Abstract
Patients with insulin resistance and early type 2 diabetes exhibit an increased propensity to develop a diffuse and extensive pattern of arteriosclerosis. Typically, these patients show elevated serum levels of the proinsulin cleavage product C-peptide and immunohistochemical data from our group revealed C-peptide deposition in early lesions of these individuals. Moreover, in vitro studies suggest that C-peptide could promote atherogenesis. This study examined whether C-peptide promotes vascular inflammation and lesion development in a mouse model of arteriosclerosis. ApoE-deficient mice on a high fat diet were treated with C-peptide or control injections for 12 weeks and the effect on lesion size and plaque composition was analysed. C-peptide treatment significantly increased C-peptide blood levels by 4.8-fold without having an effect on glucose or insulin levels, nor on the lipid profile. In these mice, C-peptide deposition in atherosclerotic plaques was significantly increased compared with controls. Moreover, lesions of C-peptide–treated mice contained significantly more macrophages (1.6 ± 0.3% versus 0.7 ± 0.2% positive area; P < 0.01) and more vascular smooth muscle cells (4.8 ± 0.6% versus 2.4 ± 0.3% positive area; P < 0.01). Finally, lipid deposition measured by Oil-red-O staining in the aortic arch was significantly higher in the C-peptide group compared with controls. Our results demonstrate that elevated C-peptide levels promote inflammatory cell infiltration and lesion development in ApoE-deficient mice without having metabolic effects. These data obtained in a mouse model of arteriosclerosis support the hypothesis that C-peptide may have an active role in atherogenesis in patients with diabetes and insulin resistance.
Keywords: C-peptide, atherosclerosis, ApoE-deficient mice
Introduction
Patients with insulin resistance and early type 2 diabetes typically develop a diffuse and extensive pattern of arteriosclerosis [1, 2]. Temporarily, these patients demonstrate elevated levels of the proinsulin cleavage product C-peptide. For years, C-peptide has been considered to be biologically inert, until recent work has demonstrated that C-peptide can activate intracellular signalling pathways in various cell types [3-6]. Previous data from our group raise the hypothesis that C-peptide might play a causal role in atherogenesis in patients with diabetes [7]: immunohistochemical analyses of early arteriosclerotic lesions from patients with diabetes revealed C-peptide deposition mainly in the subendothelial space and colocalization of C-peptide with intimal monocyte/macrophages and CD4-positive lymphocytes. In vitro migration assays, employing a modified Boyden chamber, demonstrated that C-peptide induces chemotaxis of both monocytes and CD4-positive lymphocytes [7, 8, 9]. These data raise the hypothesis that C-peptide may deposit in the subendothelial space in early lesions of patients with insulin resistance and diabetes subsequently promoting the recruitment of inflammatory cells into the vessel wall through its chemotactic action. Such mechanisms may contribute to lesion development and potentially explain why patients with diabetes develop a diffuse and extensive pattern of arteriosclerosis at a very early time-point.
In addition to its chemotactic effect on monocytes and CD4-positive lymphocytes, C-peptide also colocalized with medial vascular smooth muscle cells (VSMCs) in some diabetic patients and enhanced VSMC proliferation in vitro[10], thus potentially promoting both the development of arteriosclerotic lesions as well as neointima formation after coronary intervention.
Still, these results only refer to in vitro data and observational studies and to date the causal role of C-peptide in lesions development in experimental in vivo models of arteriosclerosis remains unexplored. Therefore, this study examined the effect of elevated C-peptide levels on lesion development in ApoE-deficient mice.
Material and methods
Animal preparation
Mice (B6.129P2-Apoetm1Unc) used for this study were purchased from Jackson lab (Bar Harbor, Maine, USA). A non-diabetic mouse model was employed to dissect direct effects of C-peptide on lesion development from potential confounding effects of insulin resistance and diabetes. To induce atherosclerosis, we fed 10-week-old male mice from each group a Western diet (SSNIFF, E 15749-34) for 13 weeks (1 week prior to subcutaneous (s.c.) injections and 12 weeks in parallel) and treated with s.c. injections of rat C-peptide [200 nmol, purchased from NEP (West Palm Beach, FL, USA), purification >95%] or water for 12 weeks. Two groups served as a controls. Mice in the chow diet control group and high fat diet control group were fed with standard chow diet and Western diet, respectively, for 13 weeks. At the beginning of the study, blood was obtained from the tail vein from each mouse to determine basal C-peptide levels in serum in each group. After 12 weeks of treatment, blood was drawn again (four hrs after last injection), to determine serum C-peptide levels after the treatment. On the next day the animals have been euthanized.
