Abstract
Irradiation impacts on the viability and differentiation capacity of tissue-borne mesenchymal stem cells (MSC), which play a pivotal role in bone regeneration. As a consequence of radiotherapy, bones may develop osteoradionecrosis. When irradiating human bone-derived MSC in vitro with increasing doses, the cells’ self-renewal capabilities were greatly reduced. Mitotically stalled cells were still capable of differentiating into osteoblasts and pre-adipocytes. As a large animal model comparable to the clinical situation, pig mandibles were subjected to fractionized radiation of 2 χ 9 Gy within 1 week. This treatment mimics that of a standardized clinical treatment regimen of head and neck cancer patients irradiated 30 χ 2 Gy. In the pig model, fractures which had been irradiated, showed delayed osseous healing. When isolating MSC at different time points post-irradiation, no significant changes regarding proliferation capacity and osteogenic differentiation potential became apparent. Therefore, pig mandibles were irradiated with a single dose of either 9 or 18 Gy in vivo, and MSC were isolated immediately afterwards. No significant differences between the untreated and 9 Gy irradiated bone with respect to proliferation and osteogenic differentiation were unveiled. Yet, cells isolated from 18 Gy irradiated specimens exhibited a reduced osteogenic differentiation capacity, and during the first 2 weeks proliferation rates were greatly diminished. Thereafter, cells recovered and showed normal proliferation behaviour. These findings imply that MSC can effectively cope with irradiation up to high doses in vivo. This finding should thus be implemented in future therapeutic concepts to protect regenerating tissue from radiation consequences.
Keywords: mesenchymal stem cells, osseous regeneration, fracture healing, radiation, osteoradionecrosis
Introduction
Radiation therapy (RT) bears the risk of osteoradionecrosis, which is the most dreaded adverse side effect in treatment of head and neck cancer, one of the most common cancers worldwide [1, 2]. During osteoradionecrosis, impairment of fibroblastic activity plays a decisive role as the bony matrix is gradually converted into fibrous tissue [3, 4]. In parallel, osteoblastogenesis becomes dysregulated leading to insufficient proliferation of osteoblasts. In consequence, this impediment often results in a high rate of myofibroblast proliferation within irradiated bone and surrounding tissues. It is generally assumed that irradiation causes a direct damage of tissue-borne multi-potent progenitor cells. In fact, due to the massive generation of reactive oxygen species (ROS), it leads to the destruction of vascular endothelium. This in turn initiates an acute immune response through cytokine release followed by an increased production of ROS via the recruitment of phagocytes. Vascular thrombosis and endothelial cell destruction eventually results in necrosis of microvascular structures, local ischaemia and consequently, tissue loss [4]. Surprisingly however, irradiated bone can be supported by exogenously applied growth factors (although the response of cells residing in bone is decreased and their supply through a degenerating vascular system remains greatly compromised) [5]. Also application of bone morphogenetic proteins is capable of enhancing bone regeneration of irradiated bone [6]. Similar to other cells, stromal cells and osteoblastic precursor cells respond to this type of cytokines [7].
ROS produced may also adversely affect the survival of stromal cells, which in many tissues contain a rare species of tissue-borne multi-potential cells, often called MSC [8]. Several studies reported that patients, who underwent RT and bone marrow transplantation thereafter, exhibited successful homing and engraftment of haematopoietic precursors to irradiated bone marrow, although infused bone marrow stromal cells refrained from doing so [9]. After treatment this cell type was found to be exclusively host derived [10, 11], indicative that MSC exhibit radioresistance. However, it remained elusive whether these cells can still exert proper functions or have lasting defects. Indeed, pelvic RT in the course of carcinoma therapy raises the risk of a hip fracture three-fold compared to the normal healthy population [12]. As of now, it is widely believed that injury to the vasculature and microenvironment affects the niches of stem cells during the recovery period [13]. Whether dormant MSC withstand radiation-induced damage and are thus able to sustainingly contribute to wound healing and tissue regeneration after irradiation, is scarcely documented [14]. To remain as close as possible to a clinical situation, we investigated this open question in pigs as a large animal experimental model. Surprisingly MSC appear to exert high radioresistant activities in vivo, hence tissue-resident MSC should be considered a potential target for future therapeutic concepts and innovative pharmaceutical formulations to effectively promote bone healing after irradiation treatment.
Materials and methods
Isolation and cultivation of MSC
Human MSC were isolated from iliac bone biopsies, cultivated in long-term culture as described previously [15]. MSC from Sus scrofa domestica were harvested from cancellous and compact bone as well as from the periosteum of the mandible and the iliac crest. Samples were reduced in size to approximately 20–100 mm3 under sterile conditions. The specimens were stored in growth medium [minimum essential medium (MEM; GIBCO-BRL, Lofer, Austria) containing 10% foetal calf serum (FCS; Invitrogen, Lofer, Austria), 100 units/ml penicillin and 100 μg/ml streptomycin] and transported at room temperature. In a sterile work cabinet, the liquid was removed and the bone pieces were inserted into a pipette tip with its tapering end sitting in a 1.5 ml reaction tube, both within a 15 ml tube. This tube was centrifuged for 1 min. at 400 χ g to collect the marrow. After centrifugation, the remaining pieces were treated with collagenase (2.5 mg/ml in growth medium; Sigma-Aldrich, Vienna, Austria) for 2–3 hrs at 37°C (Heraeus, Graz, Austria), 20% O2 and 5% CO2 to render cells free from the tight extracellular meshwork covering the bony surface. The treated specimens were again centrifuged for 1 min. at 400 χ g. The cell pellet was resuspended in growth medium as described by gentle aspiration through syringe needles of different gauge sizes. In case of agglomeration of bone, bone marrow and cells, the fluid was separated from the rest by means of a 100-μm nylon mesh filter. Thereafter, the resuspended cells were loaded on a Ficoll-Paque Plus® gradient (Amersham Biosiences, Freiburg, Germany) and centrifuged at 2500 χ g for 30 min. The ratio between the resuspended cells containing liquid and those harvested after the Ficoll-Paque Plus® gradient was 1:1. The cells were harvested from the interphase (density <1.075 g/ml). To remove the Ficoll-Paque Plus®, the cells were washed with growth medium and recovered by centrifugation at 1500 χ g for 15 min. The purified cells were further cultivated at 3% O2 and 5% CO2 (Thermo Electron Corporation, Graz, Austria) at a cell density of 0.2–0.5 χ 106 cell/cm2. After 24 hrs, the non-adherent cell fraction was removed by washing twice with PBS at 37°C. The medium was changed every 3–4 days. After the primary culture had reached approximately 30–50% confluence, the culture medium was removed and the cells were washed twice with PBS for 3 min. at 3% O2. Thereafter, PBS was removed and the cells were treated with 0.05% trypsin/1 mM ethylenediaminetetraacetic acid (GIBCO, Lofer, Austria) for 5 min. at 37°C. Cells were harvested, washed once in media and further expanded at a density of 50 cells/cm2 to assess colony formation or expansion in long-term culture. In the course of long-term culture, the number of population doublings during every passage was accounted.
