Abstract
Accumulating evidence suggests that chronic stress can be a cofactor for the initiation and progression of cancer. Here we evaluated the role of endothelial nitric oxide synthase (eNOS) in stress-promoted tumour growth of murine B16F10 melanoma cell line in C57BL/6 mice. Animals subjected to restraint stress showed increased levels adrenocorticotropic hormone, enlarged adrenal glands, reduced thymus weight and a 3.61-fold increase in tumour growth in respect to no-stressed animals. Tumour growth was significantly reduced in mice treated with the β-antagonist propranolol. Tumour samples obtained from stressed mice displayed high levels of vascular endothelial growth factor (VEGF) protein in immunohistochemistry. Because VEGF can induce eNOS increase, and nitric oxide is a relevant factor in angiogenesis, we assessed the levels of eNOS protein by Western blot analysis. We found a significant increase in eNOS levels in tumour samples from stressed mice, indicating an involvement of this enzyme in stress-induced tumour growth. Accordingly, chronic stress did not promote tumour growth in eNOS−/− mice. These results disclose for the first time a pivotal role for eNOS in chronic stress-induced initiation and promotion of tumour growth.
Keywords: eNOS melanoma, stress, NOS isoforms, B16F10
Introduction
Behavioural stress is increasingly becoming an important aspect of daily life. We react to stress by activating a cascade of physiologic adaptive responses of the central and peripheral nervous system, namely the hypothalamic–pituitary–adrenal axis and the sympathetic nervous system. Inadequate, excessive and/or protracted responses can have adverse consequences on physiologic functions such as growth, metabolism, circulation, reproduction and immune response. It has been shown that chronic stress, defined as stress that persists for long time, has deleterious effects on cell-mediated immunity [1], appearance and/or complication of allergy [2], evolution of liver-related diseases such as viral hepatitis or cirrhosis [3] and other diseases. Stress is also accepted to constitute a relevant factor in the development of cancer [4-6]. The underlying mechanism is believed to involve immunological disfunction [7]. Furthermore, β-adrenergic activation of the cyclic AMP (cAMP)-protein kinase A (PKA) signalling pathway, leading to enhanced expression of pro-angiogenic factors and tumour vascularization, has been identified as a major mechanism in chronic stress-mediated promotion of tumour growth [8]. Tumour angiogenesis is regulated, among other factors, by nitric oxide, a gaseous pleiotropic free radical synthesized from l-arginine and oxygen by four major isoforms of nitric oxide synthase (NOS): neuronal NOS, eNOS, inducible NOS (iNOS) and mitochondrial NOS (mNOS) [9, 10]. Nitric Oxide promotes angiogenesis by increasing vasodilatation, vascular permeability, endothelial cell proliferation and migration. The pro-angiogenic activity of nitric oxide is mediated by activation of soluble guanylyl cyclase, leading to cyclic guanosine 3′,5′-monophosphate accumulation and activation of its target kinases and ion [11-13]. Specifically, eNOS was shown to modulate cancer-related events (angiogenesis, apoptosis, cell cycle, invasion and metastasis) and genetic studies showed that eNOS gene polymorphisms are associated with the development of multiple cancers [14-16]. eNOS activity is regulated by adrenaline [17-19]. We therefore hypothesized that eNOS could be involved in stress-mediated regulation of tumour growth and progression. Here we report results in a model, constituted by a murine melanoma (B16F10) in C57BL/6 mice.
Materials and methods
Cells
The murine melanoma cell line, B16F10, was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in Dulbecco's modified Eagle's minimal essential medium supplemented with glutamine, essential and nonessential amino acids, vitamins, antibiotics and 10% heat-inactivated foetal bovine serum (Gibco/Invitrogen, Grand Island, NY, USA). The cells were maintained at 37°C in a humidified atmosphere of 5% CO2. All experiments were performed with cultures grown for no longer than 6 weeks after recovery from frozen stocks.
