Abstract
Consistent evidence underlines the utility of human papillomavirus (HPV) DNA testing in the management of women with equivocal cervical cytological abnormalities, but not in case of low-grade lesions. We performed a meta-analysis including studies where the high-risk probe of the Hybrid Capture-II is used to triage these two cytological categories. The triage test-positivity rate reflects the colposcopy referral workload.Data were pooled on the HPV test positivity rate in women with atypical squamous cells of undetermined significance (ASCUS/ASC-US) or low-grade squamous intraepithelial lesions (LSIL), derived from different cytological classification systems. The meta-analysis was restricted to studies, published between 1991 and 2007. A random-effect model was applied for meta-analytical pooling and the influence of covariates on the HPV positivity rate was analyzed by meta-regression. The variation by age was assessed within individual studies since age strata were not defined uniformly. On an average, 43% (95% CI: 40–46%) of women with ASCUS/ASC-US were high-risk HPV positive (range 23–74%). In women with LSIL, the pooled positivity rate was 76% (95% CI: 71–81%; range 55–89%). In spite of considerable inter-study heterogeneity, the difference in HPV positivity between the two triage groups was large and highly significant: 32% (95% CI: 27–38%). HPV rates dropped tremendously as age and cutoffs of test positivity increased. Other factors (cytological classification system, country, continent, collection method and year of publication) had no statistically significant impact, except in LSIL triage where HPV positivity was significantly lower in European compared to American studies. Women with LSIL, especially younger women, have high HPV positivity rates suggesting limited utility of reflex HPV triaging these cases. Research is needed to identify more specific methods to triage women with low-grade squamous cervical lesions.
Keywords: cervical cancer, atypical squamous cells of undetermined significance, low-grade squamous intraepithelial lesions, triage, human papillomavirus, HPV, meta-analysis
Introduction
Women with minor cytological lesions identified in a Pap smear have a small but significantly increased risk of developing cervical cancer compared to women with normal smears. Reviews of the natural history of cervical epithelial atypia or low-grade squamous lesions suggest that the 2-year cumulative risk of invasive cervical cancer is in the range of 0.10% to 0.25%[1, 2]. Therefore, careful follow-up of these lesions is warranted.
The recognition of the strong association between persistent infection with oncogenic human papillomavirus types and the subsequent development of cervical cancer has prompted the detection of HPV DNA as an alternative triage method [3]. A recent meta-analysis of the accuracy of HPV DNA detection for cervical intraepithelial neoplasia of grade 2 or worse (CIN2+), using the high-risk probe of the Hybrid Capture II assay (HC2, Qiagen, Gaithersburg, MD, USA), in case of equivocal cytology demonstrated that the pooled estimate of the sensitivity and specificity was 94.8% (95% CI [CI]: 92.7–96.9%) and 67.3% (95% CI: 58.2–76.4%), respectively [4]. The pooled sensitivity for predicting presence of CIN3+ was 96.4% (95% CI: 93.5–99.9%) and the pooled specificity was 56.5% (95% CI: 45.5–67.5%) [5]. The sensitivity of the HC2 assay was 16% (ratio = 1.16; CI: 1.04–1.29%) and 13% (ratio = 1.13; CI: 1.05–1.22%) higher than that of repeat cytology at cut-off atypical squamous cells of unspecified significance (ASCUS)/borderline dyskaryosis or worse for, respectively, CIN2+ or CIN3+. The specificity of cytological and virological triage was similar.
Until recently, the recommended policy in case of cervical equivocal (borderline) or mild (low-grade) cytological abnormalities consisted of the repetition of the smear and referral for colposcopy if the lesion persists or progresses [6, 7]. Meanwhile, the ASCUS-LSIL triage study (AITS) has demonstrated superior performance of triage of women with ASCUS by testing for high-risk HPV types compared to repetition of the Pap smear or immediate colposcopy referral. The results from the ASCUS and LSIL triage study (ALTS), corroborated by meta-analytical work, provide the evidence for current recommendations for reflex hrHPV testing in case of atypical squamous cells of undetermined significance [8–11]. Recommendations for managing low-grade cytological abnormalities are not uniform, ranging from immediate colposcopy [10] to repeat cytology and referral if cytological abnormality is persistent [12]. The utility of reflex hrHPV testing in case of LSIL or mild dyskaryosis is more controversial: it is not recommended in the most recent guidelines of the American Society for Colposocopy and Cervical Pathology [10], whereas others consider introducing it in screening programmes [13]. In Australia, reflex HPV testing to triage minor cytological abnormalities was not yet accepted, but this recommendation has been criticized [14]. In this new systematic review, we compare the HPV test positivity rate in women with equivocal and low-grade cytological abnormalities and examine how triage can be optimized by targeting different age groups.
Methods
Methods for retrieving published reports regarding the accuracy of HPV triage of women with minor cervical abnormalities were described previously [4, 15]. Studies were included if the following three criteria were fulfilled: (1) women had an index smear showing ASCUS or low-grade intra-epithelial lesions (LSIL) and study outcomes were reported separately for both triage groups, (2) the high-risk probe of the Hybrid Capture II was applied using the standard cut-off as positivity criterion (signal, expressed as relative light units [RLU] more intense than that of a control sample which contains 1 pg of HPV DNA per millilitre) and (3) all women were submitted to verification with colposcopy and colposcopy-directed biopsies and/or endocervical curettage when presence of squamous or glandualar intra-epithelial neoplasia was suspected. From the ALTS [16, 17], we used results from two of the three trial arms: women randomly assigned to immediate colposcopy and women randomly assigned to the HPV DNA testing arm, where colposcopic verification was restricted to women being HPV positive or having HSIL cytology.
In the current meta-analysis, we focus on the proportion of women with a positive HC2 test in atypical and low-grade squamous lesions. For this reason, inclusion criteria were relaxed and studies on management of minor squamous cervical lesions, distinguishing atypia and low-grade abnormalities with partial gold standard verification and/or age-stratification of the HPV status were also included.
The 1991 and 2001 versions of The Bethesda System (TBS) and the Terminology of the British Society of Clinical Cytology (BSCC) were used for classification of cytology [18–20]. In the 1991 version of TBS, ASCUS encompassed three subcategories of atypical squamous cells: (a) favour reactive (ASC-R); (b) undetermined significance and (c) neoplasia cannot be excluded. In TBS-2001, the first subcategory (ASC-R) was lumped with ‘negative for neoplasia or malignancy’, whereas the second and third sub-categories were identified as ASC-US (with hyphen; atypical squamous cells of undetermined significance) and ASC-H (atypical squamous cells, high-grade lesion cannot be excluded), respectively. For the current meta-analysis, we computed the number of ASCUS cases if possible from the respective subcategories. In publications, using TBS-2001, where this was not possible, only data on ASC-US cases were extracted. Studies reporting data exclusively on ASC-H or atypical glandular cells were excluded. The definition of low-grade squamous intraepithelial lesions (LSIL) remained unchanged in TBS-1991 and TBS-2001. The BSCC terms borderline cytology and mild dyskaryosis were considered as similar to ASCUS/AS-CUS and LSIL, respectively, but were treated separately [21].