Irradiation of cultivated MSC
Cells were grown in 25 cm2 flasks (2 cm in height) to a confluence of 50% and treated with 6 MeV photons, which are commonly used in clinical radiotherapy for treatment of cancer patients (ELEKTA Synergy Linear Accelerator; serial number: 131431, ELEKTA Oncology Systems installed at the Department of Therapeutic Radiology and Oncology/Medical University Innsbruck). For radiation of cell probes, an experimental setup was chosen which guaranteed broadly homogeneous dose delivery to the cell probes. The flasks were completely filled with medium. Four flasks were placed on a staple of Perspex as well as surrounded by Perspex and covered with a slab of 1 cm super flab material at a source–surface distance of SSD ∇ 100 cm. Cell probes were irradiated with energy doses from 3 to 18 Gy.
After irradiation, the medium was removed leaving 7 ml behind, and cells were incubated at atmospheric conditions of 3% O2 and 5% CO2. After 4 days, osteogenic differentiation was started. After 5 days, cells from a separate flask were trypsinized as described and seeded to assess the colony formation.
Flow cytometric analysis
Cell viability of human MSC was examined with the aid of an argon laser-equipped flow cytometer (FACSCanto; Becton Dickinson, Vienna, Austria) by monitoring propidium iodide (PI) fluorescence together with monitoring forward and side scattering as described previously [16]. Briefly, staining was performed as follows: cells were washed with PBS and stained. 2.5 μg/ml PI was applied for 15 min. at room temperature. Cells were washed and resuspended in 300 μl PBS, and subjected to flow cytometry analysis. The sum of G1, S and G2 can be estimated as 100% (excluding debris) as to compare the cell cycle phase distribution with decreased proliferation. Data were analysed with the aid of CellQuest Software (Becton Dickinson). Cell cycle phases were assessed 4 days post-irradiation by analysing the amount of nuclear DNA by staining permalized cells with propidium iodide: cells were detached from the dishes as described and resuspended in de-ionized water containing 0.1% Triton X-100 and 50 μg/ml propidium iodide [17]. The proportions of each cell cycle stage were calculated with the aid of Cell Quest Pro (BD Biosciences, Vienna, Austria).
For cell surface marker analysis of porcine MSC, cells were seeded at density of 50 cells/cm2 and expanded at 3% O2 for 2 weeks. Prior to flow cytometric analysis, cells were trypsinized, counted and aliquots comprising 100,000 cells were further treated. Cells were washed with PBS, and centrifuged at 400 χ g for 2 min. The cell pellet was re-suspended in 50 μl PBS, and cells were incubated with 20 μl antibody and 20 μg/ml 7-aminoactinomycin D (7-AAD; 1 mg/ml) at 4°C for 30 min. All antibodies used here were labelled with the fluorophore phycoerythrin: anti-human CD11b (msIgG1, kappa; clone ICRF44, 0.05 mg/ml; BioLegend), anti-human CD90 (msIgG1, kappa; clone 5E10, 0.02 mg/ml; Becton Dickinson) and anti-CD105 (msIgG2a, clone MEM-229, 1 mg/ml; Abcam). After antibody binding, cells were washed and resuspended in 300 μl PBS, and subjected to flow cytometric analysis (Software: FACSDiva; FACSCanto). Only 7-AAD negative cells were subjected to further analysis.
Adipogenic, osteogenic and chondrogenic differentiation in vitro
Human MSC were stimulated to differentiate in vitro as described previously [15]. Differentiation capacity was assessed in duplicates by initially growing cells for 10 days at 3% O2 and 5% CO2 (Thermo Electron Corporation) and 37°C in growth medium, and thereafter incubating the cells for 21 days with 10 mM β-glycerol phosphate disodium salt pentahydrate (Fluka, Vienna, Austria), 10 nM dexamethasone (Sigma-Aldrich), and 50 μg/ml 2-phospho-L-ascorbic acid tri-sodium salt (Fluka) in growth medium at 37°C, 20% O2 and 5% CO2 (Hera Cell 240; Heraeus). Eventually, the cultures were fixed with 4% para-formaldehyde in PBS. After 5 min. the specimens were washed twice with PBS, pH 4. Then the cell layer was stained with Alizarin RedS, pH 4.1 for 20 min. Excessive stain was removed by several washing steps with PBS, pH 4. For further analysis, the specimen was kept in PBS, pH 4.0. The following classification was used to determine the differentiation grade: grade 3-more than 60% of cells engulfed by mineralized matrix; grade 2—40–60%; grade 1—less than 40%; grade 0—no differentiation in reference to negative control [15].
