Abstract
The human gene MRS2L encodes a mitochondrial protein distantly related to CorA Mg2+ transport proteins. Constitutive shRNA-mediated knockdown of hMRS2 in human HEK-293 cell line was found here to cause death. To further study its role in Mg2+ transport, we have established stable cell lines with conditionally expressing shRNAs directed against hMRS2L. The cells expressing shRNA for several generations exhibited lower steady-state levels of free mitochondrial Mg2+ ([Mg2+]m) and reduced capacity of mitochondrial Mg2+ uptake than control cells. Long-term expression of shRNAs resulted in loss of mitochondrial respiratory complex I, decreased mitochondrial membrane potential and cell death. We conclude that hMrs2 is the major transport protein for Mg + uptake into mitochondria and that expression of hMrs2 is essential for the maintenance of respiratory complex I and cell viability.
Keywords: complex I, magnesium, shRNA, mag-fura 2
Introduction
Mg2+ uptake into the mitochondrial matrix occurs via elec-trophoretic uniport, driven by the (inside negative) membrane potential ΔΨm. Intramitochondrial Mg2+ concentrations ([Mg2+]m) are kept close to those in the cytoplasm, and thus far below electrochemical equilibrium. This is indicating that Mg2+ influx into the organelle is tightly controlled [1, 2]. Earlier we have identified a nuclear gene of the yeast Saccharomyces cerevisiae, named yMRS2, as encoding a mitochondrial inner membrane protein mediating Mg2+ influx into this organelle. Activity of this protein is essential for mitochondrial functions, but yeast cells lacking yMrs2 are viable under fermentative growth conditions [3–5]. This protein is distantly related to the bacterial CorA Mg2+ channel protein [6]. Most recently we have shown that yeast Mrs2 forms a high-capacity Mg +-selective ion channel [6, 7].
The human genome encodes a single expressed homologue (MRS2L) of the yeast protein that has been shown to be an integral protein of the mitochondrial membrane. Low levels of the transcript were detected in various tissues. Expression of the human Mrs2 protein (hMrs2) in yeast mrs2 knockout cells can partially suppress their mitochondrial dysfunction. Accordingly, yMrs2 and hMrs2 appear to be functional homologues [8].
In order to characterize the role of hMrs2 in mitochondria, we constitutively expressed shRNA directed against hMRS2 mRNA in HEK-293 cells. Because cells did not survive constitutive knockdown of hMRS2 we utilized conditional expression of shRNA [9]. We obtained clones of a HEK-293 cell line with stably integrated constructs expressing shRNAs directed against human Mrs2 mRNA under the control of doxycycline-regulated promoter. Studies on these conditional knockdown clones revealed that Mrs2 constitutes the major Mg2+ uptake system in human mitochondria. Upon Mrs2 ablation, the mitochondria lack complex I of the respiratory chain and cells die.
Materials and methods
Cell culture
Human embryonic kidney cells (HEK-293) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/l glucose, 3.7 g/l NaHCO3 and 110 mg/l pyruvate, supplemented with 8% foetal bovine serum (FBS, GibcoBRL). Additionally, DMEM (4.5 g/l glucose, 3.7 g/l NaHCO3, 8% FBS) without pyruvate and uridine and DMEM (4.5 g/l glucose, 3.7 g/l NaHCO3, 8% FBS) containing 110 mg/l pyruvate (Invitrogen) and 4 μg/ml uridine (Sigma) were used as mentioned in the text. Cultures were kept at 37°C in 5% CO2 atmosphere.
Transfection was performed using the SuperFect transfection reagent (Qiagen GmbH, Hilden, Germany). Stably transfected cells were selected in media supplemented with either 200 μg/ml neomycin (Invitrogen Ltd. Paisley UK) or 50 μg/ml hygromycin (Invitrogen) or 50 μg/ml zeocin (Invitrogen) or 2 μg/ml blasticidin (Invitrogen). The expression of shRNAs was initiated by addition of doxycycline (Sigma, Sigma-Aldrich, St Louis, MO, USA) at the concentration of 1 μg/ml.
Constructs
Synthetic DNA template sequences for shRNAs expression were as follows (complementary sequences underlined):
MRS2-E: AGATCTCCACCATGGAATGCCTGCGCTTCAA GAGAGCGCAGGC-ATTCCATGGTG TTTTTGGAAAAGCTT and
MRS2-Q: AGATCTCAGCTCGTGAGCTTAGGGTGTTCAA GAGACACCCTAA-GCTCACGAGCT TTTTTGGAAAAGCTT.
The MRS2-E and MRS2-Q templates were generated by annealing of the sense and antisense oligonucleotides and filling-in by Klenow fragment. The obtained DNA fragments were digested by BglII and HindIII and ligated either into pSuper or pTer vectors (both kindly provide by Reuven Agami) [9, 10].
Constructs for tet-inducible shRNA expression were generated by liga-tion of the HindIII-PstI fragment harbouring expression cassettes for shRNA-E or −Q from pTer vector into HindIII-PstI sites of pIND vector with hygromycin selection marker (Invitrogen) yielding ptetHsh-E and ptetHsh-Q. Constructs ptetHsh-E and ptetHsh-Q and the empty parental vector (pIND) were stably transfected into a HEK-293 cell line expressing the tet-repressor (tetR/Neo, derived from pcDNA3.1, Invitrogen, [11]) kindly provided by D. Takai. The resulting clones are referred to as MPL-E1, MPL-Q1 and MPL-O, respectively.