We used a random effect model for pooling proportions [22]. Interstudy heterogeneity was assessed with Cochrane's Q-test [23]. The percentage of total variation across studies due to heterogeneity was evaluated by the I2 measure [24]. Forest plots were drawn showing the variation of the HC2 test positivity rate among all studies together with the pooled measure [25, 26]. Subgroup meta-analyses were used to distinguish the respective cytological classification systems [27]. A random-effect was also used to pool the difference in test positivity rate between ASCUS/borderline and LSIL/mild dyskaryosis cases [28].
The change in HPV positivity rate by age category was assessed by a chi-square trend, which generalizes the Wilcoxon test to several ordered groups [29]. The influence of study characteristics on inter-study heterogeneity was explored using a multi-variate hierarchical meta-regression with the logit-transformed HPV positivity rate as the dependent variable [30–32]. Relative risks were computed from the coefficients of the meta-regression using established formulas [33, 34].
We used Stata, version 10.0 (Stata Corp., College Station, TX, USA), for statistical analysis [27, 35].
Results
We identified 32 studies enrolling all together 26,311 women with a cytological report of ASCUS, ASC-US or borderline dyskaryosis that could be included in the meta-analysis [13, 16, 36–65]. In 20 studies, high-risk HPV positivity could be derived for ASCUS defined according to TBS-1991, in six studies for AS-CUS, defined according to TBS-2001 and in six studies for borderline dyskaryosis, based on the BSCC terminology. The test-positivity rate varied between 22.8%[53] and 74.2%[55] (see Fig. 1). In spite of the wide and statistically significant inter-study heterogeneity, the pooled HPV positivity rates did not differ significantly by used cytological classification system (43.1% in ASCUS; 41.6% in ASC-US and 42.8% in borderline dyskaryosis; P for inter-group heterogeneity = 0.75). The overall pooled test positivity was 42.8% (95% CI: 39.5–46.1%).
1.

Meta-analysis of the proportion of women with ASCUS or a borderline Pap smear that have a positive Hybrid Capture II test.
Sixteen LSIL/mild dyskaryosis triage studies could be included in the meta-analysis enrolling 5,389 women [13, 17, 37, 38, 41–44, 46, 50, 51, 59, 60, 64–66]. In 12 and 4 studies, respectively, TBS and the BSCC were used. The lowest HC2-positivity rate was reported by Ronco (54.6%) [64] and the highest by Rowe (88.6%) [50] (see Fig. 2). The range of variation (highest--lowest proportion) in the low-grade group was smaller (34.0%) than in the ASCUS/borderline group (51.4%) but the inter-study heterogeneity was statistically significant in both meta-analyses (P for Cochrane's Q-test <0.001). The pooled hrHPV positivity rate did not significantly vary by cytological classification system used: 75.3% in LSIL and 78.5% in mild dyskaryosis; P= 0.32). The overall pooled HC2 positivity rate was 75.9% (95% CI: 71.2–80.6%).
2.
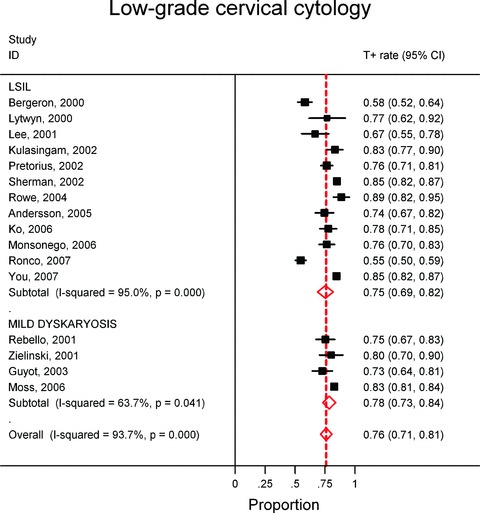
Meta-analysis of the proportion of women with LSIL or a mildly dyskaryotic Pap smear that have a positive Hybrid Capture II test.
The difference in the HC2-positivity rate between ASCUS/borderline dyskaryosis and LSIL/mild dyskaryosis always was positive and significantly different from zero in all studies except in one, where it approached significance (Fig. 3) [46]. The overall difference in positivity rate, pooled from 15 studies, was 32.2% (95% CI: 26.8–37.7%).
3.
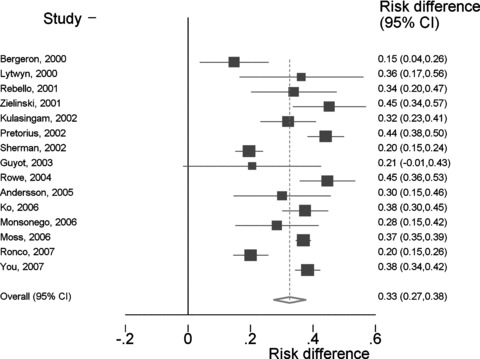
Difference in Hybrid Capture 2 test-positivity rate between LSIL/mild dyskaryosis and ASCUS/borderline dyskaryosis cases.
In 11 studies, age-specific results were provided (Table 1) [13, 17, 39, 49, 50, 54, 56, 61, 62, 64, 67]. However, no subgroup meta-analysis could be performed since the definition of the age strata was not uniform. A consistent and statistically significant negative trend with increasing age was observed in both triage groups (P value for trend test always <0.01). In general, hrHPV rates were higher than 80% among women younger than 30 years, with LSIL/mild dyskaryosis, at the exception of Italy, where 72% of women younger than 35 years were HPV positive. In a triage pilot project, conducted in the UK, a rate of 51% (95% CI: 41.9–60.6%) was observed only in women of 50 years and older with mild dyskaryosis [13].
1.