Animals, tumour implantation and treatments
Thirty eight-week-old male C57BL/6 mice were obtained from Harlan (San Pietro al Natisone, Italy). eNOS−/− mice 20 were a kind gift of Dr. Sandro De Falco (CNR-IGB, Naples, Italy). Mice were housed five per cage and maintained on a 12 hrs light:12 hrs dark cycle (lights on at 7:00 a.m.) in a temperature-controlled room (22 ± 2°C) and with food and water ad libitum at all times. The experimental protocols were in compliance with the European Communities Council directive (86/609/EEC). After a 3 weeks of acclimation to the housing conditions, mice were distributed into three groups (10 animals/group). Two groups of animals received a pre-treatment with placebo (PBS) or with propranolol for 7 days and then exposed to a physical restraint (chronic stress) as described [20]. Briefly, 1 week before the tumour injections, we placed the animals in a conic tube (Falcon), perforated on the top and the back to allow their to breathe, 2 hrs daily for a total of 21 days. S-propranolol hydrochloride, a non-selective β-adrenoreceptor antagonist, was purchased from Sigma-Aldrich (St. Louis, MO, USA) and administered at 2 mg/kg pro-die. After pre-treatments, C57BL/6 mice were injected subcutaneously (s.c.) with a suspension of B16F10 cells (3 χ 105 cells/mouse in the right hind footpad). Treatments with placebo and propanolol continued for the following 21 days.
Tumour growth was measured every 3 days and expressed as volume, according to the formula V= (aχb2)/2, where a is the largest superficial diameter and b is the smallest superficial diameter. A control group of not stressed animals also received tumour cells injection and was handled and deprived of food and water in parallel for the same time period of stressed mice. Animals were sacrificed at the indicated times after tumour implantation and organs were dissected and analysed. The results derive from four independent experiments.
High-frequency ultrasound imaging
Imaging and measuring of adrenal glands were performed by Vevo2100 high-resolution ultrasound imaging system in B-mode using the MS-550d (centre operating frequency of 40MHz, axial resolution 40 μm) probe, which gives typical frame rates of 557, positioning the mice on the platform ventral side up. Shown results are representative of four independent experiments.
Magnetic resonance imaging
Animals were subjected to magnetic resonance imaging (MRI) at 1.5 T (Magnetom Symphony, Syngo MR 2002B, Siemens, Erlangen, Germany) and a phased array coil. Mice were placed in a supine, head first position. Pre-contrast sagittal and coronal T1-weighted two-dimensional spin-echo images (TR/TE 400/13 msec.; turbo factor 5; slice thickness 2 mm; gap 10 mm; matrix 205 χ 256; FOV 100 mm; acquisition time 4 min.) and coronal T2-weighted two-dimensional turbo spin echo images (TR/TE 4000/95 msec.; turbo factor 13; slice thickness 2 mm; gap 10 mm; matrix 205 χ 256; FOV 100 mm; acquisition time 4.47 min.) of whole body were obtained. Subsequently, sagittal and coronal T1-weighted images were acquired after i.v. injection of 300 μl of a positive paramagnetic contrast medium (gadodiamide, Omniscan; GE Healthcare, Oslo, Norway). Full examination imaging time was of approximately 15 min. Shown results are representative of four independent experiments.
Histology and immunohistochemistry (IHC)
Tumours were dissected from adult animals, fixed in 4% formalin overnight at 4°C and embedded in paraffin for sectioning using standard procedures. Sections of 4 μm were stained with haematoxylin and eosin. For IHC analyses, sections were incubated for 2 hrs at room temperature with the anti-vascular endothelial growth factor (VEGF) monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by incubations with saturating amounts of biotin-labelled secondary antibody and streptavidin-peroxidase for 20 min. each. After incubation in a solution containing 0.06 mM diaminobenzidine (Dako, Basturp, Denmark) and 2 mM hydrogen peroxide in 0.05% PBS (pH 7.6) for 5 min., slides were washed, dehydrated with alcohol and xylene and mounted with cover slips using a permanent mounting medium (Permount-Proscitech, Kirwan, Australia). Sections were examined with a Nikon Axiophoto microscope (Carl Zeiss Inc., Thornwood, NY, USA) and images were acquired at 200χ magnification. Shown results are representative of four independent experiments.