For multi-lineage differentiation of porcine MSC, the following details were applied: cells were seeded at density of 50 cells/cm2 and expanded under atmospheric conditions of 3% O2, 5% CO2 for 2 weeks. For differentiation, cells were cultured at 20% O2, and treated for 3 weeks with adipogenic (DMEM high glucose supplemented with 15% horse serum, 100 nM dexamethasone and 1 mM sodium pyruvate) [18], or osteogenic differentiation medium (growth medium supplemented with 10 nM dexamethasone, 50 μg/ml 2-phospho-L-ascorbic acid tri-sodium salt, 10 mM β-glycerolphosphate disodium salt pentahydrate) [19]. For subsequent histological analysis, cells were fixed with 4% para-formaldehyde in PBS for 15 min., stained with 0.7% Oil Red O for adipogenesis or 2% Alizarin Red S for osteogenesis.
To assess the chondrogenic differentiation capacity, 500,000 cells were pelleted in 15 ml reaction tubes and treated with differentiation medium (DMEM high glucose supplemented with 10 nM dexamethasone, 37.5 μg/ml 2-phospho-L-ascorbic acid trisodium salt, 1χ ITS+, 10 ng/ml transforming growth factor-β and 1 mM sodium pyruvate) [18], or growth medium (negative control) for 4 weeks at 20% O2. Pellets were fixed in 4% para-formaldehyde for 20 min. Before embedding in paraffin, the pellets were stained with toluidine blue for a few minutes to ease visualization of the specimen. The sections were treated either with alcian blue and counterstained with iron haematoxylin, or with an anti-collagen type II antibody (msIgG1, kappa, clone COLL-II; Chemicon, Molsheim, France). After detection of bound antibody by means of a secondary horseradish peroxidase labelled antibody, the sample was counterstained with Mayer’s haemalum.
Colony formation
Cells were seeded at a density of 50 cells/cm2 in triplicates, and grown at atmospheric conditions of 3% O2, 5% CO2. After 14 days, cells were washed with PBS, fixed in acetone/methanol (1:1) and stained with 2% crystal violet for 20 min. Excess stain was removed with tap water, and colonies were counted manually.
RT-PCR analysis
RNA was isolated from two 10 cm dishes with the aid of RNA Isolation Kit Mini (Qiagen, Hilden, Germany). For RT-PCR analysis, cDNA was synthesized from total RNA using RevertAid H Minus-MMuLV-RT (Fermentas, St. Leon-Roth, Germany) and oligo(dT) primer (MWG, Munich, Germany). The assays were performed on a LightCycler 480 instrument (Roche, Vienna, Austria) with the LC480-SYBR Green I Master kit (Roche). The 16 μl reactions contained 0.9 μM forward and reverse primers. After the activation of the enzyme at 95°C for 8 min., 50 cycles at 95°C for 15 sec., 57°C for 8 sec. and 72°C for 15 sec. were performed. mRNA expression levels were calculated relative to the housekeeping gene GAPDH. Primer sequences for porcine GAPDH, osteocalcin (osteogenesis) and adipocyte fatty acid-binding protein 4 (adipogenesis), aggrecan and Sox9 (chondrogenesis) were as reported previously [18]. qPCR was performed in duplicates. As a control, cells treated with growth medium under the same conditions were used.
Mandible irradiation
All animal experiments were performed based on permission by the Austrian Government and National Ethics Committee (permission number: BMBWK-66.011/0143-BrGT/2006) and conducted in concordance to the EU directive 86/609/EEC.
To ensure broadly homogenous dose delivery in the targeted volume of pig mandible in vivo, irradiation treatment was performed according to a standardized clinical workflow: first, the pig’s head and in particular the jaws, separated with a bite block and placed in treatment position were examined by computed tomography (CT); next with the aid of the clinical treatment planning system, PrecisePLAN® (ELEKTA Oncology Systems, Crawley, UK) a standardized three-dimensional model was compiled on the basis of the CT data set to correctly juxtapose two opposing wedged fields of 7 χ 3 cm, thereby yielding a widely homogenous dose distribution during irradiation. Pig mandibles were irradiated with energy doses of 9 Gy and 1 week later another fraction of 9 Gy, which altogether corresponds to a biologically effective dose (BED) of 60 Gy applying the linear quadratic model [20, 21]. BED is an approximate quantity by which different radiotherapy fractionation regimens can be intercompared. It builds on the generally accepted assumption that full repair occurs between fractions [22] and as a consequence thereof the biological effect of each fraction exerts an equivalent effect [23, 24]. Proper positioning of the radiation field was controlled by generating electronic portal images of each radiation field.
Under general anaesthesia, a total of 20 pigs underwent an iatrogenic unilateral mandibule fracture. The fractures were stabilized with reconstruction plates and locking screws in general anaesthesia (Synthes 2.4; Synthes Austria, Salzburg, Austria). In the irradiation group (16 pigs), the fracture was set 4 weeks after irradiation, which followed the protocol described earlier. The irradiated pigs were sacrificed either right after irradiation, or 4, 5, 6, 8 or 12 weeks after irradiation. The non-irradiated pigs were sacrificed 1, 2, 4 and 8 weeks after the surgical treatment.
Histology
Biopsies were embedded in Technovit 9100 Neu (Heraeus Kulzer, Hanau, Germany) as described previously [7]. Performing the sawing and grinding technique, described by Donath et al. [25], histological sections of the fracture gaps were prepared with a thickness of 12 μm in average. Toluidine blue O staining was performed to assess the healing capacity of the irradiated and non-irradiated fracture sites.
Results
MSC are rapidly proliferating when cultured in media containing high proportions of foetal bovine serum. When grown at low density, MSC readily form colonies, and they are capable of differentiating into multiple lineages when induced by appropriate means.