Change by age group in the HPV test positivity rate (HC2 Assay high-risk probe, signal>1 pg/mL) in the ASCUS/borderline dyskaryosis group and LSIL/mild dyskaryosis group
| ASCUS/ borderline dyskaryosis | LSIL/mild dyskaryosis | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Age group (years) | N | T+ | T+ rate | N | T+ | T+ rate | |||||||||||||||||||||||||||
| Shlay 2000 | <30 | 76 | 37 | 48.7% | - | - | - | |||||||||||||||||||||||||||
| ≥ 30 | 119 | 24 | 20.2% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | ||||||||||||||||||||||||||||||||||
| Sherman, 2002 | 18–22 | 701 | 498 | 71.0% | 383 | 332 | 86.7% | |||||||||||||||||||||||||||
| 23–28 | 653 | 426 | 65.2% | 299 | 263 | 88.0% | ||||||||||||||||||||||||||||
| ≥ 29 | 844 | 263 | 31.2% | 166 | 124 | 74.7% | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | P (chi-squre trend) = 0.007 | |||||||||||||||||||||||||||||||||
| Bruner, 2004 | 40–49 | 54 | 13 | 24.1% | - | - | - | |||||||||||||||||||||||||||
| 50–59 | 27 | 6 | 22.2% | - | - | - | ||||||||||||||||||||||||||||
| 60–69 | 11 | 6 | 54.5% | - | - | - | ||||||||||||||||||||||||||||
| ≥ 70 | 1 | 0 | 0.0% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) = 0.405 | ||||||||||||||||||||||||||||||||||
| Rowe, 2004 | ≤25 | 446 | 244 | 54.7% | - | - | - | |||||||||||||||||||||||||||
| 26–40 | 372 | 134 | 36.0% | - | - | - | ||||||||||||||||||||||||||||
| 41–50 | 224 | 30 | 13.4% | - | - | - | ||||||||||||||||||||||||||||
| >50 | 243 | 30 | 12.3% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | ||||||||||||||||||||||||||||||||||
| Boardman, 2005 | <20 | 94 | 72 | 76.6% | - | - | - | |||||||||||||||||||||||||||
| 20–25 | 231 | 167 | 72.3% | - | - | - | ||||||||||||||||||||||||||||
| >25 | 202 | 118 | 58.4% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) = 0.115 | ||||||||||||||||||||||||||||||||||
| Kendall, 2005 | ≤20 | 1064 | 622 | 58.5% | - | - | - | |||||||||||||||||||||||||||
| 21–25 | 1800 | 909 | 50.5% | - | - | - | ||||||||||||||||||||||||||||
| 26–30 | 1049 | 385 | 36.7% | - | - | - | ||||||||||||||||||||||||||||
| 31–35 | 828 | 207 | 25.0% | - | - | - | ||||||||||||||||||||||||||||
| 36–40 | 847 | 138 | 16.3% | - | - | - | ||||||||||||||||||||||||||||
| 41–45 | 646 | 84 | 13.0% | - | - | - | ||||||||||||||||||||||||||||
| 46–50 | 462 | 62 | 13.4% | - | - | - | ||||||||||||||||||||||||||||
| 51–55 | 252 | 40 | 15.9% | - | - | - | ||||||||||||||||||||||||||||
| 56–60 | 169 | 20 | 11.8% | - | - | - | ||||||||||||||||||||||||||||
| >60 | 217 | 34 | 15.7% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | ||||||||||||||||||||||||||||||||||
| Bergeron, 2006 | ≤20 | 208 | 104 | 50.0% | - | - | - | |||||||||||||||||||||||||||
| 21–25 | 473 | 262 | 55.4% | - | - | - | ||||||||||||||||||||||||||||
| 26–30 | 465 | 238 | 51.2% | - | - | - | ||||||||||||||||||||||||||||
| 31–35 | 506 | 229 | 45.3% | - | - | - | ||||||||||||||||||||||||||||
| 36–40 | 403 | 150 | 37.2% | - | - | - | ||||||||||||||||||||||||||||
| 41–45 | 415 | 128 | 30.8% | - | - | - | ||||||||||||||||||||||||||||
| 46–50 | 298 | 62 | 20.8% | - | - | - | ||||||||||||||||||||||||||||
| 51–55 | 174 | 50 | 28.7% | - | - | - | ||||||||||||||||||||||||||||
| 56–60 | 60 | 13 | 21.7% | - | - | - | ||||||||||||||||||||||||||||
| >60 | 45 | 18 | 40.0% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | ||||||||||||||||||||||||||||||||||
| Moss, 2006 | 20–34 | 1924 | 1239 | 64.4% | 1335 | 1188 | 89.0% | |||||||||||||||||||||||||||
| 35–49 | 1217 | 353 | 29.0% | 373 | 259 | 69.4% | ||||||||||||||||||||||||||||
| 50–64 | 543 | 88 | 16.2% | 117 | 60 | 51.3% | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | P (chi-square trend) < 0.001 | |||||||||||||||||||||||||||||||||
| Selvaggi, 2006 | <20 | 57 | 32 | 56.1% | - | - | - | |||||||||||||||||||||||||||
| 21–25 | 189 | 94 | 49.7% | - | - | - | ||||||||||||||||||||||||||||
| 26–30 | 159 | 61 | 38.4% | - | - | - | ||||||||||||||||||||||||||||
| 31–35 | 91 | 34 | 37.4% | - | - | - | ||||||||||||||||||||||||||||
| 36–40 | 30 | 9 | 30.0% | - | - | - | ||||||||||||||||||||||||||||
| 41–45 | 32 | 8 | 25.0% | - | - | - | ||||||||||||||||||||||||||||
| 46–50 | 41 | 11 | 26.8% | - | - | - | ||||||||||||||||||||||||||||
| 51–55 | 30 | 8 | 26.7% | - | - | - | ||||||||||||||||||||||||||||
| 56–60 | 22 | 5 | 22.7% | - | - | - | ||||||||||||||||||||||||||||
| >60 | 21 | 4 | 19.0% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | ||||||||||||||||||||||||||||||||||
| Wright, 2006 | ≤25 | 446 | 244 | 54.7% | - | - | - | |||||||||||||||||||||||||||
| 26–40 | 372 | 134 | 36.0% | - | - | - | ||||||||||||||||||||||||||||
| 41–50 | 224 | 30 | 13.4% | - | - | - | ||||||||||||||||||||||||||||
| >50 | 243 | 30 | 12.3% | - | - | - | ||||||||||||||||||||||||||||
| P (chi-square trend) < 0.001 | ||||||||||||||||||||||||||||||||||
| Ronco, 2007 | <35 | 241 | 110 | 45.6% | 219 | 157 | 71.7% | |||||||||||||||||||||||||||
| ≥35 | 516 | 128 | 24.8% | 266 | 108 | 40.6% | ||||||||||||||||||||||||||||
| P (chi-square trend) = 0.001 | P (chi-square trend) = 0.002 | |||||||||||||||||||||||||||||||||
Two studies defined HPV positivity at the cut-off of 0.2 pg of HPV DNA per millilitre and were therefore not included in the general meta-analysis shown in the Figs 1 and 2[68, 69]. Nevertheless, the data of these studies and those from other included studies that provided values for higher thresholds [17, 46, 64] were included in the meta-regression. The HPV test positivity rate was 1.60 times higher (95% CI: 1.50–1.68%) in the LSIL/mild dyskaryosis group compared to the ASCUS/borderline group (see Table 2). The positivity rate decreased when the cutoff increased (−3.9%; 95% CI: −2.0 to −5.8% per additional RLU unit) (P< 0.001). The HPV positivity did not vary significantly by continent in ASCUS/borderline smears (P> 0.20); however, in LSIL/mild dyskaryisis, rates were significantly lower in European studies compared to American studies (RR = 0.83; 95% CI: 0.71–0.94%). There was no significant trend by year of publication (P= 0.33). The method of collection of cellular material used for HPV testing (Standard Transport Medium, ThinPrep [Cytyc Corporation, Boxborough, MA, USA], BD-SurePath [TriPath Imaging Inc., Burlington, NC, USA)], other and undefined methods) was not significant either. The test positivity was not significantly different in studies with complete and incomplete verification, added to the meta-analysis because of availability of age details (P= 0.36).
2.