The hMrs2-HA construct in the pcDNA3 vector has been described previously [8]. The hMrs2-HA insert of this vector was cloned into the HindIII/EcoRI sites of the expression vector pIND with the neomycin selection marker. HEK-293 clones MPL-E1 and MPL-Q1, stably transfected with the vectors pVgRXR and pIND-hMrs2-HA have been named MPL-E1/Mrs2-HA and MPL-Q1/Mrs2-HA.
Isolation of mitochondria and Mg2+ measurements
Cells were harvested by centrifugation and resuspended in cold isolation buffer (IB) (210 mM mannitol, 70 mM sucrose, 5 mM Hepes-KOH pH 7.4 and 1 mM DTT) with 0.25% BSA (fraction V) and 2 mM EGTA/KOH pH 7.4. After digitonin treatment (1 mg/ml) the cells were washed and then broken in a Dounce-homogeniser on ice. Cell debris was removed by repeated low speed centrifugation (300 g for 3 min.). Mitochondria were collected by centrifugation (10.000 g for 20 min.), washed twice with IB buffer with EGTA, recollected again by centrifugation and finally resuspended in IB buffer with EGTA, according to [12].
Measurements of [Mg2+] by spectrofluorometry
The determination of free ionised Mg2+ concentrations ([Mg2+]) in isolated mitochondria by use of mag-fura-2 was done essentially as describe in detail by [5] and in the legend to Supplementary Figure 3. Continuous recordings of fluorescent intensities at excitation 340 and 380 nm (Mg2+-bound and -free dye, respectively) were transformed into 340/380 wavelength ratios by use of the computer program FL WinLab (Perkin-Elmer) and [Mg2+]i values were calculated from the 340/380 nm ratio according to the formula of Grynkiewicz et al.[13], with a dissociation constant 1.53 for the mag-fura 2 -Mg2+ complex.
Membrane potential measurements
Cells were loaded with fluorescence dye JC-1 (Molecular Probes) by incubating them with this membrane potential-sensitive dye (final concentration 0.5 μg/ml) in DMEM with 8% FBS for 30 min. at 37°C and 5% CO2. After washing the fresh DMEM with 8% FBS was added to the cells. Zeiss Axioplan2 microscope was used with appropriate filter sets (emitter/splitter/exciter F41–001, F41–007, F41–020, AF Analysentechnik, Germany): FITC (excitation/emission 460–500 nm/520–570 nm) for JC-1 monomer monitoring (excitation/emission 485 nm/530 nm) and CY3 (excitation/emission 525–565 nm/570–645 nm) for JC-1 aggregate monitoring (excitation/emission ∼535 nm/570 nm). Images were captured using a digital charge-coupled device camera (Spot2, Diagnostic Instruments, Inc.).
Gel electrophoresis and immunoblotting
Mitochondria were isolated from cells of clones MPL-O, MPL-E1 and MPL-Q1 grown in the presence of doxycycline for 15 days. Two hundred μg of mitochondria were extracted by addition of extraction buffer (750 mM aminocaproic acid, 50 mM Bistris/HCl pH 7.0) and laurylmaltoside to a final concentration of 1%. After incubation on ice for 30 min., the samples were centrifuged at 100,000 g for 30 min. and the supernatant was supplemented with ¼ vol. of sample buffer (500 mM aminocaproic acid, 5% Serva blue G). Twenty-five μl of the solubilized protein solution was analysed by blue native (BN) electrophoresis on a 5–18% linear polyacry-lamide gradient according to [14]. The gel was blotted onto a nitrocellulose membrane, which was then either stained with Coomassi Blue reagent or analysed by immunoblotting.
To observe shRNA-dependent expression of Mrs2-HA, clones MPL-E1/Mrs2-HA and MPL-Q1/Mrs2-HA were grown first for 36 hrs either in medium with or without doxycycline and then for 24 hrs in the presence of ponasterone, inducing Mrs2-HA expression. Mitochondrial fractions isolated from these cells were separated by SDS-PAGE and immunoblotted with anti-HA and porin (Calbiochem) antisera.
To monitor effects of knockdown of MRS2 expression on complex I clones MPL-O, MPL-E1 and MPL-Q1 were grown for 0, 5, 10 or 15 days in presence of doxycycline. Mitochondria were isolated and total mitochondrial proteins were separated by SDS-PAGE and immunoblotted using an antiserum against the 39 kD subunit of complex I.
Results
Constitutively expressed shRNA directed against hMRS2
To find effective shRNAs interfering with hMRS2 expression, we have transfected HEK-293 cells with vectors constitutively expressing shRNAs directed against various regions of hMRS2 mRNA (Supplementary Fig. 1A and B). Transient transfection with the pEGFP-Hsh-E and -Q constructs had the strongest effect on the expression of a GFP reporter construct (Supplementary Fig. 1C). In an attempt to obtain stable clones constitutively expressing shRNA-E, we have transfected HEK-293 cells with a construct (pHsh-E) expressing shRNA-E under the control of the H1 promoter (Supplementary Fig. 2A). However, at early stages of post-transfectional selection growth of most clones expressing the shRNA-E was arrested after 14 days. Microscopic inspection of these clones revealed cells with aberrant morphology that were partly detached from the surface of the dish. Vital staining with the lypophilic fluorescence dye JC-1 indicated that most arrested cells were still energized, but many JC-1-loaded daughter cells showed very weak fluorescence of the JC-1 aggregate, indicating partial loss of the mitochondrial membrane potential ΔΨm (Supplementary Fig. 2B).