After treatment with increasing doses of ionizing radiation, cultured human MSC, which had been derived from bone of systemically healthy individuals and which bear the above-mentioned properties, were tested regarding cell cycle dynamics (Fig. 1A), cell death (Fig. 1B), colony forming capacity (Fig. 1C), cellular morphology (Fig. 1D), as well as adipogenic and osteogenic differentiation potential (Fig. 1E and F). Notably, with increasing doses of radiation, a decrease of G1 phases with a concomitant increase in cell destruction became apparent. Although a slight increase in S phases was noticed at effective doses up to 18 Gy, the later dose also induced a high level of cell death. Furthermore, at doses of 12 Gy and higher no colony formation was observed after reseeding irradiated cells (Fig. 1C). However, when receiving 12 Gy or more, cells still survived with most cells exhibiting a flat and large cellular phenotype, reminiscent of stress-induced premature senescent cells (Fig. 1D). However, this type of cells could actually be further differentiated into adipogenic (Fig. 1E) and osteogenic precursors (Fig. 1F) when incubated in appropriately stimulating media. Although increasing doses of radiation resulted in adipogenic differentiation potential, which decreased in a dose-dependent fashion, osteogenesis was found enhanced after irradiation; also doses up to 12 Gy showed no impact on mineralization of the extracellular space. These data suggest that irradiation with biologically effective doses of 9 Gy and higher induce irreversible damage in MSC, which are contained in cultivated MSC cultures.
Fig 1.
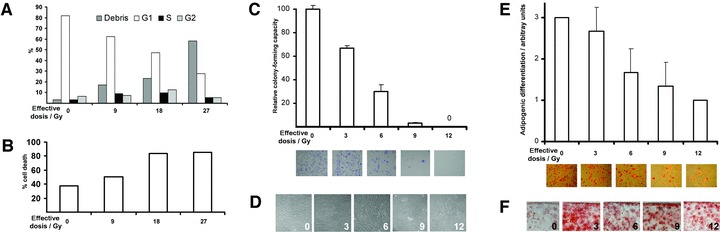
Irradiation of in vitro cultivated human mesenchymal stromal cells derived from cancellous bone of the iliac crest. (A) Irradiation of proliferating cells with the indicated dosage showed an impact on cell cycle progression of the surviving cell fraction and cell survival (B). (C) Colony formation was decreased after irradiation treatment (assessed in duplicates; a representative example displayed in the lower part of panel C). (D) High-power photomicrographs depicting cells after irradiation (doses in Gy) as indicated. (E) Adipogenic differentiation potential decreased after irradiation in vitro at indicated dosages (measured in triplicates; representative examples of Oil Red stained photomicrographs in the lower part of E); for specification of arbitrary units, see Materials and methods. (F) Osteogenic differentiation capacity was enhanced after irradiation up to doses as high as 12 Gy, shown as a set of representative examples of Alizarin Red stained photomicrographs. Experimental results in bar graphs are shown as mean ± standard deviation.
These initial findings were corroborated with MSC derived from mandibular bone of pigs, which similarly to human counterparts show firm adherence to plastic, fibroblastoid appearance, clonogenic growth, a comparable immune phenotype albeit constricted due to the lack of specific antibodies with proven cross-reactivity in swine and tri-lineage differentiation potential (Fig. 2). In contrast to what has been experienced with cultivated human MSC, subjecting explanted porcine MSC to increasing doses of irradiation in vitro resulted in a gradual loss of osteogenic differentiation potential (Fig. 3). In course of the in vivo experiment, in which the jaws of experimental animals were subjected to fractionated radiation of 2 χ 9 Gy, which closely resembles the BED of a standardized clinical treatment regimen for cancer therapy [22-24], we observed that an artificial fracture, which had been deliberately generated and subsequently treated with osteosynthesis plates and screws, showed delayed osseous healing indicating that the clinically applied irradiation yielded biological effective doses high enough to compromise subsequent regenerative processes and healing (Fig. 4).
Fig 2.
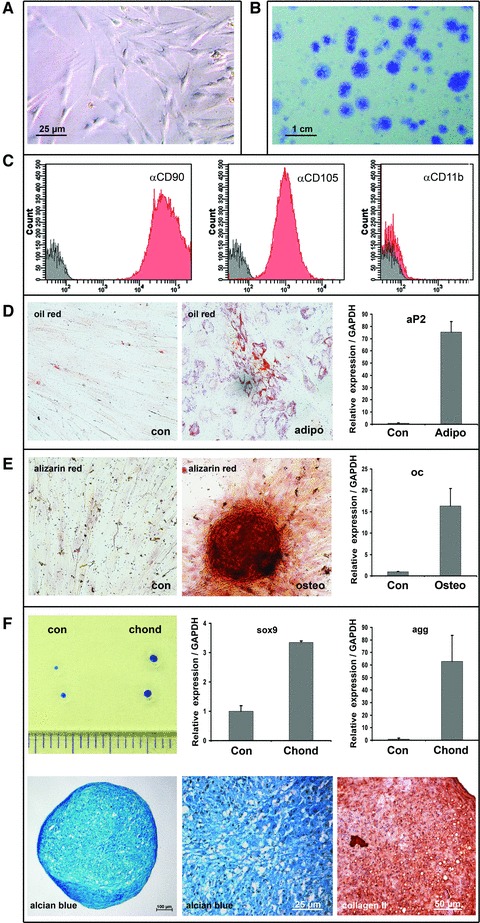
Properties of in vitro cultured porcine MSC. Fibroblastoid cells, which exhibited firm plastic adherence (A), clonogenic growth (B), a respective immune phenotype (C) and tri-lineage differentiation capacity (D–F), were isolated from mandibular bone, bone marrow and periosteum as well as from cancellous bone of the iliac crest. (C) Immune phenotyping of porcine MSC was performed taking only 7-aminoactinomycin D-negative cells into account; negative controls comprise unstained cells. Fractions highlighted in grey comprise cells stained solely with 7-AAD; fraction shown in red are cells stained with the indicated monoclonal antibody. (D) Differentiation capacity was shown for adipogenic potential (adipo), both applying histological staining with oil red, and quantitative RT-PCR analysis for the molecular marker aP2 and un-induced control cells (con). (E) Osteogenic potential (osteo) was proven by staining cultures with alizarin red S and the expression of the osteogenic marker osteocalcin (oc). (F) Chondrogenic differentiation (chond) could be demonstrated in pellet culture: in case of induced MSC the aggregates expanded in size (upper left panel), and stimulated MSC activated chondrogenic markers such as sox 9 and aggrecan (agg) (upper row, centre and right side). Paraffin sections of the aggregate culture efficiently bound the histological dye alcian blue (lower row, left and centre panels) and showed collagen II expression after immunohistochemical evaluation with a specific antibody. Experimental results in bar graphs are shown as mean ± standard deviation.