Rat-II-C-peptide (EVEDPQVAQLELGGGPGAGDLQTLALEVARQ) and mouse-II-C-peptide (EVEDPQVAQLELGGGPGAGDLQTLALEVAQQ) are identical except in one amino acid sequence. This model using non-immunogenic rat C-peptide in a diabetic mouse has previously been established by Langer et al. to evaluate the role of C-peptide on wound healing and microcirculation [15].
C-peptide serum levels were measured by RIA (Linco-Research #RCP-21K; Missouri, USA) at two different time points (before and after treatment) in blood samples drawn 4 hrs after the last subcutaneous injections. Soluble ICAM and E-selectin as well as tumour necrosis factor (TNF)-α and interleukin (IL)-6 were measured in serum using ELISA-assays (R&D Systems, Minneapolis, MN, USA).
The mice were housed individually under conventional conditions. The protocols were approved by the Regierungsprsidium Tuebingen (Germany) and all procedures were conducted in accordance with the recommendation given by the animal care facility of the University Ulm.
Aortic atherosclerotic lesion analysis
Atherosclerotic lesions were analysed in the aortic arch, and descending aorta as previously described [11]. Briefly, mice were perfused at physiological pressure with normal saline via the left ventricle, and the hearts and aortas were removed en bloc. The aortic arch was embedded in optical cutting temperature compound (Tissue Freezing Medium, Jung, Germany). To evaluate intimal lesion size, frozen sections of aortic arch were incubated with Oil-red-O (0.5% in glycerol). Immunohistochemical studies used goat anti-rat polyclonal antibody for C-peptide detection (Linco Research #4023-01; Missouri, USA; which has 100% cross-reactivity to mouse C-peptide), rat anti-mouse monoclonal antibody to Mac3, a macrophage marker (1:50; BD Pharmingen, San Diego, CA, USA), and SMC α-actin staining with anti–α-actin mouse monoclonal antibody (1:75; Sigma-Aldrich, St. Louis, MO, USA). Longitudinal cryostat sections of the aortic arch, and formalin fixed, pinned-out en face preparations of the descending aorta were prepared as described previously [12, 13]. Aortic lesions were stained with Oil-red-O according to the method of Paigen et al. [14]. We analysed mouse atherosclerotic lesions in longitudinal sections from a segment of the lesser curvature of the aortic arch (defined using a perpendicular line dropped from the right side of the innominate artery and from the left side of the left subclavian artery, as shown in Figure 2A. Images were captured by a digital system, area of staining was measured using computer-assisted image quantification (Image-Pro Plus software) and the percentage of the total area with positive colour was calculated and recorded for each mouse. Measurements and evaluation of the atherosclerotic lesions were performed in a blinded fashion.
Fig 2.
