1) mRNA and protein levels. LPS-stimulated murine macrophages were treated with 1, 5 and 10 μmol/l of AITC and SFN for 6 hrs. Following RNA isolation, HO-1 mRNA levels were determined via real-time PCR (A). Each bar represents the mean (±S.E.M.) of at least two independent experiments measured in duplicate. Means without a common letter differ significantly (P < 0.05). HO-1 protein levels were measured in whole cell extracts generated from LPS-treated RAW264.7 macrophages incubated with 10 μmol/l AITC and SFN for 24 hrs (B). One representative Western blot is shown.
Nuclear Bach1 levels were not affected by AITC and SFN treatments in our RAW264.7 macrophages (data not shown).
Effects of AITC in mice fed a pro-inflammatory high-fat diet
No significant changes in feed intake (1.89 ± 0.46 versus 1.61 ± 0.57 g/d) and body weight gain (2.04 ± 0.57 versus 1.87 ± 0.48 g) between control and AITC-treated mice were observed during the study period. A 7-day-oral-application of AITC in mice fed a high-fat diet resulted in an increase in nuclear Nrf2 protein levels in the liver (Fig. 7C). This increase in nuclear Nrf2 protein was accompanied by a significant increase in Nrf2 and HO-1 mRNA levels (Fig. 7A and B). Administration of AITC resulted also in a moderate but not significant down-regulation of IL-1β, iNOS and miR-155 levels (Fig. 8A–C) in mouse liver. TNF-α mRNA levels were not affected by AITC treatment (data not shown). In an independent experiment, mice were fed either a low-fat standard or a high-fat diet for 8 weeks resulting in an increase of IL-1β liver mRNA levels in the high-fat diet as compared to the low-fat standard diet group. This observation is in line with literature data [24, 25], indicating an induction of pro-inflammatory markers in mice consuming a high-fat diet.
Fig 7.
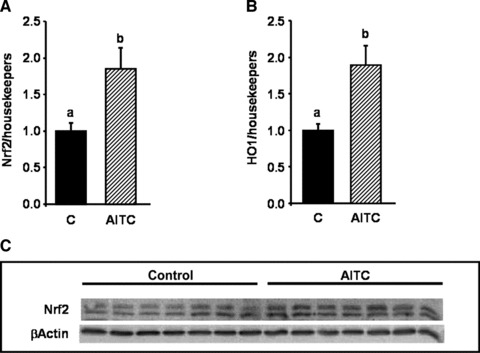
Effect of allyl-isothiocyanate (AITC) on Nrf2 and heme-oxygenase 1 (HO-1), in mice fed a pro-inflammatory high-fat diet. Female C57BL/6 mice were fed a pro-inflammatory high-fat diet for eight weeks. After seven weeks, the mice were applied 15 mg AITC per kg body weight or PBS (control) via oral gavage daily for seven days. Following RNA extraction from mouse liver levels of Nrf2 (A) and HO-1 (B) were measured by real-time PCR. Each bar represents the mean (±S.E.M.) from nine or 10 animals. Means without a common letter differ significantly (P < 0.05). Nuclear Nrf2 levels were determined in nucleic extracts derived from mouse liver via Western blotting (C). One representative Western blot with seven animals per group is shown.
Fig 8.
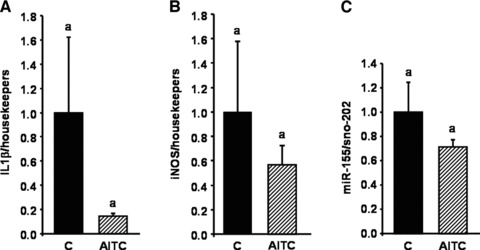
Effect of allyl-isothiocyanate (AITC) on IL-1β, iNOS and miR-155 in mice fed a pro-inflammatory high-fat diet. Female C57BL/6 mice were fed a pro-inflammatory high-fat diet for eight weeks. After seven weeks, the mice were applied 15 mg AITC per kg body weight or PBS (control) via oral gavage daily for seven days. Following RNA extraction from mouse liver levels of IL-1β (A), iNOS (B) and miR-155 (C) were measured by real-time PCR. Each bar represents the mean (±S.E.M.) from nine or 10 animals. Means without a common letter differ significantly (P < 0.05).
Discussion
There is increasing interest in the anti-inflammatory activity of isothiocyanates. Current data clearly indicate that AITC down-regulates inflammatory gene expression in LPS-stimulated murine macrophages in vitro. Although AITC was slightly less potent than SFN in down-regulating various biomarkers of inflammation it was less (∼ 25%) cytotoxic at concentrations >20 μmol/l (data not shown). As far as the concentration of the test compounds is concerned, the administered lower SFN concentrations are, by far, in the physiological range [26]. To the best of our knowledge, plasma AITC values have yet not been reported, although based on urinary excretion, a high bioavailability of AITC has been suggested [27, 28].