Fig 3.
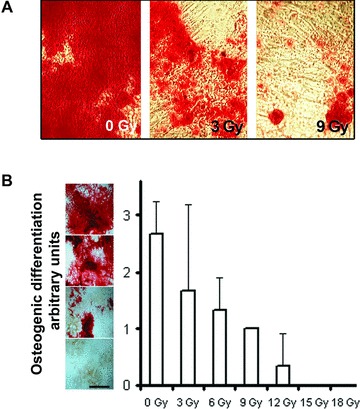
Culture and in vitro osteogenic differentiation of primary porcine mesenchymal stromal cells. (A, B) Osteogenic differentiation potential decreased after irradiation in vitro at the indicated dosage (arbitrary units were distinguished as depicted left side to the graph depicted in (B) grade 3–more than 60% of cells engulfed by mineralized matrix; grade 2—40–60%; grade 1—less than 40%; grade 0—no differentiation in reference to negative control scale bar equals 1 cm. Experimental results in bar graphs are shown as mean ± standard deviation.
Fig 4.
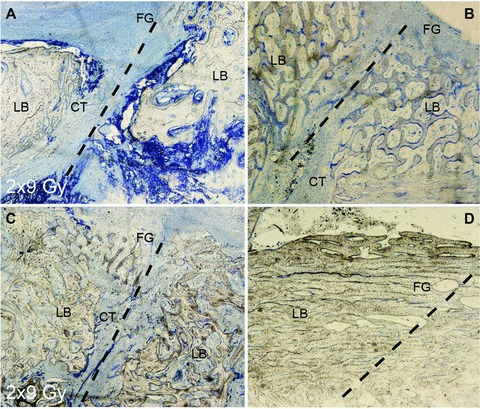
Fracture healing in irradiated mandible of Sus scrofa domestica. Bone healing was investigated 8 weeks after a fracture gap was set. Samples (A) and (C) were irradiated, (B) and (D) are untreated controls. Representative examples of slow or poor healing (A, B) juxtaposed to a more rapid course (C, D) in both irradiated (A, C) and control mandibular bone; dashed line marks the former edge of the fracture gap (FG); connective tissue (CT) stains blue, local bone is labelled LB. Experimental results in bar graphs are shown as mean ± standard deviation.
This observation prompted us to consider in vivo irradiated MSC as an indicator for vitality after treatment. Similar to the observations in vitro, MSC suffer damage, resulting in attenuation of cell intrinsic osteogenic differentiation capacity in vivo. To determine the impact of ionizing radiation on MSC that are borne within bone and bone marrow, mandible biopsies were taken from living animals directly after irradiation with a single dose of 9 or 18 Gy, the latter accounting for a BED of 60 Gy. Notably, long-term proliferation capacity of isolated MSC, which survived the radiation treatment, was comparable to those of non-irradiated counterparts (Fig. 5A). MSC, which had been isolated directly after the animals received a 9 Gy dose, exhibited no difference in colony forming capability, and also osteogenic potential was comparable to non-irradiated controls (Fig. 5B and C). These results suggested that a 9 Gy treatment is tolerated in as much pig mandible and surrounding tissue still contained viable cells, although at decreased numbers, which still exhibited long-term proliferation potential and osteogenic differentiation capacity. Moreover, the tissue showed no obvious signs of exuberant necrosis and only mild enduring inflammation became apparent. Yet, after irradiation with a single dose of 18 Gy MSC numbers were greatly reduced (Fig. 5B) and although isolated MSC still exhibited uninterrupted long-term proliferation potential (Fig. 5A), cellular properties were greatly affected as their osteogenic differentiation potential was attenuated (Fig. 5C). Conspicuously, MSC tolerate irradiation up to 9 Gy.
Fig 5.
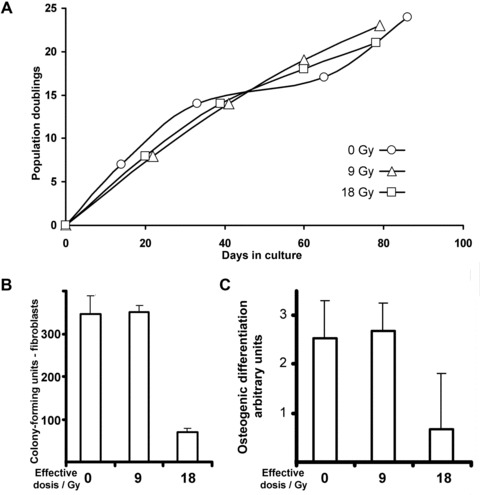
Properties of primary porcine mesenchymal stromal cells isolated from the mandible directly after irradiation with the indicated effective biological dosage. (A) The proliferation potential was monitored in long-term culture. (B) Colony formation was accounted in low-density secondary culture. (C) After irradiation and subsequent cultivation in the presence of osteogenic induction medium, the differentiation potential was assessed in triplicates (for grading, see left panel). For specification of arbitrary units, see Materials and methods. Experimental results in bar graphs are shown as mean ± standard deviation.
Therefore, the animals were treated with two fractions of 9 Gy separated by 1 week. This treatment accounts for a BED of 60 Gy, a dose, which is routinely applied in clinical radiation therapies. Next, the aforementioned experimental analyses were further corroborated by isolating MSC 4, 5 or 6 weeks post-radiation, showing that the respective MSC quantity in the animals receiving 18 Gy matched the same limited number of colony-forming units as observed in untreated controls (Fig. 6A and B). Notably, those cells also exhibited normal osteogenic differentiation potential (Fig. 6C). MSC were expanded as cell lines in long-term culture over the period of up to 100 days. The observed long-term proliferation capacities (Fig. 7) were indistinguishable from MSC isolated from bone marrow as well as from trabecular bone of non-treated control groups.
Fig 6.