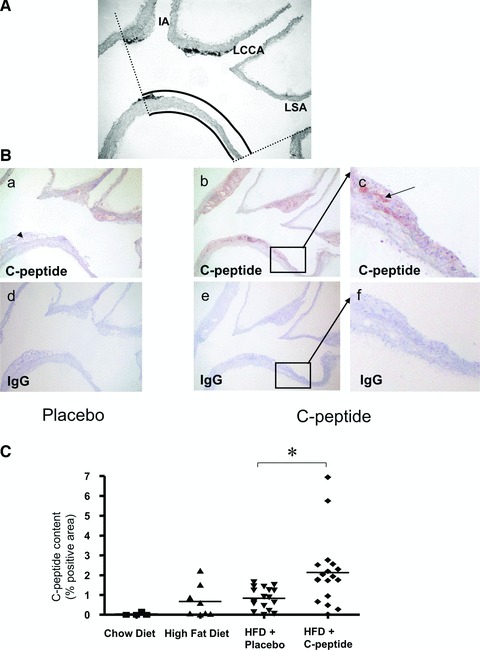
(A) Representative photograph of a mouse aortic arch longitudinal section, used for the analysis of total wall area by computer-assisted image quantification. IA: inominate artery; LCCA: left common carotid artery; LSA: left subclavian artery. (B) C-peptide deposition in the aortic arch. Representative longitudinal sections of the aortic arch show C-peptide deposition in atherosclerotic plaques in a control (a) and a C-peptide–treated mouse (b). (c) High power view of the area indicated by the rectangle in (b). Adjacent sections (d), (e) and (f) stained with identical concentrations of type and class matched IgG show no immunoreactive C-peptide. Arrow indicates C-peptide positive areas. (C) Quantitative image analysis of immunoreactive C-peptide deposition in the aortic arch expressed as % positive area. ApoE-deficient mice on chow or high fat diet alone served as controls. Each data point represents a value from a single mouse (chow diet: n= 5, high fat diet: n= 8, placebo: n= 17, C-peptide: n= 18); *P < 0.01 for comparison between groups.
Statistical analysis
Results of the experimental studies are reported as mean ± S.E.M. Differences were analysed by one-way ANOVA test. P < 0.05 was regarded as significant.
Results
Characteristics of mice
After 12 weeks of C-peptide or water s.c. injections BID (in parallel to diet) body weight and lipids (total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein) did not significantly differ between the two treatment groups (Table 1). Furthermore, glucose and insulin levels showed no differences between groups (Table 2). Moreover, serum levels of the endothelial markers soluble E-selectin and soluble ICAM as well as levels of the inflammatory markers TNF-α and soluble IL-6 showed no significant differences between groups (Table 2). No local side-effects of C-peptide injections were observed.
Table 1.
Metabolic characteristics of mice on chow diet or high fat diet, as well as placebo or C-peptide treated mice on high fat diet
| Chow diet (n= 5) | High fat diet (n= 8) | Placebo (n= 17) | C-peptide (n= 18) | P-value (placebo versusC-peptide) | |
|---|---|---|---|---|---|
| Weight (g) | 29.69 ± 0.48 | 31.49 ± 0.74 | 30.09 ± 3.05 | 30.75 ± 2.72 | 0.75 |
| Total cholesterol (mmol/l) | 7.22 ± 0.26 | 13.59 ± 1.2 | 11.92 ± 2.29 | 13.09 ± 5.54 | 0.31 |
| Triglyceride (mmol/l) | 0.79 ± 0.1 | 0.69 ± 0.11 | 0.67 ± 0.27 | 0.87 ± 0.73 | 0.38 |
| HDL (mmol/l) | 0.52 ± 0.01 | 0.32 ± 0.04 | 0.29 ± 0.07 | 0.34 ± 0.15 | 0.23 |
| LDL (mmol/l) | 2.48 ± 0.44 | 7.93 ± 1 | 6.4 ± 1.8 | 7.04 ± 1.73 | 0.26 |
Table 2.
Insulin and glucose levels in C-peptide–treated mice and controls
| Placebo (n= 17) | C-peptide (n= 18) | P-value | |
|---|---|---|---|
| Glucose (mg/dl) | 356 ± 21.59 | 280 ± 41.52 | n.s. |
| Insulin (ng/ml) | 0.49 ± 0.04 | 0.41 ± 0.03 | n.s. |
| Soluble E-selectin (ng/ml) | 75.94 ± 5.33 | 87.71 ± 2.86 | n.s. |
| Soluble ICAM (ng/ml) | 744.64 ± 30.69 | 808.18 ± 36.97 | n.s. |
| Soluble IL-6 (pg/ml) | 12.54 ± 3.73 | 13.65 ± 7.1 | n.s. |
| Soluble TNF-α (pg/ml) | 28.9 ± 16.79 | 30.57 ± 13.45 | n.s. |
Markers for endothelial function: soluble E-selectin and soluble ICAM and inflammatory markers: TNF-α and soluble IL-6; n.s.: not significant.