Western blotting
Tissues were homogenized in TNN buffer (50 mM Tris-pH 7.5, 150 mM NaCl, 0.5% NP40) supplemented with a protease inhibitor cocktail (Sigma-Aldrich) and protein extracts were obtained by using five cycles of freeze and thawing. Protein amount was determined by Bradford assay. Thirty micrograms of total protein were loaded in a SDS-PAGE and blotted on a nitrocellulose membrane. Nitrocellulose blots were blocked with 5% non-fat dried milk in TBS/Tween 20 (TBST) buffer (Tris-HCl pH 7.4, 20 mM, NaCl 500 mM and 0.01% Tween 20) and incubated with primary antibodies in TBST/5% milk overnight at 4°C. To detect VEGF-A protein levels we used a specific polyclonal antibody (Santa Cruz Biotechnology). eNOS protein levels were analysed by an anti-eNOS monoclonal antibody (BD-Biosciences). We used also an anti–β-actin monoclonal antibody, purchased from Sigma-Aldrich, to determine equal loading conditions. Immunoreactivity was detected by sequential incubation with horseradish peroxidase–conjugated secondary antibodies and enhanced chemiluminescence reagents (GE HealthCare, Piscataway, NJ, USA) following standard protocols. Scanning densitometry of the bands was performed with an Image Scan (SnapScan 1212; Agfa–Gevaert N.V.). The area under the curve related to each band was determined using Gimp2 software (http://gimp-win.sourceforge.net/index.html). Background was subtracted from the calculated values. Significance of differences between the groups was calculated by the Student's t-test. Results were obtained from tissue collected in single experiment.
Adrenocorticotropic hormone (ACTH) measure
Animals’ peripheral blood was collected by retrorbital sinus and ACTH levels were measured by a MODULAR ANALYTICS E 170 Module (Roche Diagnostics).
Statistical analysis
Normally distributed data were represented as mean ± S.E.M. One-way ANOVA and Bonferroni post-hoc analysis were used to examine the significance of differences among groups (Graph pad Prism 5.0). A probability value with P≤ 0.05 was considered to be statistically significant.
Results
Melanoma growth promotion by chronic stress involves β-adrenergic receptor signalling
In a murine model of ovarian carcinoma, β-adrenergic activation of the cAMP–PKA signalling pathway was identified as a major mechanism by which chronic stress can enhance tumour angiogenesis in vivo and thereby promote tumour growth [8]. We therefore verified the role of stress and β-adrenergic signalling in tumour growth in our model. In C57BL/6 mice carrying melanoma (B16F10) implants, we measured peripheral blood levels of ACTH. We found a significant increase in such levels in animals subjected to chronic (restraint) stress, compared to unstressed animals in three independent experiments (Fig. 1A). To assess the effect of stress on sympatho–adrenal–medullary activity, we measured the size of both adrenal glands by using Vevo2100 high-resolution ultrasound imaging system. Adrenal glands of stressed mice were larger (2.68 ± 0.32 mm) than those of control animals (1.81 ± 0.15 mm; P < 0.05). Moreover, the weight of thymuses in stressed animals was significantly lower than in control animals (P= 0.04; Fig. 1B). Graphs depict results from three separate experiments. We also evaluated the atrophy of prevalently B lymphoid organs such as liver and spleen and did not find significant differences in stressed versus control animals, confirming that chronic stress preferentially affected T, and not B, compartment [21, 22]. Then, to assess the effects of chronic stress on tumour progression, we evaluated the growth of B16F10 tumour cell implants in syngenic C57BL/6 stressed mice. As shown in Figure 2, tumour growth in chronically stressed mice was increased by 3.6-fold respect to control group; increase was 35.5-fold reduced (P < 0.0001) in animals treated with the non-specific β-antagonist propranolol. Consistent with these observations, MRI showed increased tumour growth in stressed mice respect to the other groups (Fig. 3) [23,24]. Thus, chronic stress appeared to greatly enhance tumour growth; such effect involved β-adrenergic receptor signalling. Images are representative of three separate experiments.
Fig 1.

Measurements of ACTH levels and weight of thymus in stressed mice. Measurements of ACTH levels on peripheral blood sera obtained from control and stressed animals (n= 10) revealed that the levels of ACTH in stressed+placebo mice are increased respect to control mice P < 0.05 data are represented as the mean ± S.E. (n= 10). On the contrary, in stressed+placebo mice the weight of thymus is lower than the control mice and propranolol-treated mice. Data are represented as the mean ± S.E.; P < 0.05 (n= 10). Ppl: propranolol; ACTH: adrenocorticotropic hormone.
Fig 2.