Conditional knockdown of hMrs2 expression
To generate conditional hMRS2 knockdown, we have transfected the HEK-293 cells with ptetHsh-E and -Q constructs expressing shRNA under the control of the doxycycline-regulated RNA poly-merase III promoter H1 and, in parallel, with a vector expressing the tetracycline repressor (Fig. 1A). Resulting clones MPL-E1 and MPL-Q1 were overall healthy, propagated well and exhibited normal morphology. About 10 days after the activation of shRNA transcription (by doxycycline supplementation), no morphological without doxycycline (Fig. 1B). Only when propagated in the presence of doxycycline for 25 days or more clones expressing ptetHsh-E or -Q constructs developed aberrant morphology, rounded up and detached from the surface of the dishes. Control cells transfected with the same vector lacking an shRNA template (MPL-O) stayed healthy during prolonged times of supplementation with doxycycline (Fig. 1B).
1.
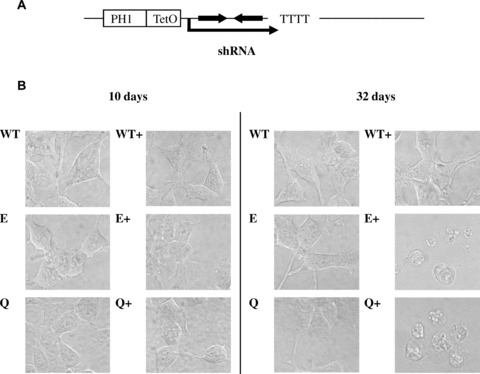
Conditional knockdown of the Mrs2. (A) Overview of ptetHsh constructs for the conditional expression of shRNA-E and shRNA-Q from a tet-regulated H1 promoter (doxycycline binding site tetO downstream of the TATA box). (B) Morphology of stably transfected HEK-293 cells carrying vector tetR/Neo and either vector pTer (without shRNA template) or vector ptetHsh-E or vector ptetHsh-Q (marked WT, E and Q, respectively). Cells were grown in the absence of the inducer doxycycline (WT, E and Q) or in the presence of doxycycline (WT+, E+ and Q+) for times indicated. Cell morphology was observed by visible light microscopy
Next, we determined the effect of shRNA-E or -Q induction on the expression of hMrs2. Because no hMrs2-specific antibodies are available, we have transfected MPL-E1 and MPL-Q1 clones with pIND-hMrs2-HA/pVgRXR construct and examined by immunoblotting the expression level of hMRS2-HA protein after 2 days’ induction of shRNA expression with doxycycline and subsequent overnight induction of Mrs2 transcription with ponasterone in isolated mitochondria. As shown in Figure 2, mitochondria isolated from the cells MPL-E1/hMrs2-HA and MPL-Q1/hMrs2-HA growing in absence of doxycycline had large amounts of Mrs2. In contrast, the clones induced with doxycycline for shRNA expression had much lower levels of Mrs2-HA. Accordingly we conclude that doxycycline-induced expression of shRNA-E or -Q strongly affected the expression of endogenous Mrs2.
2.
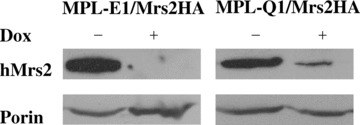
Down-regulation of Mrs2 protein level by shRNAs. Clones MPL-E1 and MPL-Q1 were transfected with vectors pVgRXR (encoding the ecdysone-VP16 and RXR receptor) and pIND-hMrs2-HA, enabling ponasterone A (Pon, analogue of ecdysone) inducible expression of hMrs2-HA. Stable transfectants were grown for 36 hrs in parallel either with doxycycline (Dox, analogue of tetracycline) or without this inducer and then additionally in the presence of ponasterone for 24 hrs to induce expression of the Mrs-HA protein. Proteins of mitochondrial fractions obtained from these cultures were separated by SDS-PAGE and analysed by immunoblotting using antisera against HA epitope and against porin (outer mitochondrial membrane protein, used as a loading control).
Determination of Mg2+ uptake rates into isolated mitochondria
Isolated yeast mitochondria have been shown to respond to a rise of external Mg2+ ([Mg2+]e), by a rapid increase of intra-mitochon-drial free Mg2+ concentrations ([Mg2+]m), which can be measured over time by use of the entrapped Mg2+-sensitive dye mag-fura 2 [5]. We have adapted this ratiometric method of [Mg2+]m determination to HEK-293 mitochondria (cf. METHODS and Supplementary Fig. 3).
Figure 3 presents continuous recordings of [Mg2+]m obtained with mitochondria isolated from clones MPL-E1, expressing shRNA-E and mitochondria from control cells MPL-O, all grown for 10 days in the presence of doxycycline. In MPL-O mitochondria [Mg2+]m was calculated to be about 1 mM at resting conditions and to rapidly rise to 2 mM with [Mg2+]e of 1 mM (Fig. 3). A further increase of [Mg2+]e to 5 mM led to a minor increase in [Mg2+]m only (not shown). The response to raising [Mg2+]e above 1 mM was somewhat variable from experiment to experiment, but generally much less pronounced than the increase observed with [Mg2+]e of 1 mM. This indicated that the mitochondrial Mg2+ uptake system was already saturated at about 2 mM [Mg2+]m. Plateau levels remained constant over extended periods of time, confirming that neither mag-fura 2 leaked in nor magnesium leaked out from the mitochondria. The rapid increase in [Mg2+]m within a few seconds was comparable to that observed with yeast mitochondria and reflected a high capacity influx driven by the (inside negative) membrane potential ΔΨm of mitochondria.