C-peptide administration increases C-peptide serum levels
Basal C-peptide levels did not differ between groups (1.7 ± 0.2 versus 1.5 ± 0.2 nmol/l for water and C-peptide-treated mice, respectively) and diet alone did not increased C-peptide levels in the control group. After 12 weeks of treatment, s.c. injections with C-peptide BID significantly increased C-peptide serum levels to 12.9 ± 1.8 nmol/l compared with 2.7 ± 0.8 nmol/l in control mice (Fig. 1). Given that patients with early diabetes and insulin resistance exhibit elevated levels of C-peptide, these data suggest that our model may be suited to mimic the pathophysiological condition in these patients.
Fig 1.
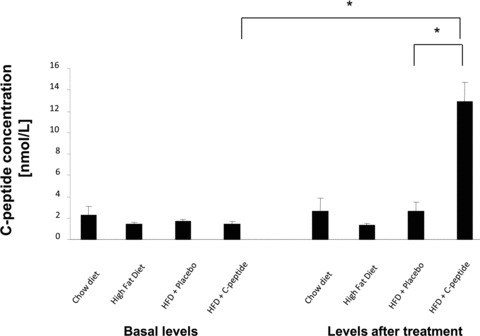
C-peptide serum levels at baseline and after 12 weeks of C-peptide (s.c. injections BID; 200 nmol C-peptide/injection) or placebo treatment in ApoE-deficient mice on a high fat diet. C-peptide levels were measured with RIA in blood samples taken four hrs after the last application of dissolved peptide. ApoE-deficient mice on chow or high fat diet alone served as controls. Bars represent mean ± S.E.M.; *P < 0.05 compared with placebo after treatment and compared with baseline in the C-peptide group; chow diet: n= 5, high fat diet: n= 7, placebo: n= 11, C-peptide: n= 16).
C-peptide deposits in atherosclerotic lesions
To examine the deposition of C-peptide in early arteriosclerotic lesions, we used longitudinal sections of the aortic arch (Fig. 2A). Immunohistochemical analyses of the aortic arch showed prominent C-peptide staining in C-peptide–treated mice with only scarce deposition in the control group. Staining of sections with isomatched IgGs at similar concentrations showed no immunoreactivity, thus affirming the specificity of the detected signals (Fig. 2B). Computer-assisted image quantification revealed significantly higher C-peptide deposition in C-peptide–treated mice compared to controls (2.1 ± 0.4% versus 0.8 ± 0.1% positive area; P < 0.01; Fig. 2C).
C-peptide treatment increases macrophage content in atherosclerotic lesions
Given our previous in vitro data showing that C-peptide induces monocyte chemotaxis [7], we next examined lesion monocyte/macrophage content by immunohistochemical statining for MAC-3. As shown in (Fig. 3), C-peptide–treated mice exhibited significantly higher macrophage content in lesions compared to control mice (1.6 ± 0.3% versus 0.7 ± 0.2% positive area; P < 0.01). Moreover, as previously seen in early arteriosclerotic plaques of patients with diabetes, monocyte/macrophages colocalized with C-peptide in these murine lesions (Fig. 4).
Fig 3.
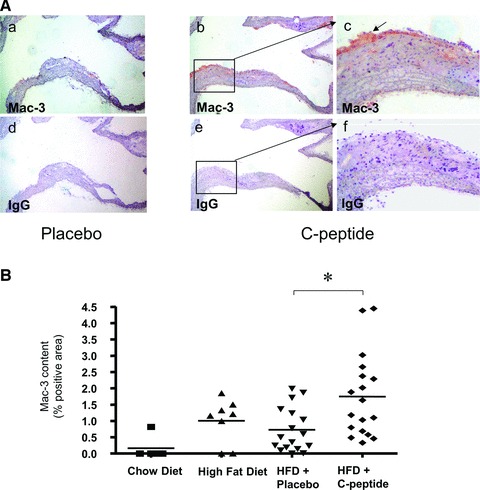
C-peptide treatment increases monocyte/macrophage content in atherosclerotic lesions. (A) Representative images of longitudinal sections of mouse aortic arches stained for the monocyte/ macrophage marker Mac-3 in a control (a) and a C-peptide treated mouse (b). (c) High power view of the area indicated by the rectangle in (b). Adjacent sections (d), (e) and (f) stained with identical amounts of type and class matched IgG show no immunoreactivity. Arrow indicates Mac-3 positive area (red). (B) Quantitative image analysis of immunoreactive Mac-3 staining in the aortic arch expressed as % positive area. ApoE-deficient mice on chow or high fat diet alone served as controls. Each data point represents a value from a single mouse (chow diet: n= 5, high fat diet: n= 8, placebo: n= 17, C-peptide: n= 18); *P < 0.01 for comparison between groups.