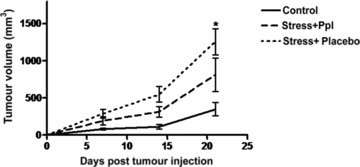
Effect of chronic stress on murine melanoma cancer growth. In vivo tumour growth of B16F10 cells was measured in C57BL/6 mice. The animals were distributed into three groups (10 animals/group). Two groups of animals received a pre-treatment with placebo (PBS) or with propranolol for seven days and then exposed to a physical restraint. After seven days, the animals were injected subcutaneously with 3 χ 105 cells/mouse in the right hind footpad B16F10 cells. Differences in growth were analysed by ANOVA (n= 10; P < 0.0001). Mean tumour volume ± S.E.M. are given.
Fig 3.
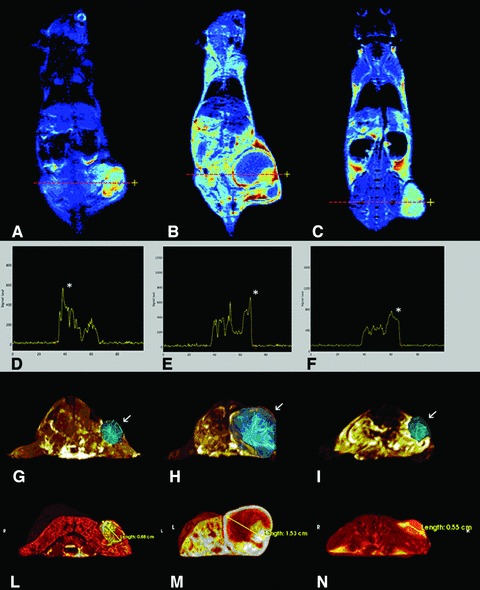
Three-dimensional measure and visualization of tumours. Coronal (A–C) post-contrast T1-weighted images (T1-w), applying intensity colouring maps of stressed + Ppl (A), stressed + placebo (B) and placebo (C). Dotted lines (A–C) and asterisks (D–F) indicate maximum enhancement plotted along X-axis on T1-w post-contrast images. MIP images show tumour lesions (arrows) and their extension throw a polygonal reconstruction of involved tissues (G–I). Volume images are representative of tumours of mice treated with stress + Ppl; stress + placebo and control. Volumes measured 0.349 cm3 (stressed + Ppl), 2.849 cm3 (stressed + placebo) and 0.1720 cm3 (control), respectively. Virtual renderings (L–N) cut on axial planes show a three-dimensional evaluation of tumours spatial extension and their relationships with normal tissues. Major axis is reported in cm in all images (G–I). Ppl: propranolol.
Chronic stress induces an increase in e-NOS levels
Catecholamine-induced expression of VEGF gene has been recognized to exert a major role in promoting tumour neoangiogenesis and thereby tumour growth [8]. By IHC and Western blotting, we analysed VEGF protein levels in tumours by stressed, compared to unstressed, animals. As shown in Figure 4(A–C), levels of VEGF protein were increased in tumours of stressed mice compared to specimens obtained from control animals. Images are representative of tissues collected from three separate experiments. These data were also confirmed by Western blot analysis (Fig. 4D) and subsequent densitometry from samples obtained from two separate experiments.
Fig 4.
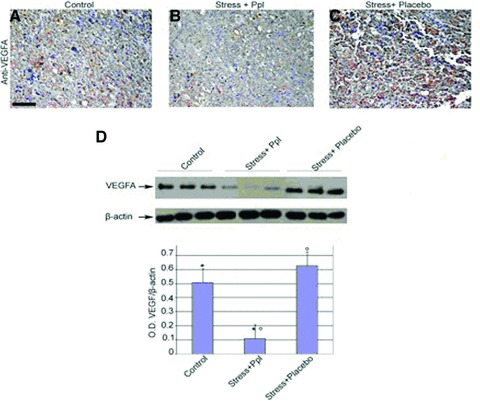
VEGFA expression in stressed mice. Melanoma sections from control and stressed mice were obtained and VEGFA levels were analysed by IHC. Protein lysated from the sections were obtained and VEGFA content was analysed by Western blotting. An anti–β-actin antibody was used as loading control (upper panel). Graphical representation of VEGFA/β-actin ratio in control and stressed mice (lower). Data are represented as the mean ± S.E.; (n= 3) *P= 0.01 versus ctrl and stress + Ppl; (n= 3) °P= 0.002 versus stress + placebo and stress + Ppl. Densitometry analysis was obtained from samples of two separate experiments. Scale bar: 100 μm in A–C. Ppl: propranolol.