3.
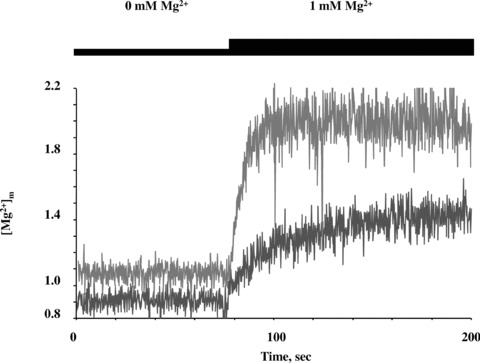
Determination of free Mg2+ concentrations [Mg2+]m in isolated mitochondria dependent on [Mg2+]e. Mitochondria isolated from clones MPL-O (with empty vector; red curve) and MPL-E1 (expressing shRNA-E; blue curve) after 10 days of doxycy-cline treatment were loaded with the Mg2+-sensitive dye mag-fura 2. Changes in free Mg2+ concentrations [Mg2+]m over time were determined upon raising extramitochondrial Mg2+ concentrations [Mg2+]e from nominally 0 mM to 1 mM. (For details of methods cf. legend to Supplementary Fig. 3 and [5]).
Mitochondria isolated from shRNA expressing cell lines MPL-E1 exhibited consistently lower [Mg2+]m values (by 53 ± 17% on average of seven experiments) than in control cells at resting conditions (Fig. 3). Measurements of total cellular Mg2+ revealed a small, but statistically not significant decrease of its concentration in clones expressing shRNA-E and shRNA-Q for 10 days (Supplementary Fig. 4). Upon a raise of [Mg2+]e to 1 mM initial rates of increase in [Mg2+]m (Δ[Mg2+]m based on the slope of tangents of the curves within the first few seconds were more than fivefold smaller with MPL-E1 mitochondria than with mitochondria isolated from MPL-O control cells. In these ‘mutant’ mitochondria the slow increase in [Mg2+]m lasted for several minutes, but plateau levels finally reached were significantly below those of control cells (not shown). Similar results were obtained with mitochondria isolated from MPL-Q1 cells expressing shRNA-Q for 10 days (not shown).
Stable clones expressing shRNA (MPL-E1, -Q1) as well as control cells (MPL-O) growing in medium supplemented with doxycycline for 10 days were adherent and healthy without any apparent morphological changes. These cells exhibited similar JC-1 fluorescence as cells lacking shRNA expression (Supplementary Fig. 5). Quantitative determination of JC-1 fluorescence signals obtained from isolated mitochondria of the mutant and control cells also revealed similar ΔΨm values (not shown). Thus, a reduction in Mg2+ uptake due to weak driving forces could be excluded. Accordingly, shRNA mediated reduction in expression of hMrs2 was regarded as resulting in strongly reduced rates of Mg2+ uptake into mitochondria of clones MPL-E1 and -Q1 after 10 days of shRNA induction.
Effects on membrane potential and mitochondrial membrane complexes
Blue native gel electrophoresis was applied to observe effects of down-regulation of Mrs2 expression on mitochondrial membrane complexes (Fig. 4). Compared to cells not induced for shRNA expression the lack of complex I (NADH:ubiquinone oxidoreduc-tase) was apparent after 15 days of shRNA expression while the pattern of other protein complexes appeared to be unchanged. Western blotting of the blue native gels involving antisera against SU1 of complex I and subunit 4 of complex IV clearly documented the absence of the former complex, but no apparent reduction of the latter. Time-course experiments revealed that amounts of the 39 kD subunit of complex I were slightly decreased 10 days after shRNA induction and strongly decreased upon prolonged shRNA expression (Fig. 5 and Supplementary Fig. 6).
4.
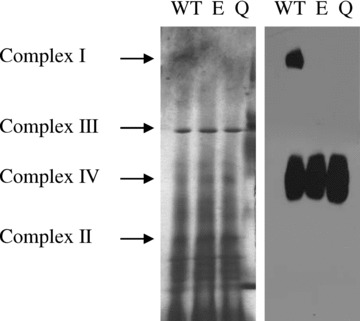
Effect of Mrs2 ablation on mitochondrial membrane complexes. Clones MPL-O (with an empty vector), MPL-E1 and MPL-Q1 (expressing ptetHshE and ptetHshQ, respectively), marked here as WT, E and Q, were grown for 15 days in the presence of doxycycline. The mitochondria were isolated and proteins were solubilized in laurylmaltoside (1%). Respiratory chain complexes were resolved by BN-PAGE. One part (left) of the gel was stained with Coomassie Blue whereas the other part (right) was transferred onto nitrocellulose membrane and immunodeco-rated with sera against the 39 kD subunit of the complex I and against subunit IV of the complex IV.
5.
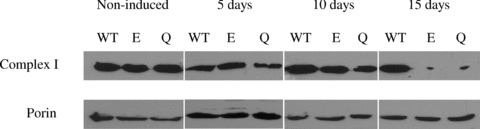
Down-regulation of Mrs2 expression decreases amounts of 39 kD subunit of complex I. HEK-293 clones MPL-O MPL-E1 or MPL-Q1 (designated as WT, E and Q) were grown for 15 days without doxycycline (non-induced) or for 5, 10 or 15 days in presence of doxycycline. Mitochondria were isolated and total mitochondrial proteins were separated by SDS-PAGE and immunoblotted using an antiserum against the 39 kD subunit of complex I (upper panel) or porin (lower panel).
