Abstract
Fibroblast apoptosis plays a crucial role in normal and pathological scar formation and therefore we studied whether the putative apoptosis-inducing factor curcumin affects fibroblast apoptosis and may function as a novel therapeutic. We show that 25-μM curcumin causes fibroblast apoptosis and that this could be inhibited by co-administration of antioxidants N-acetyl-l-cysteine (NAC), biliverdin or bilirubin, suggesting that reactive oxygen species (ROS) are involved. This is supported by our observation that 25-μM curcumin caused the generation of ROS, which could be completely blocked by addition of NAC or bilirubin. Since biliverdin and bilirubin are downstream products of heme degradation by heme oxygenase (HO), it has been suggested that HO-activity protects against curcumin-induced apoptosis. Interestingly, exposure to curcumin maximally induced HO-1 protein and HO-activity at 10–15 μM, whereas, at a concentration of >20-μM curcumin HO-1-expression and HO-activity was negligible. NAC-mediated inhibition of 25-μM curcumin-induced apoptosis was demonstrated to act in part via restored HO-1-induction, since the rescuing effect of NAC could be reduced by inhibiting HO-activity. Moreover pre-induction of HO-1 using 5-μM curcumin protected fibroblasts against 25-μM curcumin-induced apoptosis. On a functional level, fibroblast-mediated collagen gel contraction, an in vitro wound contraction model, was completely prevented by 25-μM curcumin, while this could be reversed by co-incubation with NAC, an effect that was also partially HO-mediated. In conclusion, curcumin treatment in high doses (>25 μM) may provide a novel way to modulate pathological scar formation through the induction of fibroblast apoptosis, while antioxidants, HO-activity and its effector molecules act as a possible fine-tuning regulator.
Keywords: fibroblast apoptosis, wound healing, wound contraction, scar formation, heme oxygenase, antioxidant, bilirubin, curcumin, reactive oxygen species
Introduction
During the normal wound healing process, three distinct but overlapping phases can be distinguished, namely the inflammatory, proliferation and remodelling phase. During the remodelling phase, fibroblasts that have migrated into the wound area and deposited new extracellular matrix molecules (ECM) eventually go through the process of apoptosis. This results in a relatively acellular scar, which is the end-point of normal wound healing. Conversely, during abnormal or pathological wound healing, fibroblasts do not undergo apoptosis, resulting in a hypertrophic scar [1]. Such a scar contains increased amounts of ECM and fibroblasts compared to a normal scar. On rare occasions, the scar may even extend beyond the boundaries of the original wound (keloid scar). Irrespective of the type of scar that is eventually formed, significant problems for the patient can arise since scars are not only disfiguring but can also cause significant functional impairment.
The polyphenol curcumin has shown to increase wound contraction and decrease wound-healing time in full-thickness wound models in rodents [2]. Following curcumin treatment, the wounds showed enhanced fibronectin and collagen expression combined with increased collagen maturation and collagen cross-linking, which resulted in increased tensile strength. Also in diabetic animals, curcumin beneficially affected skin wound healing as shown by increased formation of granulation tissue that eventually resulted in increased neo-vascularization and faster re-epithelization [3, 4]. Similarly, curcumin demonstrated to improve wound healing after gamma-irradiation or to protect against radiation-induced damage in animal models [5].
It has been described in detail that curcumin induces apoptosis in a wide variety of cell lines [6–10]. Although the mechanism by which curcumin initiates cell death is unclear and strongly depends on the cell type, reactive oxygen species (ROS) seem to be a common mediator. Curcumin-induced ROS formation in human hepatoma cells induced the nuclear factor-erythroid 2-related factor 2 (Nrf2) transcription factor, which could be blocked by the antioxidants N-acetyl-l-cysteine (NAC) and vitamin E [11]. Atsumi et al. have also demonstrated that treatment with NAC blocks curcumin-induced apoptosis in human gingival fibroblasts and leukemia cells [6, 12].
Panchatcharam et al. observed that curcumin induced the activity of antioxidant enzymes superoxide dismutase, catalase and glutathione peroxidase [2] and detoxifying enzymes such as cytochrome P450 [13] and glutathione s-transferase [14]. For instance, H2O2-induced damage in keratinocytes and fibroblasts could be inhibited by curcumin [15]. Furthermore, curcumin can also induce the cytoprotective enzyme heme oxygenase-1 (HO-1) in several cell types [16–18]. HO is the enzyme that can break down heme thereby generating carbon monoxide (CO), free ferrous iron and biliverdin. The latter is immediately converted to bilirubin by biliverdin reductase (BVR). Bilirubin represents one of the most powerful endogenous antioxidants known so far [19]. Two isoforms of HO have been characterized, of which HO-2 is considered to be constitutively expressed and responsible for normal heme capturing and catabolism. On the other hand, HO-1, which is normally expressed at a low level, is highly inducible by its substrate heme, various stress-related stimuli and tissue injury [20].