Among effects of VEGF, there is induction of eNOS activity. Furthermore, immobilization stress is known to produce the activation of AP-1 transcription factor, that induces the expression of eNOS gene [25], a modulator of neoangiogenesis and other cancer-related events (apoptosis, cell cycle, invasion and metastasis) [14-16]. We therefore evaluated the expression of eNOS protein by Western blot analysis in tumours of stressed and control mice. A significant increase in eNOS levels was detected in tumours from stressed mice (Fig. 5A and B). On the other hand, iNOS levels were not different in the two groups of tumours (data not shown), indicating that this latter form of NOS was not modulated by chronic stress.
Fig 5.
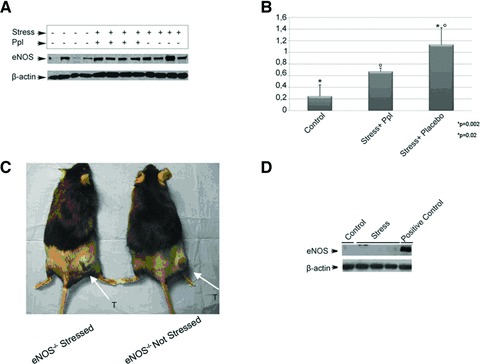
eNOS expression in stressed mice. (A) Western blot analysis with anti-eNOS on tumour lysates from control and stressed mice revealed an increased level of eNOS in stressed + placebo mice compared to control; *°P= 0.02 and stressed + Ppl mice; *P= 0.002 (n= 4). β-Actin protein was analysed as loading control. (B) Graphical representation of eNOS/β-actin ratio in control and stressed mice. The results were obtained from two independent experiments. (C) No differences in tumour growth are observed in eNOS−/− stressed and no stressed mice. (D) Tissue samples from B16F10 tumours implanted in eNOS−/− mice either untreated or subjected to physical stress were processed and analyzed by Western blot for eNOS contents. This analysis revealed the presence of eNOS protein only in the positive control. Ppl: propranolol.
eNOS exerts a pivotal role in chronic stress-promoted tumour growth
To investigate whether eNOS-increased levels were required for tumour growth promotion by chronic stress, we evaluated B16F10 melanoma growth in eNOS−/− mice. In a separate experiment, we used 20 animals eNOS−/− and divided into two groups of pre-treatments: control (not stressed) and chronically stressed mice. After seven days, tumour cells were injected as described earlier. In stressed animals (ACTH = 291 ± 15.23 pg/ml), we could not detect any significant increase in tumour growth, compared to unstressed animals: indeed, after 21 days of restraint stress, mean volumes of tumours stressed and unstressed mice were 17 ± 13 and 65 ± 4.3 mm3 (P > 0.05), respectively (Fig. 5C).
These results indicate eNOS as a final effector of pathway activated by chronic stress and disclose for the first time a pivotal role of eNOS in chronic stress-induced promotion of tumour growth.
Discussion
Until recently, the majority of research on the tumour-promoting effects of stress was focused on the neuroendocrine regulation of the immune response [26, 27]. In last years, more direct neuroendocrine effects on cancer cell proliferation, growth and invasion have been proved [27]; the identification of specific molecules responsible for such effects is presently an important target of oncologic research. Growing evidence indicate that tumour promotion by chronic stress rely largely on induction of catecholamine release. Indeed, substantial amounts of norepinephrine and epinephrine are produced during chronic stress due to the activation of sympathoadrenal medullary axis. Catecholamines in turn stimulate in several tissues, including various tumour types and surrounding cells, the production of different proteins that can help tumour development: these include metalloproteinases, growth factors and cytokines [28-30]. The specific contributions and roles of these proteins in tumour growth and metastatization need to be dissected, to understand the mechanism of stress-induced tumour promotion and to identify effective targets for therapy. In an orthotopic mouse model of stress-promoted ovarian carcinoma, the promoting effect of stress was recently shown to be mediated primarily through activation of the tumour cell cyclic AMP (cAMP)–protein kinase A (PKA) signalling pathway by the β2-adrenergic receptor and, specifically, by resulting neovascularization. In this context, catecholamine-induced increase in the tumour levels of the VEGF-A was identified as an important step downstream of the activation of cAMP-PKA signalling. These results constituted a major advancement in the understanding of stress effect on in vivo tumour development.