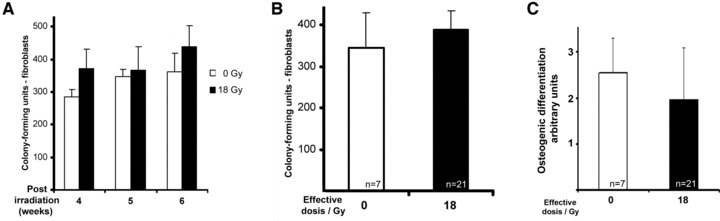
Clonogenic growth of porcine mesenchymal stromal cells isolated from the mandible after fractionated irradiation with 2 χ 9 Gy. (A) Colony formation of primary cultivated cells isolated 4–6 weeks post-irradiation and (B) integration of data accounted from all primary cultures isolated at the latter time points. (C) Integration of data accounted from all assessments of osteogenic differentiation with MSC isolated at time points indicated in (A). Specification of arbitrary units, see Materials and methods. Experimental results in bar graphs are shown as mean ± standard deviation.
Fig 7.
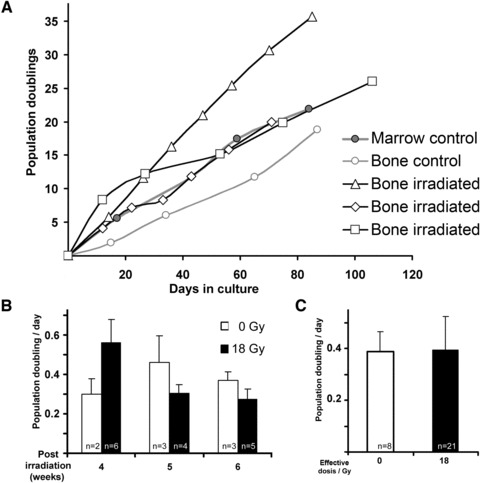
Proliferation potential of porcine mesenchymal stromal cells. (A) Growth kinetics of cells were isolated from trabecular bone of the mandible 4 weeks after fractionated irradiation with 2 χ 9 Gy, and grown in long-term culture (representative examples). As controls, MSC were also harvested from trabecular bone as well as bone marrow of unirradiated animals. (B) Proliferation index of cells isolated 4–6 weeks post-irradiation during their early stages of long-term cultures and integration of data accounted from all long-term cultivations; white bars show unirradiated controls, black bars are MSC isolated after radiation treatment with 18 Gy. (C) Integration of data accounted from all long-term cultures cultures isolated at the latter time points as indicated. Experimental results in bar graphs are shown as mean ± standard deviation.
Conclusively, these results indicate that irradiation of pig mandibles in a fashion likewise to a clinical routine treatment regimen in human induces retarded bone healing, yet endogenous MSC appear to remain unaffected.
Discussion
MSC exhibit high proliferation potential and multi-potent differentiation capacity [8]. In recent years, many scientists were able to isolate MSC from a large variety of specialized tissues. This naïve cell type could also be successfully differentiated in vitro into various tissue-specific precursors with phenotypes closely resembling somatic cell types, mainly of mesodermal origin. Inevitable damages during life-time, or other, yet intended harmful events during medical therapies may activate dormant stem cells in their niches, and it is also assumed that MSC contribute to the regeneration of bone and bone marrow after injury in vivo through proliferation and controlled differentiation.
Harmful biological effects of irradiation are mediated via highly reactive radicals, such as the water ion H2O+ or the hydroxyl radical OH˙, both of which are freely diffusible over cellular membranes and thus can damage any biomolecular entity, most important in this context DNA [26]. Cells are more radiosensitive during the M and G2 phase of the cell cycle, yet being most resistant in the late S phase [27]. The cell cycle of cancer cells is shorter than that of normal cells. Interestingly, cells residing in oxygenized tissues are two to three times more sensitive to radiation than cells at anoxic conditions [28], and in their regeneration phase after irradiation, normal somatic cells often proliferate faster.
In the treatment of head and neck cancer, osteoradionecrosis is a common consecutive complication of irradiation, which preferably occurs in the mandible [29]. It is conceivable to stimulate healing of irradiated bone through the application of cytokines such as bone morphogenetic protein (BMP), or vascular endothelial growth factor (VEGF) because it is well-known that osseous bone healing in vivo is greatly enhanced by such bioactive factors. It is generally accepted that MSC are responsive to BMP [30-33]. As the influence of irradiation on the fate and proliferation of MSC is only scarcely investigated, we first monitored the changing properties of cultured MSC, which were derived from porcine irradiated bone, by assessing their clonogenic growth potential, which after low-density seeding serves as a reliable method to quantify the cell pool that bears stem cell-like qualities. This is considered a good quantitative measure for the so-called stemness. Compared to non-irradiated controls, secondary colony-forming potential steadily decreased with increasing dosage, whereas the osteogenic differentiation of irradiated cells remained greatly unimpaired after the application of a high dose of 18 Gy, which corresponds to a BED of 60 Gy. These results are in good concordance with recently reported observations with murine MSC irradiated with 0, 2, 6 and 12 Gy in vitro [34]. The cellular proliferation was clearly diminished after application of 12 Gy, although adipogenic and osteogenic differentiation could still be achieved. It has however been reported that human MSC when irradiated with a single dose of 2, 4, 8 and 12 Gy in suspension and not as an adherent monolayer first ceased growth but restored their proliferation rate to normal levels after 2 weeks [35]. Osteogenic and adipogenic potentials were decreased with increasing doses of radiation [35]. Yet comparable in vivo data, in particular, results conveyable to a clinical situation are missing up to now.