Fig 4.
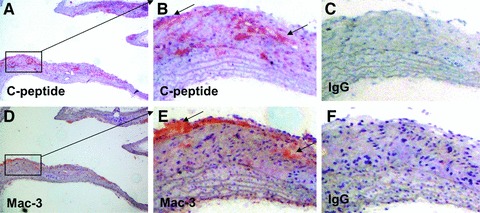
C-peptide colocalizes with monocytes/macrophages in arteriosclerotic lesions of ApoE-deficient mice treated with C-peptide. (A, B) High power view of rectangle in (A) sections of C-peptide–treated mice show immunoreactive C-peptide in the intima (stained in red, indicated by arrows). Adjacent section stained with anti–Mac-3 antibody demonstrates colocalizing monocytes/macrophages (stained in red; indicated by arrows) in the intimal area (D) and (E); high power view of rectangle in (D). (C, F) Represent IgG-matching controls.
C-peptide treatment increases vascular SMC content
Because C-peptide can act as a mitogen in aortic VSMCs in vitro[10], we next examined the effect of C-peptide on VSMC content in the aortic arch of treated mice. Staining for smooth muscle α-actin within lesions was significantly increased in C-peptide–treated mice compared with controls (4.8 ± 0.6 versus 2.4 ± 0.3% positive area; P < 0.01; Fig. 5). Moreover, staining of lesions for Ki-67, a proliferation marker, revealed a trend towards a higher cell proliferation rate in C-peptide treated mice (0.06 ± 0.04 versus 0.67 ± 0.31, P= 0.05).
Fig 5.
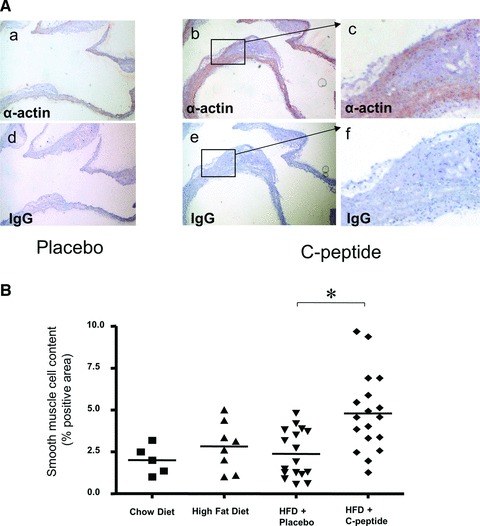
C-peptide treatment increases smooth muscle cell content in the aortic arch of C-peptide treated mice. (A) Representative images of longitudinal sections of mouse aortic arches stained for smooth muscle cell α-actin (in red) in a control (a) and a C-peptide–treated mouse (b). (c) High power view of the area indicated by the rectangle in (b). Adjacent sections (d), (e) and (f) stained with identical amounts of type and class matched IgG show no immunoreactivity. (B) Quantitative image analysis of immunoreactive α-actin staining in the aortic arch expressed as % positive area. ApoE-deficient mice on chow or high fat diet alone served as controls. Each data point represents a value from a single mouse (chow diet: n= 5, high fat diet: n= 8, placebo: n= 17, C-peptide: n= 18); *P < 0.01 for comparison between groups.