Here we confirm that the activation of the β2-adrenergic receptor is responsible for stress-induced tumour growth in another model, that is syngeneic ortothopic melanoma, and, importantly, add a piece of information, by demonstrating that, among potential VEGF targets, eNOS represents a key factor ultimately responsible for stress effect. Indeed, stress induced an increase of tumour eNOS levels in wild-type animals, as clearly demonstrated by Western blot analysis of melanoma tissues; as definitely, as a crucial evidence of eNOS role in this pathway of tumour promotion, stress was not able to promote melanoma growth in eNOS−/− mice. These results provide a novel element in the understanding of the pathway that links stress with cancer progression. More in general, they contribute to recognize eNOS as a pivotal enzyme in the neoplastic process. Nitric oxide is a critical downstream mediator of potent angiogenic agents such as VEGF [15], basic fibroblast growth factor [16], Ang-1 [31], insulin-like growth factor [29] and transforming growth factor (TGF)-β[15]. Previous studies attributed to iNOS, a major role in tumourigenesis [23, 32]. However, recent results assigned a prevalent role to eNOS in several cancer models [32-35], including liver tumours [32], prostate adenocarcinomas [35] and others [17]. Finally, oxidative stress has been recently correlated also with the response of patients affected by hepatocellular carcinoma to sorafenib combined with a somatostatin analogue (octreotide) [36]. Therefore, it appears that stress can play a role also in the determination of the clinical outcome of cancer patients.
Moreover, our results in orthotopic melanoma lend support to validation of eNOS as an important molecule in tumour development and a possible target of anti-neoplastic therapy.
Acknowledgments
This work was supported in part by funds from the Italian Association for Cancer Research (A.I.R.C.). We thank Antonio Luciano for technical assistance; Marialiusa Vecchione for immunohistochemistry analysis and a special thank to Prof. Maria Caterina Turco for helpful discussions and manuscript revision. Alessandra Trocino for providing excellent bibliographic service and assistance.
Conflict of interest
There is not any conflict of interests.
References
- 1.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 2.Montoro J, Mullol J, Jauregui I, et al. Stress and allergy. J Investig Allergol Clin Immunol. 2009;19:40–7. [PubMed] [Google Scholar]
- 3.Vere CC, Streba CT, Streba LM, et al. Psychosocial stress and liver disease status. World J Gastroenterol. 2009;15:2980–6. doi: 10.3748/wjg.15.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoni MH, Lutgendorf SK, Cole SW, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–8. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chida Y, Hamer M, Wardle J, et al. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466–75. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 6.Desaive P, Ronson A. Stress spectrum disorders in oncology. Curr Opin Oncol. 2008;20:378–85. doi: 10.1097/CCO.0b013e328302166a. [DOI] [PubMed] [Google Scholar]
- 7.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5:617–25. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 8.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumour growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 9.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–14. [PubMed] [Google Scholar]
- 10.Forstermann U, Pollock JS, Tracey WR, et al. Isoforms of nitric-oxide synthase: purification and regulation. Methods Enzymol. 1994;233:258–64. doi: 10.1016/s0076-6879(94)33029-8. [DOI] [PubMed] [Google Scholar]
- 11.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–34. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 12.Roberts DD, Isenberg JS, Ridnour LA, et al. Nitric oxide and its gatekeeper thrombospondin-1 in tumour angiogenesis. Clin Cancer Res. 2007;13:795–8. doi: 10.1158/1078-0432.CCR-06-1758. [DOI] [PubMed] [Google Scholar]
- 13.Singh JP. Dimethylarginine dimethylaminohydrolase: a new therapeutic target for the modulation of nitric oxide and angiogenesis. Curr Opin Investig Drugs. 2007;8:736–41. [PubMed] [Google Scholar]