Under the conditions investigated, LPS treatment of RAW264.7 macrophages resulted in an up-regulation of miR-155. Our results in murine macrophages regarding the LPS-mediated induction of miR-155 are in accordance with previous findings in human THP1 cells [10]. In macrophages and monocytes, a broad range of inflammatory mediators increased miR-155 expression via MyD88 (myeloid differentiation factor 88) or TRIF (TIR-domain-containing adapter-inducing interferon-β) signalling [14]. Furthermore, the expression of miR-155 is regulated via NF-κB and JNK signalling [11]. Importantly, the translation of TNF-α is induced by miR-155 [29] and the transfection of miR-155 enhanced the expression of iNOS in murine macrophages [30].
Thus, we hypothesize that the inhibition of TNF-α production and iNOS gene expression due to AITC and SFN, as observed in this study, may be partly mediated by a miR-155 – dependent mechanism. To prove the role of AITC in miR-155 – mediated signal transduction pathways, further experiments, which ultimately silence miR-155 in the absence and presence of AITC, are warranted. Ippoushi et al. found a decrease of LPS-induced iNOS expression but at the same time an increase in TNF-α production in LPS-stimulated J774.1 macrophages treated with organosulfur compounds [31]. These results are partly in contrast with the present findings. Differences between our and literature data may be related to differences in the cell type (RAW264.7 versus J774.1 cells) and/or LPS concentrations (10 ng/ml versus 10 μg/ml) used.
AITC treatment of RAW264.7 cells resulted in a significant increase in Nrf2 levels, a transcription factor, which is known to antagonize NF-κB – dependent gene expression [32]. Nrf2 nuclear translocation due to AITC treatment was accompanied by a significant induction of HO-1, which has been previously shown to mediate anti-inflammatory activity [33, 34]. Collectively, the regulation of Nrf2, NF-κB and miR-155 due to AITC may have contributed to a down-regulation of inflammatory gene expression in our cultured macrophages.
O'Connell et al. recently demonstrated a significant decrease in Bach1 mRNA levels in RAW264.7 cells overexpressing miR-155 [35]. However, in this study nuclear Bach1 levels remained unchanged by LPS as well as AITC and SFN treatment. Thus, the isothiocyanate-mediated induction of Nrf2 and its target gene HO-1 in our RAW264.7 cells was possibly not mediated via changes in nuclear Bach1 levels.
Similar to our results in cultured RAW264.7 cells in vitro AITC also increased nuclear Nrf2 protein levels and HO-1 gene expression in mouse liver in vivo. Furthermore, we found significantly higher Nrf2 mRNA levels in our AITC supplemented mice as compared to control mice. It has been previously shown that Nrf2 regulates its own gene expression on the mRNA-level [36]. Interestingly, IL-1β, iNOS and miR-155 levels were 80%, 40% and 30% lower in the AITC treated mice as compared to the controls. However, this difference was not statistically significant most likely due to a high interindividual variation between animals within one group. Discrepancies between the in vitro and in vivo data may be also related to differences in the cell type (macrophages versus hepatocytes) investigated. Furthermore, cultured macrophages were activated with LPS, known to be a potent pro-inflammatory stimulus, whereas in the mouse model inflammation was induced due to a high-fat diet, a rather moderate stimulus of inflammatory gene expression. Mice were supplemented only for 7 days with AITC. It needs to be established if and to what extent long-term AITC administration and higher AITC doses may affect Nrf2 signalling and inflammatory gene expression in vivo.
In conclusion, this data indicate that AITC exhibits potent anti-inflammatory properties in cultured macrophages in vitro but only moderate anti-inflammatory effects in the livers of mice in vivo, and emphasizes the importance of verifying cell culture data using appropriate in vivo models.
Acknowledgments
A.E.W., C.B.-S. and G.R. designed the study. G.S. designed and approved the animal trial. A.E.W., C.B.-S. and J.D. conducted research and analysed data. A.E.W. and G.R. wrote the paper. The authors thank Anne-Rose Nissen, Annegret Roesen and Gaby Steinkamp for excellent technical help. We are grateful to the DFG Cluster ‘Inflammation at Interfaces’ and the Danone foundation for financial support. The funding bodies had no role in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol Nutr Food Res. 2010;54:127–35. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EFSA. Scientific opinion on the safety of allyl isothiocyanate for the proposed uses as food additive. EFSA Journal. 2010;8:40. [Google Scholar]
- 3.Woo HM, Kang JH, Kawada T, et al. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007;80:926–31. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Jeong WS, Kim IW, Hu R, et al. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004;21:661–70. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 5.Ernst IM, Wagner AE, Schuemann C, et al. Allyl-, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol Res. 2011;63:233–40. doi: 10.1016/j.phrs.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Boesch-Saadatmandi C, Loboda A, Wagner AE, et al. Effect of quercetin and its metabolites isorhamnetin and quercetin-3-glucuronide on inflammatory gene expression: role of miR-155. J Nutr Biochem. 2011;22:293–9. doi: 10.1016/j.jnutbio.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Gaedicke S, Zhang X, Schmelzer C, et al. Vitamin E dependent microRNA regulation in rat liver. FEBS Lett. 2008;582:3542–6. doi: 10.1016/j.febslet.