Mitochondrial membrane potential ΔΨm was only marginally reduced after 15 days of shRNA expression and more dramatically after 25 days (not shown). Lack of complex I alone is neither reducing ΔΨm dramatically nor causing cell death in media with abundant glucose [15] because ΔΨm can be maintained via ATP hydrolysis by ATP synthase in its reverse mode of activity [16].
Cell death, however, is known to occur upon loss of mitochondrial DNA or blockade of the respiratory chain (though not at complex I) except when media with abundant glucose are supplemented with pyruvate and uridine [17, 18]. We have observed the fate of cells depleted of Mrs2 grown in glucose-containing medium without uridine and pyruvate or in the same medium additionally supplemented with uridine and pyruvate. Both clones MPL-E and MPL-Q showed identical onset of cell death after about 25 days of shRNA expression (not shown). Cell death therefore is not likely due to loss of mtDNA or total loss of respiratory chain components in addition to complex I, but to so far unknown effects of Mrs2 depletion.
Discussion
By using the fluorescent Mg2+-sensitive dye mag-fura 2 we have established conditions for ratiometric measurements of free ionized Mg2+ concentrations [Mg2+]m in isolated human mitochondria, which allowed us to detect rapid changes in [Mg2+]m within short time intervals. Mitochondria of Mrs2 expressing HEK-293 cells responded to the addition of external Mg2+ with a twofold increase in [Mg2+]m which reached plateau levels within a few seconds. We interpret this increase as reflecting rapid influx of Mg2+ into the organelles. Maximal [Mg2+]m levels (of about 2 mM) stayed far below electrochemical equilibrium indicating a tight control of [Mg2+]m.
Earlier attempts to determine changes of [Mg2+]m dependent on [Mg2+]e[2], revealed similar large and rapid increases in [Mg]m, but only in the presence of the K+/H+ ionophore nigericin which converts ApH to Aij; in respiring mitochondria. In our assays the membrane potential (as determined by JC-1) as well as [Mg]m increased only slightly upon addition of nigericin, indicating that mitochondria used here were fully energized.
Knockdown of MRS2L in human cell culture clearly resulted in a strong reduction of Mg2+ uptake. Initial rates of uptake in response to increases in [Mg2+]e were about fivefold lower than in the cells not expressing the shRNA. Steady-state [Mg2+]m values were also lower in mitochondria of knockdown cells, but only by a factor of two. The more modest reduction in steady state levels than in Mg2+ influx is explained by the observation that residual Mg2+ uptake activity in hMrs2 knockdown mitochondria continues over an extended period of time at a low rate before it finally reaches saturation at a somewhat lower level than in control mitochondria. Knockdown of hMrs2 expression in HEK-293 cells thus had similar effects as mrs2Δ knockout mutations in yeast [5].
While the decrease of Mrs2 expression for 10 days caused a considerable loss in Mg2+ uptake capacity into mitochondria, cells stayed fully energized, morphologically unchanged and vital. Only when expression of shRNA-E or -Q was induced during 25 days or more, massive cells death could be observed, while doxycy-cline-treated control cells propagated further and stayed morphologically unaltered for at least 50 days. Mrs2 depletion led to a near complete lack of complex I of the respiratory chain, but has no obvious effects on amounts of other complexes.
In fact, synthesis of mitochondrially made proteins was completely unaffected (our unpublished results). Accordingly, Mrs2 ablation is likely to affect either assembly or maintenance of the large complex I in the inner mitochondrial membrane which is composed of seven mitochondrially encoded and a much larger number of nuclearly encoded proteins. A direct comparison of Mrs2 depletion effects in human and yeast cells is not feasible because yeast lacks a classical complex I of the respiratory chain. Yet yeast mrs2Δ cells were found to have a moderate reduction of complex IV of the respiratory chain, resulting either from defects in complex assembly or maintenance in the membrane (our unpublished results).
Mrs2-depleted cells propagated for a considerable period of time in media with abundant glucose after they had essentially lost complex I. Like respiratory deficient human cell lines with mutations in complex I [10, 11] they are assumed to gain ATP by gly-colysis and to limit the extent of mitochondrial depolarization by reversal of ATP synthase action. Why Mrs2-depleted cells finally died about 25 days after shRNA induction remained obscure. Loss of mitochondrial DNA (rho0 mutation) appeared unlikely because addition of pyruvate and uridine to growth media of Mrs2-depleted cells did not prevent their cell death while it is known to prevent cell death of rho0 cells [12]. Accordingly, so far unknown mitochondrial deficiencies may have caused the phenomenon of delayed cell death in Mrs2-depeleted human cells.
The drop of [Mg2+]m upon Mrs2 depletion observed here in human cells or previously in yeast cells [5, 6] is moderate, but has surprisingly dramatic physiological effects. Yet, similar or even milder reductions in [Mg2+]i, have previously been shown to cause major functional changes. Lowered [Mg2+]i caused a bacterial mRNA to undergo conformational changes resulting in transcrip-tional arrest [19]. Mrs2-depleted yeast cells were reported to arrest mitochondrial RNA splicing [3, 4, 20, 21]. The effects of low free Mg2+ concentrations on RNA processing and on the assembly or stability of protein complexes may reflect one of the major known roles of magnesium in biology, namely to stabilize structures of macromolecules. It comes as a surprise that apparently some of these structures would be seriously affected by relatively minor changes in [Mg2+]i.