C-peptide treatment increases lipid deposition
Finally, we investigated lipid deposition in lesions using Oil-red-O staining. In C-peptide–treated mice, lipid deposition in the aortic arch was significantly higher compared with controls (14.8 ± 1.5 versus 9.5 ± 1.6; P < 0.05; Fig. 6A). Moreover, there was a trend—albeit not significant—towards increased lipid deposition in en face preparations of the abdominal and thoracic aorta in C-peptide–treated mice compared to control mice (Fig. 6B).
Fig 6.
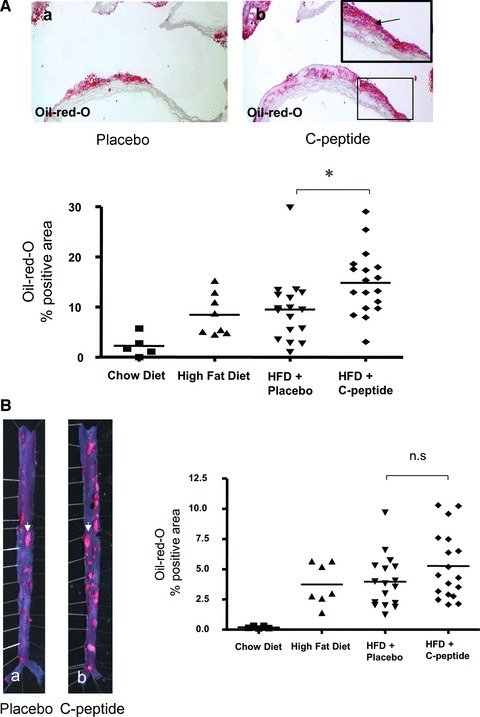
(A) C-peptide treatment increases lipid content in the aortic arch. Upper: representative Oil-red-O stained sections of the aortic arch show lipid deposition in a placebo (a) and a C-peptide–treated mouse (b). Insert: high power view of the area indicated by the rectangle in (b). Lower: Quantitative image analysis of Oil-red-O positivity in the aortic arch expressed as % positive area. ApoE-deficient mice on chow or high fat diet alone served as controls. Each data point represents a value from a single mouse (chow diet: n= 5, high fat diet: n= 8, placebo: n= 17, C-peptide: n= 18); *P < 0.05 for comparison between groups. (B) C-peptide treatment increases plaque formation in mice. Left: Representative microphotographs of thoracic and abdominal aortic specimen stained for lipid deposition with Oil-red-O in a control (a) and a C-peptide–treated mouse (b). Arrows indicate fat rich plaques. Right: Quantitative image analysis of Oil-red-O positivity in the en face aorta expressed as % positive area. ApoE-deficient mice on chow or high fat diet alone served as controls. Each data point represents a value from a single mouse (chow diet: n= 5, high fat diet: n= 8, placebo: n= 17, C-peptide: n= 18); P is not significant for comparison between groups.
Discussion
This study demonstrates for the first time that elevated serum levels of C-peptide increase macrophage and VSMC content in the vessel wall of ApoE-deficient mice, thus leading to increased lesion formation in this model.
Previously, observational data from our group have demonstrated that C-peptide deposits in the subendothelial space in early lesions of patients with diabetes, colocalizing there with inflammatory cells such as monocytes and lymphocytes [7, 8]. Moreover, C-peptide–induced chemotaxis of these cells in vitro, raising the hypothesis that C-peptide could contribute to early atherogenesis in patients with diabetes and insulin resistance: in these patients, during the phase of high C-peptide levels and the presence of endothelial dysfunction, C-peptide could deposit in the subendothelial space and through its chemotactic activity facilitate the recruitment of monocytes and T cells into the vessel wall, thus promoting the inflammatory response in the vasculature. This study now bolsters this hypothesis by demonstrating in an in vivo model that elevated C-peptide concentrations lead to C-peptide deposition in the vessel wall as well as to an increase in monocyte/macrophage and SMC content in arteriosclerotic lesions. Interestingly, as previously seen in early plaques of patients with diabetes, we also found colocalization of monocytes/macrophages in these murine lesions, suggesting that C-peptide deposition may potentially facilitate monocyte recruitment through its chemotactic effect on these cells. To distinguish the effect of C-peptide on vessel wall inflammation and plaque formation from potentially confounding metabolic effects in diabetes and insulin resistance, we chose the model of non-diabetic ApoE-deficient mice and increased C-peptide concentration by twice daily s.c. injection. A similar approach has previously been used by Langer et al. showing a robust increase in C-peptide serum levels in mice [15]. In our model, C-peptide injections were well-tolerated without any side effects, leading to a four- to five-fold increase in serum C-peptide levels, thus mimicking elevated concentrations in patients with insulin resistance and early type 2 diabetes. Interestingly, in contrast to previous reports, C-peptide treatment of these mice did not affect glucose or insulin levels nor the lipid profile. This difference to other studies may be due to the different animal models used [16].