Relative risk of hrHPV positivity computed from a metaregression
| All minor lesions | RR | lcib | ucib |
|---|---|---|---|
| Cytological category | |||
| Atypical cervical cytology (= reference) | 1 | ||
| Low-grade cervical cytology | 1.60 | 1.50 | 1.68 |
| Year of publication (ref = 1998) | 0.99 | 0.96 | 1.01 |
| RLU | 0.96 | 0.94 | 0.98 |
| Atypical cervical cytology | |||
| Cytological subcategory | |||
| ASCUS (= reference) | 1 | ||
| ASC-US | 0.95 | 0.67 | 1.25 |
| Borderline dyskaryosis | 1.03 | 0.74 | 1.35 |
| Continent | |||
| America (= reference) | 1 | ||
| Asia | 1.26 | 0.87 | 1.64 |
| Europe | 0.97 | 0.72 | 1.24 |
| Low-grade cervical cytology | |||
| Cytological subcategory | |||
| LSIL (= reference) | 1.00 | ||
| Mild dyskaryosis | 1.10 | 1.01 | 1.15 |
| Continent | |||
| America (= reference) | 1 | ||
| Asia | 0.96 | 0.82 | 1.06 |
| Europe | 0.83 | 0.71 | 0.94 |
ASCUS: atypical squamous cell of undetermined significance (defined according to 1992 version of The Bethesda System); ASC-US: atypical squamous cell of undetermined significance (defined according to 2002 version of The Bethesda System); LSIL: low-grade squamous intraepithelial lesion; RLU: relative light units (relative unit to express viral load); RR: relative risk; lcib: lower 95% confidence interval bound; ucib: upper 95% confidence interval bound.
Discussion
Substantially higher HPV rates were observed in women with LSIL than among women with ASCUS. This high rate compromises the clinical utility of HPV triaging of LSIL. In the ALTS trial, further enrolment of women with LSIL was interrupted early, when preliminary analyses revealed a HC2-positivity rate of 83%[70, 71]. Testing for high-risk HPV at 12 months after the first observation of LSIL and a negative colposcopy yielded a sensitivity to predict subsequent CIN2 or CIN3 of 92% while referring 55% for repeat colposcopy [72].
The American Society for Colposcopy and Cervical Pathology (ASCCP) recommends that women with low-grade intraepithelial lesions in cytology should be referred to colposcopy [10]. It recommends that large loop excision of the transformation zone is acceptable only when CIN2 or CIN3 is found in histopathology. When colposcopy and biopsies are normal or reveal only CIN1, HPV testing after 12 months is recommended [3, 73]. The ASCCP did not recommend reflex HPV testing in case of LSIL because of the excessive hrHPV test-positivity rates observed in the ALTS study. The pooled results of our meta-analysis are consistent with the ALTS findings.
A British pilot study also showed that the great majority of women with mild dyskaryosis were infected with high-risk papillo-mavirusses, precluding efficient triage by general hrHPV DNA testing. Eighty-four percent of women with mild dyskaryosis had a positive HC2 result. In women younger than 35 years, the test positivity rate even reached 89%[13]. To reduce the excessive number of colposcopies, the triage policy was revised in two of the three participating laboratories and women younger than 35 years were referred only when, 6 months later, they remained HPV positive or showed mild dyskaryosis or worse. The option to postpone HPV triage in women with mild dyskaryosis aged 35 or older was not explored nor simulated in the cost-effect analysis, in spite of the fact that seven to eight out of ten were hrHPV positive [74]. However, in The Netherlands, Bais and Berkhof showed that delayed HPV and repeat cytology testing in patients with borderline or mild dyskaryosis after 6 and 18 months is both safe and more cost-effective than immediate HPV triage [75, 76]. Postponing triage, allows viral clearance, which over a period of 6 to 12 months can vary from 18% to 45%[75] and therefore reduces the need for colposcopy.
From a recent Italian trial, it was suggested that hrHPV triage could be useful in women with LSIL above 35 years of age [64]. However, in this study, rates in hrHPV rates were out-lying (lowest of all studies and 22% lower than the pooled average).
In the case of borderline lesions, where HPV test positivity ranges, on average, between 40% and 50%, the number of colposcopies can be reduced considerably by virological triage. Nevertheless, in women younger than 30–35 years, the specificity and the positive predictive value are low as well [9, 62, 67, 77].
Management of women with minor cytological cervical lesions could be made more specific by increasing the cut-off of a positive HC2 test, but to date insufficient data are available to allow definite conclusions on whether raising of cut-off can be done without compromising sensitivity.
Recent data indicate that typing for the most oncogenic HPV types, in particular for HPV16, has the potential to select women with minor cytological lesions that have a high risk for having or developing CIN3 [78–80]. The ROC curve, in Fig. 4, illustrates the change in the trade-off in sensitivity and specificity (using CIN3+ as outcome) by targeting increasingly more HPV types using ASCUS triage data from the ALTS study [78]. At each step, the type was chosen that yielded the largest gain in sensitivity and the lowest loss in specificity when the gain in sensitivity was equal for more types. HPV16 was the first type to be included, showing a sensitivity of 56% for a specificity of 89%. Adding HPV31 improved the sensitivity by 8% and decreased the specificity by 5%. The number of triage positive women increased considerably by testing for more than 10 types, with only a very small additional detection of CIN3 cases. Figure 4 also contains the point corresponding with the accuracy of HC2 [8]. HC2 targets 13 high-risk types, but also cross-reacts with certain other high- and low-risk types [81]. Therefore, HC2 could reach sensitivity for CIN3+ of 92% for a specificity of only 51%[8]. In the Guanacaste cohort, Castle found a 39% absolute risk for prevalent or incident CIN3 in LSIL women infected with HPV16, whereas women infected with one of the other 12 types included in the high-risk probe of the HC2 assay expressed a risk for CIN3+ of only 10%[82]. These data suggest that focused management of ASCUS cases carrying HPV16 or some other types (located in the left part of the ROC curve in Fig. 4) and more conservative follow-up of ASCUS cases infected with other types (near the right part of the curve) could increase triaging efficiency.
4.
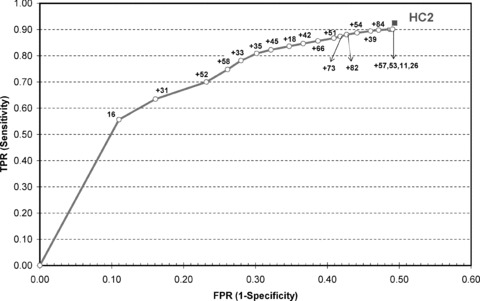
ROC curve representing the true positivity rate (TPR = sensitivity) for CIN3 or worse disease as a function of the false-positivity rate (FPR = 1-specificity) for increasingly sensitive combinations of HPV genotypes. The plot is constructed using data received from the ASCUS-LSIL triage study, and concerns the accuracy of an HPV multi-typing assay applied on women with ASCUS [82]. The square in the upper right corner corresponds with the sensitivity and specificity for CIN3+ of the Hybrid Capture II assay [8].