Acknowledgments
Material for shRNA expression was kindly provided by Reuven Agami, Daiya Takai, Masaru Okabe and David R.Engelke. This work was supported by the Austrian Science Fund FWF.
Supporting Information
Effect of the shRNA on the Mrs2 expression.(A) Sequences of the shRNA-E and -Q directed to MRS2 mRNA.The shRNA-E and −Q were targeted to regions from −4 to+15 bp and from +917 to +945 bp(5′ end and central part of coding sequence, respectively) ofMRS2L (accession number Q9HD23) mRNA sequence. (B) Schematicpresentation of the construct pEGFP-Hsh-E used for the expressionof shRNA-E from Pol III promoter H1 and of EGFP mRNA from Pol IIpromoter CMV. The latter transcript contained the MRS2-E sequenceas a target for mRNA interference by shRNA-E. The EGFP reporterconstruct pEGFP/Hsh-E for monitoring of shRNA interferingefficiency was generated by ligation of HindIII-BamHIfragment harbouring the expression cassette from pSuper vector intoHindIII-BamHI sites of pEGFP-C1 vector with neomycinselection marker (Clontech). (C) Fluorescence microscopy ofHEK-293 cells revealing shRNA-dependent down-regulation of GFPfluorescence. Confluent cell populations were transientlytransfected either with the empty pEGFP-C1 vector or with thepEGFP-Hsh-E constructs. Intensities of EGFP fluorescence wereobserved 36 hrs after transfection. While pEGFP-C1 transfectantsshowed bright green fluorescence, pEGFP-shRNA transfectantsrevealed only barely detectable green fluorescence consistent withthe notion that shRNA-E interfered with the reporter GFP-mRNAvia its MRS2 target sequence. Only a faint signal could be observed upon pro-longed exposure of the cells transfected with the pEGFP-Hsh-E construct. Similar results were obtained with the pEGFP-Hsh-Q construct (not shown).
Constitutive Mrs2 knockdown. (A) Schematicrepresentation of the construct pHsh-E used for constitutiveexpression of the shRNA under the constitutive Pol III promoter H1.(B) Microscopy of the HEK-293 cells constitutivelyexpressing shRNA-E. Hygromycin selection of pHsh-E transfectantsfor 14 days resulted in colonies with aberrant morphology (B, leftpanel). When stained with the lypophilic dye JC-1, most transfectedcells showed bright JC-1 fluorescence with a FITC filter (monomericform of the dye; middle panel) and Cy3 filter (JC-1 aggregates,right panel), essentially reflecting loading of cells with the dyeand aggregate formation dependent on the mitochondrial membranepotential ΔΨm, respectively. Some daughtercells (circled) showed reduced FITC signals and lacked Cy3 signalsindicating loss of mitochondrial membrane potential.
Original records of the Mg2+uptake measurements. HEK-293 cells stably transfected with (empty)NeoR/Tet and pTer plas-mids were grown for 10 days indoxycycline-supplemented medium. Mitochondrial fractions wereisolated and loaded with the Mg2+-sensitivefluorescent dye mag-fura 2 (furaptra). (A) Loading ofmitochondria with the 5 μM membrane permeable mag-fura 2-AM(acetoxymethyl ester) and 5% permeation facilitatorPluronic acid F-127 (both purchased from Molecular Probes) was donein IB buffer with EGTA and 0.5 mM ATP at 25°C for 30 min.Mitochondria were collected by centrifugation (10.000 g for 2min.), washed twice with IB buffer (without EGTA) and recollectedagain by centrifugation. The measurements were performed at25°C in the Perkin-Elmer LS-55 spectrofluorometer with fastfilter accessory (FFA), which allowed fluorescence to be measuredat 20 ms intervals with emission at 509 nm of free mag-fura 2 andMg +-bound mag-fura 2 excited at 380 and 340nm, respectively, with stirring in 3 ml cuvettes containing 2 ml ofisolated mitochondria. Initially the mitochondria were kept innominally Mg +-free IB buffer (restingconditions). The stepwise increase of the external[Mg2+] to finalconcentrations of 1 and 5 mM caused increases in the 340 nm anddecreases in the 380 nm excitation curves, reflecting changes ofthe ion concentration [Mg2+]in mitochondria. For the calibration of the fluorescence signals atthe end of each experiment 20 mM Mg2+ was addedtogether with triton 80 to rupture the mitochondrial membrane.Release of mag-fura 2 to the buffer led to saturation of the dyewith Mg2+ that resulted in maximum of340/380 nm ratio (Rmax). Finally, addition ofEDTA sufficient to chelate all divalent metal ions resulted inminimum 340/380 nm wavelength ratio (Rmin).(B) Continuous recordings of fluorescence intensities at 340and 380 nm were transformed into 340/380 wavelength ratiosby use of the computer program FL WinLab (Perkin-Elmer).
Total magnesium concentration of MPL-O, MPL-E1and MPL-Q1 clones. Cells of the MPL-O, MPL-E1 and MPL-Q1 clonesgrown in the presence of doxycycline for 10 days were resuspendedin PBS buffer and permeabilized with digitonin (0.1 mg/ml).Magnesium concentration (± S.D.) of the supernatant obtained after centrifugation at 15,000 rpm was measured by atomic absorption spectroscopy.