In our experimental setting 10-week-old ApoE-deficient mice were put on a Western type diet for 1 week to trigger lesion development. After this week, mice were treated with C-peptide for 12 weeks in parallel to a high fat diet, as shown before [17, 18]. Because we were interested to explore the effect of C-peptide on early lesion development, we choose a moderate diet and harvested animals already after 13 weeks of diet.
The increase in lesion monocyte/macrophages as well as VSMCs was paralleled by significantly enhanced plaque lipid accumulation in the aortic arch, suggesting that the effect of C-peptide on monocyte recruitment and SMC proliferation translates into an increase in lesion formation. In addition to that, we found a clear, albeit not significant trend to increased plaque formation in the en face preparation in the aorta. Conflicting in vitro data exist on the role of C-peptide in SMC proliferation [10, 19, 20] depending on the origin of SMCs as well as the experimental conditions chosen. The data shown here suggest that C-peptide increases SMC content in lesions in vivo, confirming that C-peptide could act as a mitogen in aortic SMCs. Our previous in vitro data clearly demonstrated an effect of C-peptide on CD4-positive lymphocyte chemotaxis but due to methodological limitations with lack of an appropriate antibody for CD4-positive cells for immunohistochemistry in mice, we were not able to examine the effect of C-peptide on CD4-positive lesion lymphocyte content, but we see no differences for CD3-positive lesion lymphocyte content in our model. Moreover, our study showed no differences in E-selectine and ICAM-1 levels. This data are in contrast to several findings in which C-peptide reduce up-regulation of cell adhesion molecules under inflammatory conditions [21, 22]. The use of solvent (water) in control mice is a limitation of our study and treatment with scrambled C-peptide or peptide with endothelium affinity may have been a more appropriate control. Still, previous in vitro data from our group demonstrated that scrambled C-peptide does not exhibit any effect in vascular cells. However, future experiments should use scrambled C-peptide or peptide with endothelium affinity as a negative control. In addition, further work is warranted to assess the effect of C-peptide on vessel wall chemokine expression to potentially better explain the increase in monocyte/macrophage content on lesions of C-peptide– treated mice.
Taken together, this study demonstrates that elevated C-peptide concentrations increase monocyte recruitment into the vessel wall in vivo, thus promoting lesion formation in a mouse model of arteriosclerosis. Our findings illustrate for the first time a hitherto unapparent causal link between high levels of serum-C-peptide and the progression of atherosclerotic lesions in an animal model, thus giving further momentum to the hypothesis that C-peptide may contribute to lesion development in patients with insulin resistance and early type 2 diabetes.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft (SFB 451, B11, DFG WA2145/1-1) to Prof. Dr. Daniel Walcher as well as a grant from the Else-Krner-Fresenius-Stiftung to Prof. Dr. Daniel Walcher.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia. 2007;50:503–9. doi: 10.1007/s00125-006-0559-y. [DOI] [PubMed] [Google Scholar]