Triage with a poorly specific test unnecessarily labels women as being at risk for cervical cancer, may induce anxiety and over-treatment and possible subsequent adverse obstetrical effects [83, 84]. Certain molecular markers of early carcinogenic transformation could make triage more specific. One small LSIL triage study using tests for messenger-RNA for viral oncoproteins E6 or E7 with a follow-up of 2 years, showed a low-test positivity rate (27%) and a high specificity (93%) for subsequent high grade CIN [85]. Triage with GP5+/CP6+ PCR showed a positivity rate of 74%, a specificity of 29%, whereas the sensitivity (80%) was equal to that of mRNA testing. Over-expression of certain cell cycle regulator proteins, integration of HPV DNA sequences in the human genome or determination of certain genetic or immunologic profiles are potential candidates for adjunct triage testing [86–91]. A recent meta-analysis established a clear correlation between p16 over-expression and the severity of cytological lesions, however the variation of p16 positivity was extremely large (ranging between 10% and 100% in ASCUS and between 24% and 86% in LSIL), underlining lack of standardization in immuno-staining, interpretation and reporting [92]. Nevertheless, in experienced hands and using clearly defined criteria, p16 immuno-staining has shown excellent results with sensitivities for CIN2+ similar to HC2, remarkably lower positivity rates (27% in ASCUS, 24% in LSIL) and consequently substantially higher specificities (84% and 81%, in ASCUS and LSIL, respectively) [93]. These promising results invite for more powerful well-designed studies to evaluate the role of p16 and other biomarkers that identify progressive cervical lesions and/or transforming HPV infections. Currently, we must acknowledge the lack of good triage studies comparing p16 with currently used alternative strategies to triage minor cytological lesions. We note only one recent Italian study, where p16-immunostaining was used in the background of HPV screening to triage HPV-positive women. P16-enhanced cytology showed a higher sensitivity and similar positive predictive value for high-grade CIN compared to non-stained conventional cytology [94]. In order to explore the potential to use p16 over-expression as a progression marker in triage, we propose to set up an international workshop to standardize issues of sample processing and to define clear criteria for categorizing levels of positivity.
Our meta-analysis also provides substance for the use of hrHPV testing with a standardized assay as quality control method in cytopathology which could become an alternative for established re-screening practices [95–97]. High-risk HPV postivity rates could be used to identify laboratories or cytotechnologists that overcall or undercall equivocal or low-grade abnormalities. If we should use the range −/+ two standard deviations around the pooled test-positivity rates derived from our meta-analysis, we could consider 25–61% as benchmark for equivocal squamous cytology and 57–95% for low-grade lesions. In that case, one study could be hypothesized as overcalling ASC-US [53], two studies as under-calling ASCUS [52, 55] and one study as over-calling LSIL [64]. Certainly, more research and debate is necessary before this idea can be translated into evidence-based guidelines.
Conclusion
Around three quarters of women with LSIL are hrHPV positive compared to less than half with equivocal cytological abnormalities. The pooled results of our meta-analysis are in agreement with ALTS findings and indicate that reflex hrHPV testing is insufficiently discriminative in case of LSIL. In older women with LSIL, hrHPV testing could be useful, but currently no obvious age threshold can be defined by lack of reported age-specific data.
More meta-analytical work is needed, based on 5-year age groups or using individual patient data, also including other HPV testing assays, to provide guidance on LSIL triage. For this purpose, we are currently obtaining age-stratified data from published triage studies. This effort generating clinically relevant evidence will require collaboration between systematic reviewers and the principal investigators of published or ongoing studies [98].
Acknowledgments
Financial support was received from (1) the European Commission (Directorate of SANCO, Luxembourg, Grand-Duchy of Luxembourg), through the ECCG (European Cooperation on development and implementation of Cancer screening and prevention Guidelines, IARC, Lyon, France); (2) European Research Network of Excellence Cancer Control using Population Registries and Biobanking, funded by the 6th Framework programme (Brussels, Belgium) through the University of Lund (Malmö, Sweden); (3) the Gynaecological Cancer Cochrane Review Collaboration (Bath, UK); (4) the Belgian Cancer Foundation (Belgische Stichting tegen Kanker, Brussels, Belgium) and (5) IWT (Institute for the Promotion of Innovation by Science and Technology in Flanders, project number 060081).
We thank Mark Shiffman (National Cancer Institute, Bethesda, USA) for the crude aggregated type-specific data from the ALTS study allowing us to construct the ROC curve in Fig. 4.
References
- 1.Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural History of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–35. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 2.Holowaty P, Miller AB, Rohan T, To T. Natural History of Dysplasia of the Uterine Cervix. J Natl Cancer Inst. 1999;91:252–8. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 3.Wright TC, Cox JT, Massad LS, Wilkinson EJ. 2001 Consensus guidelines for the management of women with cervical cyto-logical abnormalities. JAMA. 2002;287:2120–9. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M, Buntinx F, Van Ranst M, Paraskevaidis E, Martin-Hirsch P, Dillner J. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. J Natl Cancer Inst. 2004;96:280–93. doi: 10.1093/jnci/djh037. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Dillner J, Van Ranst M, Buntinx F, Martin-Hirsch P, Paraskevaidis E. Re: Have we resolved how to triage equivocal cervical cytology? J Natl Cancer Inst. 2004;96:1401–2. doi: 10.1093/jnci/djh277. [DOI] [PubMed] [Google Scholar]
- 6.Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH National Cancer Institute. Interim guidelines for management of abnormal cervical cytology. JAMA. 1994;271:1866–9. [PubMed] [Google Scholar]
- 7.Coleman D, Day N, Douglas G, Farmery E, Lynge E, Philip J, Segnan N. European Guidelines for Quality Assurance in Cervical Cancer Screening. Europe against cancer programme. Eur J Cancer. 1993;29A:S1–S38. [PubMed] [Google Scholar]
- 8.ASCUS-LSIL Triage Study Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188:1383–92. doi: 10.1067/mob.2003.457. [DOI] [PubMed] [Google Scholar]