Considering the fact that bone marrow is a complex in vivo environment with many interactions of different cell types at various stages of differentiation, and secondly the marrow cavity being a complex three-dimensional structure, a scene that could be hardly re-enacted with cells in culture, we next studied the fate of MSC after irradiation in vivo. For that purpose, pig mandibles were irradiated with either 9 or 18 Gy, dosages which resulted in a greatly delayed osseous healing at the site of an artificial fracture. To examine the rate of damage in tissue-borne MSC, the animals were sacrificed immediately after irradiation and cells were isolated. The self-renewal property and osteogenic potential of MSC was clearly diminished after irradiation with a dose of 18 Gy. Nine Gy had only little impact on the MSC, which is in stark contrast to our observation regarding radiation sensitivity of in vitro cultured human MSC. During common radiotherapy in our clinic, patients are subjected to a fractionized treatment regimen thereby receiving a BED of 60 Gy. Working along these lines, we expected that an equivalent dose would lead to a sustaining and lasting effect on MSC in the animal model. In line with this assumption, we actually accounted delayed bone healing during the recovery phase after treatment and also noticed 4 weeks post-radiation that the blood vessel density was greatly reduced in bone and muscle at irradiated sites (unpublished results). Yet, viable MSC could be successfully isolated at several time points up to 8 weeks post-radiation. The MSC number was comparable to non-irradiated control samples; their long-term proliferation potential closely resembled that of MSC from untreated bone and the cells also differentiated effectively along the osteogenic lineage. Given these observations, radiation sensitivity appears to be greatly attenuated in MSC in a large animal model in vivo, which may be either due to intrinsic preventive measures such as enhanced repair mechanisms, or due to exogenous protective means of the stem cell niche. Cellular mechanisms of radiotolerance have been proposed for MSC as they appear to exhibit high antioxidant ROS scavenging capacities together with an enhanced activity of the DNA double-strand break repair system [36].
Fibrosis is considered key in the development of irradiation-related changes of bone [37]. In this context, hypoxia does not appear to be critical but is more a consequence of fibrosis in irradiated tissue [4]. Yet this study suggests that tissues tarnished by irradiation contain fully functional MSC. It is therefore conceivable that these cells may still contribute to bone healing and regeneration and may also take an active part in supporting and regulating haematopoiesis thereby sustaining organismic immune function.
Consistent with the notion that MSC survive RT, it has been reported earlier that fibroblastic colony-forming units (CFU-F) reached normal values 25 days after whole body irradiation [38]. The notion of enhanced in vivo radioresistance of MSC has further been substantiated by the observation that mesenchymal cells remained host specific in patients, who after irradiation underwent allogeneic bone marrow transplantation, and virtually no transplanted stroma cell was capable of homing and engrafting into the recipient’s bone marrow; at the same time, the entire haematopoietic system could be restored by donor-derived cells [39].
Besides these observations yet another conceptional view emerged, which refers to the possibility that undisturbed MSC residing in distant body parts are being mobilized, and in a targeted fashion may engraft into lesioned tissues and empty niches. Mice that have been subjected to total body irradiation with 3.5 Gy and subsequently received hMSC intravenously showed indeed enhanced engraftment into bone marrow, muscle, brain, heart, lungs and liver when compared to unirradiated litter mates [40]. Similar observations have been reported after low-dose irradiation of tumours, where the recruitment of MSC into the tumour microenvironment was also increased [41].
A recently proposed concept [42, 43], which takes into account evidence that MSC-like cells reside in or close to the vessel wall, not only elegantly explains the broad tissue distribution of MSC. Yet in extrapolation of this privileged position, it is thus also conceivable that MSC contribute to vessel stability and, generally spoken, to tissue homeostasis [44]. In turn it is highly likely that during degeneration of blood vessels, MSC are being released into injured tissue. This is in line with our findings, which demonstrate that MSC themselves behave unaffected after irradiation [45]. It is further highly likely that in this case MSC become activated and proliferate whereby they also generate soluble bioactive factors. Besides increasing cellular mass, specifically secreted trophic and/or similarly active biomolecules secreted by MSC may in turn contribute to repair and/or regeneration of the injured tissue. By now, it became a well-accepted paradigm that MSC are capable of modulating immune surveillance, thus controlling negative interferences of intruding T- and B-lymphocytes within the injury site [45]. By this token, MSC may not only be a key in repair but even more in preservation of the affected tissue.
In conclusion, the here presented observations on cellular properties of MSC after irradiation encompass analyses performed in vitro, in vivo and ex vivo, clearly demonstrating that MSC bear in vivo radioprotective activities higher than commonly believed. This evidence supports the notion that tissue-resident MSC can be effectively induced to promote bone healing after irradiation treatment, and thus the radioprotective property of MSC should be further considered in the context of future therapeutic concepts. Further research is however required to determine whether the protective activity is based on intrinsic mechanisms or due to structural determinants of the niche or the surrounding tissues, or finally, whether MSC from undisturbed sites are being activated to migrate and engraft to irradiated lesions.