Here we report that curcumin treatment can induce human dermal fibroblast apoptosis and inhibit fibroblast-mediated collagen gel contraction via a ROS-mediated process, since apoptosis could be completely blocked by antioxidants such as NAC, biliverdin and bilirubin. Interestingly, enhanced levels of HO-1 protect against effects of curcumin and therefore, HO-effector molecules could possibly function as regulators of curcumin-induced fibroblast apoptosis and contraction, thereby enabling fine-tuning of the balance between acellular and hypertrophic wound healing.
Materials and methods
Fibroblast culture
Human foreskin-derived fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM) with high glucose, containing 10% foetal calf serum (MP Biomedicals, Uden, the Netherlands) and 1% penicillin/streptomycin/amphotericin B (Invitrogen life sciences, Breda, the Netherlands) at 37°C in a 5% CO2 atmosphere. Medium was refreshed every 2–3 days and when the cells reached ∼90% confluence they were subcultured using trypsin-EDTA (Invitrogen) at a 1:3 dilution.
Preparation of porphyrin and biliverdin/bilirubin solutions
Heme (FePP), cobalt protoporphyrin (CoPP), stannous (tin) mesoporphyrin (SnMP), biliverdin and bilirubin were all obtained from Frontier Scientific, Carnforth, UK. All solutions were freshly prepared as previously described [21]. In short, the substances were dissolved together with Trizma base in a 0.1-M NaOH solution and further diluted in H2O. After the pH was adjusted to pH 8 with HCl, H2O was added to obtain a 2-mM solution. The solutions were then filter-sterilized, protected from light and directly used.
Detection of apoptosis induced by curcumin using FACS analysis
In this study, FACS analysis was used to differentiate between living, early apoptotic, late apoptotic/necrotic and necrotic cells by staining with Annexin V-FITC and propidium iodide (PI). Fibroblasts were plated at 70% confluence in 6-well plates and allowed to adhere for at least 6 hrs before the cells were treated with varying doses of curcumin (2.5–25 μM, Sigma-Aldrich, Zwijndrecht, the Netherlands) for 48 hrs.
To investigate whether the observed curcumin-induced apoptosis was mediated by reactive oxygen species (ROS) we incubated the cells with the known antioxidants N-acetyl-l-cysteine (NAC, 6 mM, Sigma-Aldrich), or with the HO-effector molecules biliverdin or bilirubin (1 μM) in the presence of 25 μM curcumin. To block HO-activity during curcumin + NAC treatment we used the previously described specific non-porphyrin HO-activity inhibitor QC-15 at a dose of 50 μM [22, 23]. Since we observed that 50-μM SnMP in combination with 25-μM curcumin affected HO-1 expression and therefore could act as a possible confounder, we opted for the QC-15 inhibitor to study the effect of HO-activity in all experiments where 25-μM curcumin was used. No effect of QC-15 alone or in combination with curcumin on the expression of HO-1 was found in this study.
We also studied whether moderately enhanced expression of HO-1 protects cells against apoptosis. In order to induce HO-1 expression, adherent fibroblasts were pre-treated for 24 hrs with varying doses (2.5; 5 or 10 μM) of curcumin alone or not treated, followed by a 48-hr incubation period with 25 μM curcumin that normally causes apoptosis. In addition, pre-conditioning using 5 μM of curcumin was performed in the absence or presence of the HO-activity inhibitor SnMP (20 μM), followed by exposure to 25 μM curcumin.
Early apoptosis was demonstrated by determining phosphatidyl serine (PS) exposure on the outside of the cell membrane via Annexin V-FITC staining. After incubation, all cells were collected and 100,000 cells were resuspended in 100 μl binding buffer containing Annexin V-FITC and PI according to the manufacturers recommendations (Biovision Inc, Uithoorn, the Netherlands). Next, samples were incubated for 15 min. at room temperature (RT) in the dark. As a positive control for the Annexin V-FITC and PI staining, fibroblasts fixed with 4% paraformaldehyde and permeabilized by 0.1% saponine were used. Quantification of Annexin V-FITC and PI binding was performed by a FACScan (BD Biosciences) using channels FL-1 (Annexin V-FITC) and FL-3 (PI). Cellquest Pro was used to perform quadrant analysis. All experiments were at least repeated 3 times.