- 9.Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine. 2006;24(S3):78–89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- 10.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Jordan J, Martin-Hirsch P, Arbyn M, Schenck U, Baldauf JJ, Anttila A, Nieminen P, Prendiville W. In: European Guidelines for Quality Assurance in Cervical Cancer Screening. 2nd ed. Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, Wiener H, Daniel J, Von Karsa L, editors. Luxembourg: Office for Official Publications of the European Communities; 2008. pp. 191–232. [Google Scholar]
- 12.European Commission. European Guidelines for Quality Assurance in Cervical Cancer Screening. 2nd ed. Luxembourg: Office for Official Publications of the European Communities; 2008. [Google Scholar]
- 13.Moss S, Gray A, Legood R, Vessey M, Patnick J, Kitchener HC. Effect of testing for human papillomavirus as a triage during screening for cervical cancer: observational before and after study. BMJ. 2006;332:83–5. doi: 10.1136/bmj.38701.440961.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llewellyn H. Re: An alternative cost-effectiveness analysis of ThinPrep in the Australian setting. Aust N Z J Obstet Gynaecol. 2006;46:67. doi: 10.1111/j.1479-828X.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 15.Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN. An update of pooled evidence. Gynecol Oncol. 2005;99:7–11. doi: 10.1016/j.ygyno.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Solomon D, Schiffman MA, Tarone B. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance (ASCUS): baseline results from a randomized trial. J Natl Cancer Inst. 2001;93:293–9. doi: 10.1093/jnci/93.4.293. [DOI] [PubMed] [Google Scholar]
- 17.Sherman ME, Schiffman MA, Cox JT. Effects of age and human papilloma viral load on colposcopy triage: data from the randomised atypical squamous cells of undetermined significance/low-grade intraepithelial lesion triage study (ALTS) J Natl Cancer Inst. 2002;94:102–7. doi: 10.1093/jnci/94.2.102. [DOI] [PubMed] [Google Scholar]
- 18.Luff RD National Cancer Institute. The revised Bethesda system for reporting cervical/vaginal cytological diagnoses. Report of the 1991 Bethesda Workshop. Acta Cytol. 1992;36:273–6. [PubMed] [Google Scholar]
- 19.Solomon D, Davey D, Kurman R, Moriarty A, O’Connor D, Prey M, Raab S, Sherman ME, Wilbur D, Wright TC, Young N. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 20.Evans DMD, Hudson EA, Brown CL, Boddington MM, Hughes HE, Mackenzie EFD, Marshall T. Terminology in gynaecological cytopathology: report of the working party of the BSCC. J Clin Pathol. 1986;39:933–44. doi: 10.1136/jcp.39.9.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudding N, Sutton J. BSCC terminology conference, koilocytosis and mild dyskaryosis. Cytopathology. 2002;13:379–81. doi: 10.1046/j.1365-2303.2002.00448_1.x. [DOI] [PubMed] [Google Scholar]
- 22.Dersimonian R, Laird NM. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29. [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Light RJ, Singer JD, Willet JB. In: The handbook of research synthesis. Cooper H, editor. New York: Russel Sage Foundation; 1994. pp. 439–54. [Google Scholar]
- 26.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester, UK: J. Wiley and Sons; 2000. [Google Scholar]
- 27.Harris R, Bradburm M, Deeks J, Harbord R, Altman D, Sterne J. Metan: fixed- and random-effects meta-analysis. The Stata Journal. 2008;8:3–26. [Google Scholar]
- 28.Bradburn M, Deeks JJ, Altman DG. Metan – an alternative meta-analysis command. Stata Technical Bulletin. 1999;8:86–100. [Google Scholar]
- 29.Sasieni P, Stepniewska K, Altman D. Test for trend across ordered groups revisited. Stata Technical Bulletin. 1996;6:193–6. [Google Scholar]
- 30.Lau J, Ioannidis JPA, Schmid CH. Summing up evidence: one answer is not always enough. Lancet. 1998;351:123–7. doi: 10.1016/S0140-6736(97)08468-7. [DOI] [PubMed] [Google Scholar]
- 31.Sharp S. Meta-analysis regression. Stata Technical Bulletin. 1998;7:148–55. [Google Scholar]
- 32.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 34.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–3. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 35.Sharp S, Sterne J. Meta-analysis. Stata Technical Bulletin. 1997:9–14. [Google Scholar]
- 36.Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh-Ngai J, Kurman RJ, Ransley JE, Fetterman ME, Hartinger JS, Mclntosh KM, Pawlick GF, Hiatt RA. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal papanicolaou results. JAMA. 1999;281:1605–10. doi: 10.1001/jama.281.17.1605. [DOI] [PubMed] [Google Scholar]
- 37.Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol. 2000;95:821–7. doi: 10.1016/s0029-7844(00)00795-x. [DOI] [PubMed] [Google Scholar]
- 38.Lytwyn A, Sellors JW, Mahony JB. Comparison of human papillomavirus DNA testing and repeat Papanicolaou test in women with low-grade cervical cytologic abnormalities: a randomized trial. CMAJ. 2000;163:701–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Shlay JC, Dunn T, Byers T, Baron AE, Douglas JM., Jr Prediction of cerviacal intraepithelial neoplasia grade 2–3 using risk assessement and human papillo-mavirus testing in women with atypia on papanicolaou smears. Obstet Gynecol. 2000;96:410–6. doi: 10.1016/s0029-7844(00)00907-8. [DOI] [PubMed] [Google Scholar]
- 40.Morin C, Bariati C, Bouchard C, Fortier M, Roy M, Moore L, Meisels A. Managing atypical squamous cells of undetermined significance in Papanicolaou smears. J Reprod Med. 2001;46:799–805. [PubMed] [Google Scholar]
- 41.Rebello G, Hallam N, Smart G, Farquharson D, McCafferty J. Human papillomavirus testing and the management of women with mildly abnormal cervical smears: an observational study. BMJ. 2001;322:894–5. doi: 10.1136/bmj.322.7291.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zielinski GD, Snijders PJ, Rozendaal L, Voorhorst FJ, Runsink AP, De Schipper FA, Meijer CJLM. High-risk HPV testing in women with borderline and mild dyskaryosis: long-term follow-up data and clinical relevance. J Pathol. 2001;195:300–6. doi: 10.1002/path.981. [DOI] [PubMed] [Google Scholar]
- 43.Kulasingam SL, Hughes JP, Kiviat NB, Mao C, Weiss NS, Kuypers JM, Koutsky LA. Evaluation of human papillomavirus testing in primary screening cervical abnormalities. Comparison of sesitivity, specificity, and frequency of referral. JAMA. 2002;288:1749–57. doi: 10.1001/jama.288.14.1749. [DOI] [PubMed] [Google Scholar]
- 44.Pretorius RG, Peterson P, Novak S, Azizi F, Sadeghi M, Lorincz AT. Comparison of two signal-amplification DNA tests for high-risk HPV as an aid to colposcopy. J Reprod Med. 2002;47:290–6. [PubMed] [Google Scholar]
- 45.Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener HC, Luesley D, McGoogan E, Menon U, Terry G, Edwards R, Brooks C, Desai M, Gie C, Ho L, Jacobs I, Pickles C, Sasieni P. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362:1871–6. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 46.Guyot A, Karim S, Kyi MS, Fox J. Evaluation of adjunctive HPV testing by hybrid capture II(R) in women with minor cytological abnormalities for the diagnosis of CIN2/3 and cost comparison with colposcopy. BMC Infectious Diseases. 2003;3:1–7. doi: 10.1186/1471-2334-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lonky NM, Felix JC, Naidu YM, Wolde Tsadik G. Triage of atypical squamous cells of undetermined significance with Hybrid Capture II: colposcopy and histo-logic human papillomavirus correlation. Obstet Gynecol. 2003;101:481–9. doi: 10.1016/s0029-7844(02)02715-1. [DOI] [PubMed] [Google Scholar]