Acknowledgments
We are most grateful for the technical assistance by Christian Gritsch (deceased during the course of the study), and Shasta Pelzer for her professional work with histology. The support by the Jubilee Fund of the Austrian National Bank (OeNB, 2006, project number: 12246, title: Reversing impaired healing of irradiated bone by immobilized growth factors on nanostructured osteosynthesis material) and the industrial support by Synthes Austria are greatly acknowledged. G.L.’s work is supported by the Austrian Science Fund, by the EC through FP7 integrated project VascuBone and the Tyrolean Future Fund Translational Research Project ‘Smart Implants’ together with R.G.; R.B. is a DOC-fForte fellow of the Austrian Academy of Sciences.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Chrcanovic BR, Reher P, Sousa AA, et al. Osteoradionecrosis of the jaws—a current overview. Part 1: Physiopathology and risk and predisposing factors. Oral Maxillofac Surg. 2011;14:3–16. doi: 10.1007/s10006-009-0198-9. [DOI] [PubMed] [Google Scholar]
- 2.Teng MS, Futran ND. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg. 2005;13:217–21. doi: 10.1097/01.moo.0000170527.59017.ff. [DOI] [PubMed] [Google Scholar]
- 3.Bianchini C, Ciorba A, Pelucchi S, et al. Head and neck cancer: the possible role of stem cells. Eur Arch Otorhinolaryngol. 2008;265:17–20. doi: 10.1007/s00405-007-0478-7. [DOI] [PubMed] [Google Scholar]
- 4.Lyons A, Ghazali N. Osteoradionecrosis of the jaws: current understanding of its pathophysiology and treatment. Br J Oral Maxillofac Surg. 2008;46:653–60. doi: 10.1016/j.bjoms.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Arvidson K, Abdallah BM, Applegate LA, et al. Bone regeneration and stem cells. J Cell Mol Med. 2011;15:718–46. doi: 10.1111/j.1582-4934.2010.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer IN, Niehoff P, Acil Y, et al. BMP-2 and bFGF in an irradiated bone model. J Craniomaxillofac Surg. 2008;36:210–7. doi: 10.1016/j.jcms.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kloss FR, Gassner R, Preiner J, et al. The role of oxygen termination of nanocrystalline diamond on immobilisation of BMP-2 and subsequent bone formation. Biomaterials. 2008;29:2433–42. doi: 10.1016/j.biomaterials.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 9.Odorfer KI, Egerbacher M, Unger NJ, et al. Hematopoietic bone marrow cells participate in endothelial, but not epithelial or mesenchymal cell renewal in adult rats. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01216.x. ; doi: 10.1111/j.1582-4934.2010.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieger K, Marinets O, Fietz T, et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33:605–11. doi: 10.1016/j.exphem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Dickhut A, Schwerdtfeger R, Kuklick L, et al. Mesenchymal stem cells obtained after bone marrow transplantation or peripheral blood stem cell transplantation originate from host tissue. Ann Hematol. 2005;84:722–7. doi: 10.1007/s00277-005-1067-8. [DOI] [PubMed] [Google Scholar]
- 12.Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587–93. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Wu X, Frassica D, et al. Irradiation induces bone injury by damaging bone marrow microenvironment for stem cells. Proc Natl Acad Sci USA. 2011;108:1609–14. doi: 10.1073/pnas.1015350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alves H, Munoz-Najar U, De Wit J, et al. A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J Cell Mol Med. 2010;14:2729–38. doi: 10.1111/j.1582-4934.2009.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymla stem cells and prolongs their life span. Aging Cell. 2007;6:745–57. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Laschober GT, Brunauer R, Jamnig A, et al. Leptin receptor/CD295 is upregulated on primary human mesenchymal stem cells of advancing biological age and distinctly marks the subpopulation of dying cells. Exp Gerontol. 2009;44:57–62. doi: 10.1016/j.exger.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Nicoletti I, Migliorati G, Pagliacci MC, et al. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 18.Zou L, Lv N, Feng W. Characteristics of osteoblastic differentiation in mesenchymal stem cells from porcine bone marrow in vitro. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21:1222–7. [PubMed] [Google Scholar]
- 19.Bosch P, Pratt SL, Stice SL. Isolation, characterization, gene modification, and nuclear reprogramming of porcine mesenchymal stem cells. Biol Reprod. 2006;74:46–57. doi: 10.1095/biolreprod.105.045138. [DOI] [PubMed] [Google Scholar]
- 20.Lee SP, Leu MY, Smathers JB, et al. Biologically effective dose distribution based on the linear quadratic model and its clinical relevance. Int J Radiat Oncol Biol Phys. 1995;33:375–89. doi: 10.1016/0360-3016(95)00162-R. [DOI] [PubMed] [Google Scholar]
- 21.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 22.Jones B, Tan LT, Dale RG. Derivation of the optimum dose per fraction from the linear quadratic model. Br J Radiol. 1995;68:894–902. doi: 10.1259/0007-1285-68-812-894. [DOI] [PubMed] [Google Scholar]
- 23.Withers HR. Biologic basis for altered fractionation schemes. Cancer. 1985;55:2086–95. doi: 10.1002/1097-0142(19850501)55:9+<2086::aid-cncr2820551409>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Hall E. Radiobiology for the radiobiologist. 5th ed. Philadelphia: Lippincott William & Wilkins; 2000. [Google Scholar]
- 25.Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues: the Sge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–26. doi: 10.1111/j.1600-0714.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 26.Bolus NE. Basic review of radiation biology and terminology. J Nucl Med Technol. 2001;29:67–73. [PubMed] [Google Scholar]
- 27.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928–42. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007:241–8. doi: 10.1007/s10555-007-9056-0. [DOI] [PubMed] [Google Scholar]
- 29.Reuther T, Schuster T, Mende U, et al. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients-a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32:289–95. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 30.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 31.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 32.Diefenderfer DL, Osyczka AM, Reilly GC, et al. BMP responsiveness in human mesenchymal stem cells. Connect Tissue Res. 2003;44:305–11. [PubMed] [Google Scholar]
- 33.Satija NK, Gurudutta GU, Sharma S, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16:7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 34.Clavin NW, Fernandez J, Schonmeyr BH, et al. Fractionated doses of ionizing radiation confer protection to mesenchymal stem cell pluripotency. Plast Reconstr Surg. 2008;122:739–48. doi: 10.1097/PRS.0b013e318180edaa. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Kwong DL, Chan GC. The effects of various irradiation doses on the growth and differentiation of marrow-derived human mesenchymal stromal cells. Pediatr Transplant. 2007;11:379–87. doi: 10.1111/j.1399-3046.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen MF, Lin CT, Chen WC, et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys. 2006;66:244–53. doi: 10.1016/j.ijrobp.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 37.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73:119–31. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 39.Koc ON, Peters C, Aubourg P, et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol. 1999;27:1675–81. doi: 10.1016/s0301-472x(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 40.Francois S, Bensidhoum M, Mouiseddine M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020–9. doi: 10.1634/stemcells.2005-0260. [DOI] [PubMed] [Google Scholar]
- 41.Klopp AH, Spaeth EL, Dembinski JL, et al. Tumour irradiation increases the recruitment of circulating mesenchymal stem cells into the Tumour microenvironment. Cancer Res. 2007;67:11687–95. doi: 10.1158/0008-5472.CAN-07-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 43.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 44.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]