Measurement of reactive oxygen species (ROS)
To detect ROS formation induced by curcumin, we used the ROS-specific labelling dye 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (H2DCFDA, Invitrogen). Fibroblasts were cultured at 80% confluency in DMEM without phenol red and with 10% FCS in a 96-wells plate suitable for fluorescence measurement (ThermoFischer Scientific, Vantaa, Finland). Cells were washed once with Hanks' Balanced Salt Solution (HBSS, Invitrogen) prior to labelling with 10-μM H2DCFDA in HBSS for 15 min. at 37°C. Subsequently cells were washed once with HBSS and treated for 90 min. with different concentrations of curcumin (5, 10 and 25 μM) in the presence or absence of 6-mM NAC or 50-μM bilirubin DMEM without phenol red and 1% FCS. As a negative control unlabelled cells were used. Afterwards, cells were washed twice with HBSS and fluorescence was determined using a Fluostar Galaxy fluorometer at excitation 485 nm and emission 520 nm (BMG Lab Technologies, Offenburg, Germany).
Glutathione assay
Total glutathione (GSH) and oxidized glutathione (GSSG) were determined according to the method of Tietze and Griffith [24, 25]. In short, adherent fibroblasts were treated for 24 hrs with 6-mM NAC, 25-μM curcumin, the combination of curcumin and NAC or were not treated. Cells were harvested by trypsin/EDTA and 25,000 cells were resuspended in 200 μl 10 mM HCl, lysed by 3 freeze-thaw cycles and cell debris was pelleted by centrifugation. Proteins were precipitated using 5-sulfosalicylic acid (1% final concentration, Sigma-Aldrich) for 5 min. on ice, after which samples were centrifuged at 4°C for 5 min. The supernatant was neutralized with NaOH and divided over 2 eppendorf cups. To determine the level of oxidized GSH 2-vinylpyridine (end concentration 2%, Sigma-Aldrich) was added to one of the two cups, mixed and incubated for 1 hr at RT. Total GSH and oxidized GSH was determined in 10 μl samples to which NADPH (final concentration 0.42 mM, Roche Applied Science), 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, final concentration 1.2 mM, Sigma-Aldrich) and 0.5 U glutathione reductase (Sigma-Aldrich) were added in such a way that the total volume was 100 μl. Immediately after addition of glutathione reductase, the extinction was measured every 10 sec. at 412 nm for 4 min. using the BioRad Benchmark Plus microplate spectrophotometer. Concentration of GSH was calculated from the slope of the GSH standards.
HO-1 Western procedure
The effect of exposure of fibroblasts to increasing doses of curcumin on the HO-1 protein expression was studied. Therefore, fibroblasts were seeded in 6-well plates at 70% confluence, allowed to adhere and subsequently treated for 24 hrs with curcumin (2.5–27.5 μM) or 10 μM of the known HO-1 inducers heme and CoPP. Furthermore, the effect of 6-mM NAC and the combination of 25-μM curcumin with 6-mM NAC on HO-1 expression was investigated. After treatment, cells were harvested using trypsin-EDTA, pelleted by centrifu-gation and lysed for 30 min. on ice using lysis buffer (1 mM EDTA, 0.5% Triton-X-100, 25 μg/ml leupeptin, 25 μg/ml pepstatin, 100 μM phenyl-methanesulphonylfluoride, 3 μg/ml aprotinin in PBS, pH 7.2, all Sigma-Aldrich). Cell lysates were centrifuged for 10 min. at 20,000 ×g at 4°C and 20-μg total protein was separated by SDS/PAGE using a 12.5% gel. Subsequently, proteins were blotted onto a nitrocellulose membrane using a BioRad wet blotting system. Afterwards, the membrane was blocked overnight at 4°C using Odyssey Blocking Buffer (Westburg BV, Leusden, the Netherlands). The blot was then incubated for 1 hr at RT with rabbit-anti-HO-1 Ab (1:5000; Stressgen/ITK, Uithoorn, the Netherlands). Mouse-anti-β-actin Ab was simultaneously incubated to serve as a protein loading control (1:100,000, Sigma-Aldrich). Antibodies were diluted in Odyssey Blocking Buffer containing 0.1% Tween-20. Afterwards, the membrane was washed three times for 10 min. with PBS containing 0.1% Tween-20. The secondary antibodies, goat-anti-mouse Alexa Fluor 680 (1:20,000, Rockland, Heerhugowaard, the Netherlands) and goat-anti-rabbit IRDye 800 (1:20,000 Sigma-Aldrich) were incubated for 45 min. at RT in Odyssey Blocking Buffer containing 0.1% Tween-20 and 0.01% SDS. After thorough washing, the membrane was scanned using the Odyssey Infrared Imaging System (LI-COR Biosciences). Expression of HO-1 was assessed using channel 800 and β-actin expression was determined using channel 700. Intensity of the bands was determined using the Odyssey application software.