- 14.Choi JY, Lee KM, Noh DY, et al. Genetic polymorphisms of eNOS, hormone receptor status, and survival of breast cancer. Breast Cancer Res Treat. 2006;100:213–8. doi: 10.1007/s10549-006-9245-5. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Wei Q, Bondy ML, et al. Promoter polymorphism (−786t > C) in the endothelial nitric oxide synthase gene is associated with risk of sporadic breast cancer in non-Hispanic white women age younger than 55 years. Cancer. 2006;107:2245–53. doi: 10.1002/cncr.22269. [DOI] [PubMed] [Google Scholar]
- 16.Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407–10. doi: 10.1158/0008-5472.CAN-06-2149. [DOI] [PubMed] [Google Scholar]
- 17.Seya Y, Fukuda T, Isobe K, et al. Effect of norepinephrine on RhoA, MAP kinase, proliferation and VEGF expression in human umbilical vein endothelial cells. Eur J Pharmacol. 2006;553:54–60. doi: 10.1016/j.ejphar.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 18.Kou R, Michel T. Epinephrine regulation of the endothelial nitric-oxide synthase: roles of RAC1 and beta3-adrenergic receptors in endothelial NO signaling. J Biol Chem. 2007;282:32719–29. doi: 10.1074/jbc.M706815200. [DOI] [PubMed] [Google Scholar]
- 19.Figueroa XF, Poblete I, Fernandez R, et al. NO production and eNOS phosphorylation induced by epinephrine through the activation of beta-adrenoceptors. Am J Physiol Heart Circ Physiol. 2009;297:H134–43. doi: 10.1152/ajpheart.00023.2009. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan JF, Feng NG, Bonneau RH, et al. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J Neuroimmunol. 1991;31:245–55. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- 21.Thaker PH, Sood AK. Neuroendocrine influences on cancer biology. Semin Cancer Biol. 2008;18:164–70. doi: 10.1016/j.semcancer.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frick LR, Arcos ML, Rapanelli M, et al. Chronic restraint stress impairs T cell immunity and promotes tumour progression in mice. Stress. 2009;12:134–43. doi: 10.1080/10253890802137437. [DOI] [PubMed] [Google Scholar]
- 23.Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001;2:149–56. doi: 10.1016/S1470-2045(00)00256-4. [DOI] [PubMed] [Google Scholar]
- 24.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Sabban EL, Hebert MA, Liu X, et al. Differential effects of stress on gene transcription factors in catecholaminergic systems. Ann N Y Acad Sci. 2004;1032:130–40. doi: 10.1196/annals.1314.010. [DOI] [PubMed] [Google Scholar]
- 26.Nanni S, Benvenuti V, Grasselli A, et al. Endothelial NOS, estrogen receptor beta, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest. 2009;119:1093–108. doi: 10.1172/JCI35079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben-Eliyahu S, Page GG, Schleifer SJ. Stress, NK cells, and cancer: still a promissory note. Brain Behav Immun. 2007;21:881–7. doi: 10.1016/j.bbi.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Yang EV, Sood AK, Chen M, et al. Norepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumour cells. Cancer Res. 2006;66:10357–64. doi: 10.1158/0008-5472.CAN-06-2496. [DOI] [PubMed] [Google Scholar]
- 29.Babaei S, Teichert-Kuliszewska K, Zhang Q, et al. Angiogenic actions of angiopoietin-1 require endothelium-derived nitric oxide. Am J Pathol. 2003;162:1927–36. doi: 10.1016/S0002-9440(10)64326-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fukumura D, Gohongi T, Kadambi A, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–9. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziche M, Morbidelli L, Choudhuri R, et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest. 1997;99:2625–34. doi: 10.1172/JCI119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lechner M, Lirk P, Rieder J. Inducible nitric oxide synthase (iNOS) in tumour biology: the two sides of the same coin. Semin Cancer Biol. 2005;15:277–89. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Decker NK, Abdelmoneim SS, Yaqoob U, et al. Nitric oxide regulates tumour cell cross-talk with stromal cells in the tumour microenvironment of the liver. Am J Pathol. 2008;173:1002–12. doi: 10.2353/ajpath.2008.080158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lim KH, Ancrile BB, Kashatus DF, et al. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–9. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lahdenranta J, Hagendoorn J, Padera TP, et al. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res. 2009;69:2801–8. doi: 10.1158/0008-5472.CAN-08-4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caraglia M, Giuberti G, Marra M, et al. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis. 2011;2:e150. doi: 10.1038/cddis.2011.34. ; doi: 10.1038/ cddis.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]