Mitochondrial membrane potential of the cellswith down-regulated Mrs2. Microscopy of the HEK-293 cells loadedwith JC-1. Stably transfected clones MPL-O, MPL-E1 or MPL-Q1 weregrown for 10 days in medium supplemented with doxycycline beforethe cells were loaded with JC-1 to determine their mitochondrialmembrane potential ΔΨm. The filters FITC andCY3 were used to monitor the monomeric and oligomeric(ΔΨm-dependent) forms of JC-1, respectively.
Amounts of complex I at different time-points after shRNA expression. Mitochondria of clone MPL-E1 (designated here as E) non-induced or grown for different times (10, 11, 12, 13, 14 and 15 days) with doxycycline were isolated and total proteins were separated by SDS-PAGE. Immunoblotting with serum against the 39 kD subunit of complex I (upper panel) or porin (lower panel) was used to see effect of shRNA expression on the amount of complex I.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Romani AM, Scarpa A. Regulation of cellular magnesium. Front Biosci. 2000;5:720–34. doi: 10.2741/romani. [DOI] [PubMed] [Google Scholar]
- 2.Jung DW, Panzeter E, Baysal K, Brierley GP. On the relationship between matrix free Mg2+ concentration and total Mg2+ in heart mitochondria. Biochim Biophys Acta. 1997;1320:310–20. doi: 10.1016/s0005-2728(97)00036-4. [DOI] [PubMed] [Google Scholar]
- 3.Bui DM, Gregan J, Jarosch E, Ragnini A, Schweyen RJ. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J Biol Chem. 1999;274:20438–43. doi: 10.1074/jbc.274.29.20438. [DOI] [PubMed] [Google Scholar]
- 4.Gregan J, Kolisek M, Schweyen RJ. Mitochondrial Mg(2+) homeostasis is critical for group II intron splicing in vivo. Genes Dev. 2001;15:2229–37. doi: 10.1101/gad.201301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major elec-trophoretic Mg2+ influx system in mitochondria. EMBO J. 2003;22:1235–44. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maguire ME. The structure of CorA: a Mg(2+)-selective channel. Curr Opin Struct Biol. 2006;16:432–8. doi: 10.1016/j.sbi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Schindl R, Weghuber J, Romanin C, Schweyen RJ. Mrs2p forms a high conductance Mg2+ selective channel in mitochondria. Biophys J. 2007;93:3872–83. doi: 10.1529/biophysj.107.112318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zsurka G, Gregan J, Schweyen RJ. The human mitochondrial Mrs2 protein functionally substitutes for its yeast homologue, a candidate magnesium transporter. Genomics. 2001;72:158–68. doi: 10.1006/geno.2000.6407. [DOI] [PubMed] [Google Scholar]
- 9.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 10.Van De Wetering M, Oving I, Muncan V, Pon Fong MT, Brantjes H, Van Leenen D, Holstege FC, Brummelkamp TR, Agami R, Clevers H. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 2003;4:609–15. doi: 10.1038/sj.embor.embor865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsukura S, Jones PA, Takai D. Establishment of conditional vectors for hairpin siRNA knockdowns. Nucleic Acids Res. 2003;31:e77. doi: 10.1093/nar/gng077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondr-ial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 13.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 14.Schagger H, Von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–31. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 15.Yadava N, Potluri P, Smith EN, Bisevac A, Scheffler IE. Species-specific and mutant MWFE proteins. Their effect on the assembly of a functional mammalian mitochon-drial complex I. J Biol Chem. 2002;277:21221–30. doi: 10.1074/jbc.M202016200. [DOI] [PubMed] [Google Scholar]
- 16.Rego AC, Vesce S, Nicholls DG. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1–7 neural cells. Cell Death Differ. 2001;8:995–1003. doi: 10.1038/sj.cdd.4400916. [DOI] [PubMed] [Google Scholar]
- 17.King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989;246:500–3. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- 18.Loffler M, Jockel J, Schuster G, Becker C. Dihydroorotat-ubiquinone oxidoreductase links mitochondria in the biosynthesis of pyrimidine nucleotides. Mol Cell Biochem. 1997;174:125–9. [PubMed] [Google Scholar]
- 19.Cromie MJ, Shi Y, Latifi T, Groisman EA. An RNA sensor for intracellular Mg(2+) Cell. 2006;125:71–84. doi: 10.1016/j.cell.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 20.Wiesenberger G, Waldherr M, Schweyen RJ. The nuclear gene MRS2 is essential for the excision of group II introns from yeast mitochondrial transcripts in vivo. J Biol Chem. 1992;267:6963–9. [PubMed] [Google Scholar]
- 21.Gregan J, Bui DM, Pillich R, Fink M, Zsurka G, Schweyen RJ. The mitochondr-ial inner membrane protein Lpe10p, a homologue of Mrs2p, is essential for magnesium homeostasis and group II intron splicing in yeast. Mol Gen Genet. 2001;264:773–81. doi: 10.1007/s004380000366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of the shRNA on the Mrs2 expression.(A) Sequences of the shRNA-E and -Q directed to MRS2 mRNA.The shRNA-E and −Q were targeted to regions from −4 to+15 bp and from +917 to +945 bp(5′ end and central part of coding sequence, respectively) ofMRS2L (accession number Q9HD23) mRNA sequence. (B) Schematicpresentation of the construct pEGFP-Hsh-E used for the expressionof shRNA-E from Pol III promoter H1 and of EGFP mRNA from Pol IIpromoter CMV. The latter transcript contained the MRS2-E sequenceas a target for mRNA interference by shRNA-E. The EGFP reporterconstruct pEGFP/Hsh-E for monitoring of shRNA interferingefficiency was generated by ligation of HindIII-BamHIfragment harbouring the expression cassette from pSuper vector intoHindIII-BamHI sites of pEGFP-C1 vector with neomycinselection marker (Clontech). (C) Fluorescence microscopy ofHEK-293 cells revealing shRNA-dependent down-regulation of GFPfluorescence. Confluent cell populations were transientlytransfected either with the empty pEGFP-C1 vector or with thepEGFP-Hsh-E constructs. Intensities of EGFP fluorescence wereobserved 36 hrs after transfection. While pEGFP-C1 transfectantsshowed bright green fluorescence, pEGFP-shRNA transfectantsrevealed only barely detectable green fluorescence consistent withthe notion that shRNA-E interfered with the reporter GFP-mRNAvia its MRS2 target sequence. Only a faint signal could be observed upon pro-longed exposure of the cells transfected with the pEGFP-Hsh-E construct. Similar results were obtained with the pEGFP-Hsh-Q construct (not shown).