- 48.Wensveen C, Kagie M, Veldhuizen R, De Groot C, Denny L, Zwinderman K, Trimbos B. Detection of cervical intraep-ithelial neoplasia in women with atypical squamous or glandular cells of undetermined significance cytology: a prospective study. Acta Obstet Gynecol Scand. 2003;82:883–9. doi: 10.1034/j.1600-0412.2003.00231.x. [DOI] [PubMed] [Google Scholar]
- 49.Bruner KS, Davey DD. ASC-US and HPV testing in women aged 40 years and over. Diagn Cytopathol. 2004;31:358–61. doi: 10.1002/dc.20144. [DOI] [PubMed] [Google Scholar]
- 50.Rowe LR, Aldeen W, Bentz JS. Prevalence and typing of HPV DNA by Hybrid Capture II in women with ASCUS, ASC-H, LSIL, and AGC on ThinPrep Pap tests. Diagn Cytopathol. 2004;30:426–32. doi: 10.1002/dc.20052. [DOI] [PubMed] [Google Scholar]
- 51.Andersson S, Dillner L, Elfgren K, Mints M, Persson M, Rylander E. A comparison of the human papillomavirus test and Papanicolaou smear as a second screening method for women with minor cytolog-ical abnormalities. Acta Obstet Gynecol Scand. 2005;84:996–1000. doi: 10.1111/j.0001-6349.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 52.Dalla Palma P, Pojer A, Girlando S. HPV triage of women with atypical squamous cells of undetermined significance: a 3-year experience in an Italian organized programme. Cytopathology. 2005;16:22–6. doi: 10.1111/j.1365-2303.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 53.Giovannelli L, Capra G, Lama A, Bustinto T, Genco A, Valenti FM, Pinto G, Matranga D, Ammatuna P. Atypical squamous cells of undetermined significance-favour reactive compared to atypical squamous cells of undetermined significance-favour dysplasia: association with cervical intraepithelial lesions and human papillomavirus infection. J Clin Virol. 2005;33:281–6. doi: 10.1016/j.jcv.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Kendall BS, Bush AC, Olsen CH, Zahn CM. Reflex high-risk human papillomavirus testing for women with atypical squamous cells of undetermined significance in cytologic smears: effects since implementation in a large clinical practice. Am J Clin Pathol. 2005;123:524–8. doi: 10.1309/YTM2-HQA5-0XUW-86YC. [DOI] [PubMed] [Google Scholar]
- 55.Nieh S, Chen SF, Chu TY, Lai HC, Lin YS, Fu E, Gau CH. Is p16(INK4A) expression more useful than human papillomavirus test to determine the outcome of atypical squamous cells of undetermined significance-categorized Pap smear? A comparative analysis using abnormal cervical smears with follow-up biopsies. Gynecol Oncol. 2005;97:35–40. doi: 10.1016/j.ygyno.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 56.Bergeron C, Cas F, Fagnani F, Contrepas A, Wadler R, Poveda JD. Assessment of human papillomavirus testing on liquid-based CYTO-screen system for women with atypical squamous cells of undetermined significance. Effect of age. Gynecol Obstet Fertil. 2006;34:312–6. doi: 10.1016/j.gyobfe.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Kelly D, Kincaid E, Fansler Z, Rosenthal DL, Clark DP. Detection of cervical high-grade squamous intraepithelial lesions from cytologic samples using a novel immunocytochemical assay (ProExtrade mark C) Cancer. 2006;108:494–500. doi: 10.1002/cncr.22288. [DOI] [PubMed] [Google Scholar]
- 58.Kiatpongsan S, Niruthisard S, Mutirangura A, Trivijitsilp P, Vasuratna A, Chaithongwongwatthana S, Lertkhachonsuk R. Role of human papillomavirus DNA testing in management of women with atypical squamous cells of undetermined significance. Int J Gynecol Cancer. 2006;16:262–5. doi: 10.1111/j.1525-1438.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 59.Ko V, Tambouret RH, Kuebler DL, Black-Schaffer WS, Wilbur DC. Human papillomavirus testing using hybrid capture II with surepath collection: initial evaluation and longitudinal data provide clinical validation for this method. Cancer. 2006;108:468–74. doi: 10.1002/cncr.22285. [DOI] [PubMed] [Google Scholar]
- 60.Monsonego J, Pintos J, Semaille C, Beumont M, Dachez R, Zerat L, Bianchi A, Franco E. Human papillomavirus testing improves the accuracy of colposcopy in detection of cervical intraepithelial neoplasia. Int J Gynecol Cancer. 2006;16:591–8. doi: 10.1111/j.1525-1438.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 61.Selvaggi SM. ASC-US and high-risk HPV testing: performance in daily clinical practice. Diagn Cytopathol. 2006;34:731–3. doi: 10.1002/dc.20547. [DOI] [PubMed] [Google Scholar]
- 62.Wright JD, Rader JS, Davila R, Powell MA, Mutch DG, Gao F, Gibb RK. Human papillomavirus triage for young women with atypical squamous cells of undetermined significance. Obstet Gynecol. 2006;107:822–9. doi: 10.1097/01.AOG.0000207557.30226.25. [DOI] [PubMed] [Google Scholar]
- 63.Cuschieri KS, Graham C, Moore C, Cubie HA. Human Papillomavirus testing for the management of low-grade cervical abnormalities in the UK-Influence of age and testing strategy. J Clin Virol. 2007;38:14–8. doi: 10.1016/j.jcv.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 64.Ronco G, Cuzick J, Segnan N, Brezzi S, Carozzi F, Folicaldi S, Palma PD, Mistro AD, Gillio-Tos A, Giubilato P, Naldoni C, Polla E, Iossa A, Zorzi M, Confortini M, Giorgi-Rossi P. HPV triage for low grade (L-SIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology. Eur J Cancer. 2007;43:476–80. doi: 10.1016/j.ejca.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 65.You K, Liang X, Qin F, Guo Y, Geng L. High-risk human papillomavirus DNA testing and high-grade cervical intraepithelial lesions. Aust N Z J Obstet Gynaecol. 2007;47:141–4. doi: 10.1111/j.1479-828X.2007.00701.x. [DOI] [PubMed] [Google Scholar]
- 66.Lee NW, Kim D, Park JT, Kim A. Is the human papillomavirus test in combination with the Papanicolaou test useful for management of patients with diagnoses of atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions? Arch Pathol Lab Med. 2001;125:1453–7. doi: 10.5858/2001-125-1453-ITHPTI. [DOI] [PubMed] [Google Scholar]
- 67.Boardman LA, Stanko C, Weitzen S, Sung J. Atypical squamous cells of undetermined significance: human papillomavirus testing in adolescents. Obstet Gynecol. 2005;105:741–6. doi: 10.1097/01.AOG.0000157126.12678.a6. [DOI] [PubMed] [Google Scholar]
- 68.Ferris DG, Wright TC, Jr, Litaker MS, Richart RM, Lorincz AT, Sun XW, Woodward L. Comparison of two tests for detecting carcinogenic HPV in women with Papanicolaou smear reports of ASCUS and LSIL. J Fam Pract. 1998;46:136–41. [PubMed] [Google Scholar]
- 69.Lin CT, Tseng CJ, Lai CH, Hsueh S, Huang HJ, Law KS. High-risk HPV DNA detection by Hybrid Capture II. An adjunc-tive test for mildly abnormal cytologic smears in women > or = 50 years of age. J Reprod Med. 2000;45:345–50. [PubMed] [Google Scholar]
- 70.ALTS group, Anonymous. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst. 2000;92:397–402. doi: 10.1093/jnci/92.5.397. [DOI] [PubMed] [Google Scholar]