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Valencia-Sanchez MA, Liu J, Hannon GJ, et al. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–24. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 9.O'Connell RM, Taganov KD, Boldin MP, et al. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–9. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taganov KD, Boldin MP, Chang KJ, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsitsiou E, Lindsay MA. microRNAs and the immune response. Curr Opin Pharmacol. 2009;9:514–20. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thai TH, Calado DP, Casola S, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–8. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 14.Moon DO, Kim MO, Kang SH, et al. Sulforaphane suppresses TNF-alpha-mediated activation of NF-kappaB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3. Cancer Lett. 2009;274:132–42. doi: 10.1016/j.canlet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Yanaka A, Fahey JW, Fukumoto A, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila Pa) 2009;2:353–60. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 16.Heiss E, Herhaus C, Klimo K, et al. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–15. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 17.Wierinckx A, Breve J, Mercier D, et al. Detoxication enzyme inducers modify cytokine production in rat mixed glial cells. J Neuroimmunol. 2005;166:132–43. doi: 10.1016/j.jneuroim.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Jakubikova J, Sedlak J, Bod'o J, et al. Effect of isothiocyanates on nuclear accumulation of NF-kappaB, Nrf2, and thioredoxin in caco-2 cells. J Agric Food Chem. 2006;54:1656–62. doi: 10.1021/jf052717h. [DOI] [PubMed] [Google Scholar]
- 19.Babich H, Zuckerbraun HL, Wurzburger BJ, et al. Benzoyl peroxide cytotoxicity evaluated in vitro with the human keratinocyte cell line, RHEK-1. Toxicology. 1996;106:187–96. doi: 10.1016/0300-483x(95)03189-m. [DOI] [PubMed] [Google Scholar]
- 20.Valacchi G, Rimbach G, Saliou C, et al. Effect of benzoyl peroxide on antioxidant status, NF-kappaB activity and interleukin-1alpha gene expression in human keratinocytes. Toxicology. 2001;165:225–34. doi: 10.1016/s0300-483x(01)00430-9. [DOI] [PubMed] [Google Scholar]
- 21.Wagner AE, Ernst I, Iori R, et al. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp Dermatol. 2010;19:137–44. doi: 10.1111/j.1600-0625.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 22.Kang KW, Lee SJ, Park JW, et al. Phosphatidylinositol 3-kinase regulates nuclear translocation of NF-E2-related factor 2 through actin rearrangement in response to oxidative stress. Mol Pharmacol. 2002;62:1001–10. doi: 10.1124/mol.62.5.1001. [DOI] [PubMed] [Google Scholar]
- 23.Boesch-Saadatmandi C, Wagner AE, Graeser AC, et al. Ochratoxin A impairs Nrf2-dependent gene expression in porcine kidney tubulus cells. J Anim Physiol Anim Nutr (Berl) 2009;93:547–54. doi: 10.1111/j.1439-0396.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- 24.Luyendyk JP, Sullivan BP, Guo GL, et al. Tissue factor-deficiency and protease activated receptor-1-deficiency reduce inflammation elicited by diet-induced steatohepatitis in mice. Am J Pathol. 176:177–86. doi: 10.2353/ajpath.2010.090672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wouters K, van Gorp PJ, Bieghs V, et al. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–86. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 26.Ye L, Dinkova-Kostova AT, Wade KL, et al. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 27.Ioannou YM, Burka LT, Matthews HB. Allyl isothiocyanate: comparative disposition in rats and mice. Toxicol Appl Pharmacol. 1984;75:173–81. doi: 10.1016/0041-008x(84)90199-6. [DOI] [PubMed] [Google Scholar]
- 28.Bollard M, Stribbling S, Mitchell S, et al. The disposition of allyl isothiocyanate in the rat and mouse. Food Chem Toxicol. 1997;35:933–43. doi: 10.1016/s0278-6915(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 29.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–4. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Zhao Q, Matta R, et al. Inducible nitric-oxide synthase expression is regulated by mitogen-activated protein kinase phosphatase-1. J Biol Chem. 2009;284:27123–34. doi: 10.1074/jbc.M109.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ippoushi K, Itou H, Azuma K, et al. Effect of naturally occurring organosulfur compounds on nitric oxide production in lipopolysaccharide-activated macrophages. Life Sci. 2002;71:411–9. doi: 10.1016/s0024-3205(02)01685-5. [DOI] [PubMed] [Google Scholar]
- 32.Liu GH, Qu J, Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim Biophys Acta. 2008;1783:713–27. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Ashino T, Yamanaka R, Yamamoto M, et al. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol Immunol. 2008;45:2106–15. doi: 10.1016/j.molimm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Cheung KL, Khor TO, Kong AN. Synergistic effect of combination of phenethyl isothiocyanate and sulforaphane or curcumin and sulforaphane in the inhibition of inflammation. Pharm Res. 2009;26:224–31. doi: 10.1007/s11095-008-9734-9. [DOI] [PubMed] [Google Scholar]
- 35.O'Connell RM, Rao DS, Chaudhuri AA, et al. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205:585–94. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak MK, Itoh K, Yamamoto M, et al. Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol. 2002;22:2883–92. doi: 10.1128/MCB.22.9.2883-2892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