Constitutive Mrs2 knockdown. (A) Schematicrepresentation of the construct pHsh-E used for constitutiveexpression of the shRNA under the constitutive Pol III promoter H1.(B) Microscopy of the HEK-293 cells constitutivelyexpressing shRNA-E. Hygromycin selection of pHsh-E transfectantsfor 14 days resulted in colonies with aberrant morphology (B, leftpanel). When stained with the lypophilic dye JC-1, most transfectedcells showed bright JC-1 fluorescence with a FITC filter (monomericform of the dye; middle panel) and Cy3 filter (JC-1 aggregates,right panel), essentially reflecting loading of cells with the dyeand aggregate formation dependent on the mitochondrial membranepotential ΔΨm, respectively. Some daughtercells (circled) showed reduced FITC signals and lacked Cy3 signalsindicating loss of mitochondrial membrane potential.
Original records of the Mg2+uptake measurements. HEK-293 cells stably transfected with (empty)NeoR/Tet and pTer plas-mids were grown for 10 days indoxycycline-supplemented medium. Mitochondrial fractions wereisolated and loaded with the Mg2+-sensitivefluorescent dye mag-fura 2 (furaptra). (A) Loading ofmitochondria with the 5 μM membrane permeable mag-fura 2-AM(acetoxymethyl ester) and 5% permeation facilitatorPluronic acid F-127 (both purchased from Molecular Probes) was donein IB buffer with EGTA and 0.5 mM ATP at 25°C for 30 min.Mitochondria were collected by centrifugation (10.000 g for 2min.), washed twice with IB buffer (without EGTA) and recollectedagain by centrifugation. The measurements were performed at25°C in the Perkin-Elmer LS-55 spectrofluorometer with fastfilter accessory (FFA), which allowed fluorescence to be measuredat 20 ms intervals with emission at 509 nm of free mag-fura 2 andMg +-bound mag-fura 2 excited at 380 and 340nm, respectively, with stirring in 3 ml cuvettes containing 2 ml ofisolated mitochondria. Initially the mitochondria were kept innominally Mg +-free IB buffer (restingconditions). The stepwise increase of the external[Mg2+] to finalconcentrations of 1 and 5 mM caused increases in the 340 nm anddecreases in the 380 nm excitation curves, reflecting changes ofthe ion concentration [Mg2+]in mitochondria. For the calibration of the fluorescence signals atthe end of each experiment 20 mM Mg2+ was addedtogether with triton 80 to rupture the mitochondrial membrane.Release of mag-fura 2 to the buffer led to saturation of the dyewith Mg2+ that resulted in maximum of340/380 nm ratio (Rmax). Finally, addition ofEDTA sufficient to chelate all divalent metal ions resulted inminimum 340/380 nm wavelength ratio (Rmin).(B) Continuous recordings of fluorescence intensities at 340and 380 nm were transformed into 340/380 wavelength ratiosby use of the computer program FL WinLab (Perkin-Elmer).
Total magnesium concentration of MPL-O, MPL-E1and MPL-Q1 clones. Cells of the MPL-O, MPL-E1 and MPL-Q1 clonesgrown in the presence of doxycycline for 10 days were resuspendedin PBS buffer and permeabilized with digitonin (0.1 mg/ml).Magnesium concentration (± S.D.) of the supernatant obtained after centrifugation at 15,000 rpm was measured by atomic absorption spectroscopy.
Mitochondrial membrane potential of the cellswith down-regulated Mrs2. Microscopy of the HEK-293 cells loadedwith JC-1. Stably transfected clones MPL-O, MPL-E1 or MPL-Q1 weregrown for 10 days in medium supplemented with doxycycline beforethe cells were loaded with JC-1 to determine their mitochondrialmembrane potential ΔΨm. The filters FITC andCY3 were used to monitor the monomeric and oligomeric(ΔΨm-dependent) forms of JC-1, respectively.
Amounts of complex I at different time-points after shRNA expression. Mitochondria of clone MPL-E1 (designated here as E) non-induced or grown for different times (10, 11, 12, 13, 14 and 15 days) with doxycycline were isolated and total proteins were separated by SDS-PAGE. Immunoblotting with serum against the 39 kD subunit of complex I (upper panel) or porin (lower panel) was used to see effect of shRNA expression on the amount of complex I.