- 4.Wahren J, Shafqat J, Johansson J, et al. Molecular and cellular effects of C-peptide-new perspectives on an old peptide. Exp Diabesity Res. 2004;5:15–23. doi: 10.1080/15438600490424479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitamura T, Kimura K, Jung BD, et al. Proinsulin C-peptide rapidly stimulates mitogen-activated protein kinases in Swiss 3T3 fibroblasts: requirement of protein kinase C, phosphoinositide 3-kinase and pertussis toxin-sensitive G-protein. Biochem J. 2001;355:123–9. doi: 10.1042/0264-6021:3550123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitamura T, Kimura K, Jung BD, et al. Proinsulin C-peptide activates cAMP response element-binding proteins through the p38 mitogen-activated protein kinase pathway in mouse lung capillary endothelial cells. Biochem J. 2002;366:737–44. doi: 10.1042/BJ20020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marx N, Walcher D, Raichle C, et al. C-peptide colocalizes with macrophages in early arteriosclerotic lesions of diabetic subjects and induces monocyte chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2004;24:540–5. doi: 10.1161/01.ATV.0000116027.81513.68. [DOI] [PubMed] [Google Scholar]
- 8.Walcher D, Aleksic M, Jerg V, et al. C-peptide induces chemotaxis of human CD4-positive cells: involvement of pertussis toxin-sensitive G-proteins and phosphoinositide 3-kinase. Diabetes. 2004;53:1664–70. doi: 10.2337/diabetes.53.7.1664. [DOI] [PubMed] [Google Scholar]
- 9.Aleksic M, Walcher D, Giehl K, et al. Signalling processes involved in C-peptide-induced chemotaxis of CD4-positive lymphocytes. Cell Molec Life Sci. 2009;66:1974–84. doi: 10.1007/s00018-009-9057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walcher D, Babiak C, Poletek P, et al. C-peptide induces vascular smooth muscle cell proliferation: involvement of SRC-kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase 1/2. Circ Res. 2006;99:1181–7. doi: 10.1161/01.RES.0000251231.16993.88. [DOI] [PubMed] [Google Scholar]
- 11.Gotsman I, Grabie N, Gupta R, et al. Impaired regulatory T cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–55. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 12.Buono C, Pang H, Uchida Y, et al. B7-1/B7-2 co-stimulation regulates plaque antigen-specific T cell responses and atherogenesis in LDLR-deficient mice. Circulation. 2004;109:2009–15. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 13.Buono C, Binder CJ, Stavrakis G, et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci USA. 2005;102:1596–601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paigen B, Morrow A, Holmes PA, et al. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987;68:231–40. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- 15.Langer S, Born F, Breidenbach A, et al. Effect of C-peptide on wound healing and microcirculation in diabetic mice. Eur J Med Res. 2002;25:502–08. [PubMed] [Google Scholar]
- 16.Hills CE, Brunskill NJ. Cellular and physiological effects of C-peptide. Clin Sci. 2009;116:565–74. doi: 10.1042/CS20080441. [DOI] [PubMed] [Google Scholar]
- 17.An G, Miwa T, Song WL, et al. CD59 but not DAF deficiency accelerates atherosclerosis in female ApoE knockout mice. Mol Immunol. 2009;46:1702–9. doi: 10.1016/j.molimm.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veillard NR, Steffens S, Burger F, et al. Differential expression patterns of proinflammatory and antiinflammatory mediators during atherogenesis in mice. Arterioscler Thromb Vasc Biol. 2004;24:2339–44. doi: 10.1161/01.ATV.0000146532.98235.e6. [DOI] [PubMed] [Google Scholar]
- 19.Cifarelli V, Luppi P, Tse HM, et al. Human proinsulin C-peptide reduces high glucose-induced proliferation and NF-kappaB activation in vascular smooth muscle cells. Atherosclerosis. 2008;201:248–57. doi: 10.1016/j.atherosclerosis.2007.12.060. [DOI] [PubMed] [Google Scholar]
- 20.Mughal RS, Scragg JL, Lister P, et al. Cellular mechanisms by which proinsulin C-peptide prevents insulin-induced neointima formation in human sphenous vein. Diabetologia. 2010;53:1761–71. doi: 10.1007/s00125-010-1736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scalia R, Coyle KM, Levine BJ, et al. C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. FASEB J. 2000;14:2357–64. doi: 10.1096/fj.00-0183com. [DOI] [PubMed] [Google Scholar]
- 22.Luppi P, Cifarelli V, Tse H, et al. Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-kappa B pathway. Diabetologia. 2008;51:1534–43. doi: 10.1007/s00125-008-1032-x. [DOI] [PubMed] [Google Scholar]