- 71.ASCUS-LSIL Triage Study Group. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol. 2003;188:1393–400. doi: 10.1067/mob.2003.462. [DOI] [PubMed] [Google Scholar]
- 72.Guido R, Schiffman MA, Solomon D, Burke L. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA-positive atypical squamous cells of undetermined significance: a two-year prospective study. Am J Obstet Gynecol. 2003;188:1401–5. doi: 10.1067/mob.2003.456. [DOI] [PubMed] [Google Scholar]
- 73.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neo-plasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197:340–5. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 74.Legood R, Gray A, Wolstenholme J, Moss S. Lifetime effects, costs, and cost effectiveness of testing for human papillo-mavirus to manage low grade cytological abnormalities: modelling study. BMJ. 2006;332:79–85. doi: 10.1136/bmj.38698.458866.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bais AG, Rebolj M, Snijders PJ, De Schipper FA, Van Der Meulen DA, Verheijen RH, Voorhorst F, Van Ballegooijen M, Meijer CJ, Helmerhorst TJ. Triage using HPV-testing in persistent borderline and mildly dyskaryotic smears: proposal for new guidelines. Int J Cancer. 2005;116:122–9. doi: 10.1002/ijc.20958. [DOI] [PubMed] [Google Scholar]
- 76.Berkhof J, De Bruijne MC, Zielinski GD, Bulkmans NW, Rozendaal L, Snijders PJ, Verheijen RH, Meijer CJ. Evaluation of cervical screening strategies with adjunct high-risk human papillomavirus testing for women with borderline or mild dyskaryosis. Int J Cancer. 2006;118:1759–68. doi: 10.1002/ijc.21513. [DOI] [PubMed] [Google Scholar]
- 77.Sawaya GF. A 21-year-old woman with atypical squamous cells of undetermined significance. JAMA. 2005;294:2210–8. doi: 10.1001/jama.294.17.2210. [DOI] [PubMed] [Google Scholar]
- 78.Schiffman M, Khan MJ, Solomon D, Herrero R, Wacholder S, Hildesheim A, Rodriguez AC, Bratti MC, Wheeler CM, Burk RD. A study of the impact of adding HPV types to cervical cancer screening and triage tests. J Natl Cancer Inst. 2005;97:147–50. doi: 10.1093/jnci/dji014. [DOI] [PubMed] [Google Scholar]
- 79.Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1157–64. doi: 10.1158/1055-9965.EPI-04-0812. [DOI] [PubMed] [Google Scholar]
- 80.Franceschi S, Clifford GM. Re: A study of the impact of adding HPV types to cervical cancer screening and triage tests. J Natl Cancer Inst. 2005;97:938–9. doi: 10.1093/jnci/dji159. [DOI] [PubMed] [Google Scholar]
- 81.Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papil-lomavirus DNA in women with equivocal or mildly abnormal papanicolaou smears. Am J Clin Pathol. 2005;124:722–32. doi: 10.1309/E067-X0L1-U3CY-37NW. [DOI] [PubMed] [Google Scholar]
- 82.Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97:1066–71. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 83.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intra-epithelial or early invasive cervical lesions: a systematic review and meta-analysis of the literature. Lancet. 2006;367:489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 84.Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, Prendiville W, Paraskevaidis E. Peri-natal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: a meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Molden T, Nygard JF, Kraus I, Karlsen F, Nygard M, Skare GB, Skomedal H, Thoresen SO, Hagmar B. Predicting CIN2+ when detecting HPV mRNA and DNA by PreTect HPV-proofer and consensus PCR: A 2-year follow-up of women with ASCUS or LSIL pap smear. Int J Cancer. 2005;114:973–6. doi: 10.1002/ijc.20839. [DOI] [PubMed] [Google Scholar]
- 86.Arias-Pulido H, Narayan G, Vargas H, Mansukhani M, Murty VV. Mapping common deleted regions on 5p15 in cervical carcinoma and their occurrence in precan-cerous lesions. Mol Cancer. 2002;1:3. doi: 10.1186/1476-4598-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bibbo M, Klump WJ, DeCecco J, Kovatich AJ. Procedure for immunocytochemical detection of P16INK4A antigen in thin-layer, liquid-based specimens. Acta Cytol. 2002;46:25–9. doi: 10.1159/000326711. [DOI] [PubMed] [Google Scholar]
- 88.Kadish AS, Timmins P, Wang Y, Ho GY, Burk RD, Ketz J, He W, Romney SL, Johnson A, Angeletti R, Abadi M. Regression of cervical intraepithelial neoplasia and loss of human papillomavirus (HPV) infection is associated with cell-mediated immune responses to an HPV type 16 E7 peptide. Cancer Epidemiol Biomarkers Prev. 2002;11:483–8. [PubMed] [Google Scholar]
- 89.Von Knebel DM. New markers for cervical dysplasia to visualise the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer. 2002;38:2229–42. doi: 10.1016/s0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 90.Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 91.Solomon D. Chapter 14: Role of triage testing in cervical cancer screening. J Natl Cancer Inst Monogr. 2003:97–101. doi: 10.1093/oxfordjournals.jncimonographs.a003489. [DOI] [PubMed] [Google Scholar]
- 92.Tsoumpou I, Arbyn M, Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Paraskevaidis E. p16INK4a immunostaining in cytological and histological specimens from the uterine cervix: a systematic review and meta-analysis. Cancer Treat Rev. 2009 doi: 10.1016/j.ctrv.2008.10.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wentzensen N, Bergeron C, Cas F, Vinokurova S, Von Knebel DM. Triage of women with ASCUS and LSIL cytology: use of qualitative assessment of p16INK4a positive cells to identify patients with high-grade cervical intraepithelial neoplasia. Cancer. 2007;111:58–66. doi: 10.1002/cncr.22420. [DOI] [PubMed] [Google Scholar]
- 94.Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Gillio-Tos A, De Marco L, Giorgi-Rossi P, Pontenani G, Rosso S, Sani C, Sintoni C, Segnan N, Zorzi M, Cuzick J, Rizzolo R, Ronco G New Technologies for Cervical Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol. 2008;9:937–45. doi: 10.1016/S1470-2045(08)70208-0. [DOI] [PubMed] [Google Scholar]
- 95.Ko V, Nanji S, Tambouret RH, Wilbur DC. Testing for HPV as an objective measure for quality assurance in gynecologic cytology: positive rates in equivocal and abnormal specimens and comparison with the ASCUS to SIL ratio. Cancer. 2007;111:67–73. doi: 10.1002/cncr.22488. [DOI] [PubMed] [Google Scholar]
- 96.Arbyn M, Schenck U, Ellison E, Hanselaar A. Metaanalysis of the accuracy of rapid prescreening relative to full screening of pap smears. Cancer. 2003;99:9–16. doi: 10.1002/cncr.10921. [DOI] [PubMed] [Google Scholar]
- 97.Depuydt CE, Arbyn M, Benoy IH, Vandepitte J, Vereecken A, Bogers JJ. Quality control for normal liquid based cytology: rescreening, high-risk HPV targeted reviewing and/or high-risk HPV detection? J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00379.x. doi: 10.1111/j.1582-4934.2008.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arbyn M, Cuzick J. International agreement to join forces in synthesizing evidence on new methods for cervical cancer prevention. Cancer Lett. 2008 doi: 10.1016/j.canlet.2008.09.002. in press. [DOI] [PubMed] [Google Scholar]