HO-activity assay
To determine whether the curcumin-induced HO-1 proteins were active, we performed an HO-activity assay. Therefore, the cells were treated as described in the previous section and 100,000 cells were resuspended in 89.9 μl 0.1 M phosphate buffer, 2 mM MgCl2 (pH 7.4) and subsequently lysed by 3 freeze-thaw cycles. To the cell lysate, 10.1-μl master mix was added that consisted of 2.5 μl 80 mM glucose-6-phosphate, 0.1 μl 0.5 U/μl glucose 6-phospate dehydrogenase in 5 mM glycine (both Sigma-Aldrich), 5 μl 32 mM NADPH tetrasodium salt (Roche Applied Science, Almere, the Netherlands) and 2.5 μl 2 mM heme. The samples were incubated for 45–60 min. at 37°C in the dark. Next, 100 μl methanol was added and the samples were centrifuged at 13,000 ×g. A 50-μl aliquot of this sample was run on an HPLC (Spectra-Physics Analytical, Spectrasystem SCM400) equipped with a 5-μm Discovery C18 column and a Discovery C18 Supelguard Cartridge 5 μm particle size precolumn (both Sigma-Aldrich). Separation was done by a mixture of 80% solvent A (40% 100 mM NH4Ac, pH 5.5, 5% methoxy ethanol and 50% methanol [all Sigma-Aldrich]) and 20% solvent B (5% methoxy ethanol and 95% methanol) over a 20-min. time interval at a flow rate of 1 ml/min. Biliverdin and bilirubin were detected at a wavelength of 377 and 450 nm, respectively. Standards of biliverdin and bilirubin (both Frontier Scientific) were also run in order to quantify the amount of biliverdin/bilirubin found in the samples.
Collagen gel contraction assay
We tested if curcumin affected fibroblast-mediated gel contraction. Collagen gels consisted of 0.14% rat-tail collagen type I (Serva Electrophoresis, Brunschwig chemie, Amsterdam, the Netherlands), 1x MEM, 0.1 M HEPES and 30 mM sodium bicarbonate and the pH was neutralized with NaOH. Per ml collagen gel 50,000 cells were incorporated. One ml of the collagen-cell mix was pipetted carefully in a well of a 24-wells plate, which was previously blocked with 1% BSA, and the gel was allowed to gelate at 37°C and 5% CO2. After 45 min., DMEM + 10% FCS was added containing the tested substances. Gel contraction was determined after 24 hrs by scanning the 24 wells plate using a flat bed scanner and a computer. The surface area was determined using image analysis software (ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/, 1997–2007). At the end of the experiment, the wet weight of the gels was determined.
Statistics
Statistics were performed using GraphPad Prism 4.03 software via a oneway ANOVA followed by the Newman-Keuls multiple comparison test. Differences between groups were stated to be statistically significant when P < 0.05.
Results
High doses of curcumin induce dermal fibroblast apoptosis
Curcumin has shown to influence the wound healing process in vivo. We investigated the effect of different doses of curcumin on dermal fibroblast morphology and cell death by microscopy. Treatment with 5- and 10-μM curcumin for 48 hrs did not affect fibroblast morphology, whereas treatment with 25-μM curcumin caused rounding of the cells and cell death (Fig. 1A–D). Treatment of fibroblasts with 25-μM curcumin for 24 hrs induced a too mild apoptosis-inducing effect (<15% of the cells positive for Annexin V-FITC) to be reproducible. After 48 hrs of treatment with 25-μM curcumin, a far more reproducible level of apoptosis of ∼60% could be measured and therefore this time point was taken to further study the effect of curcumin on fibroblast apoptosis.
1.
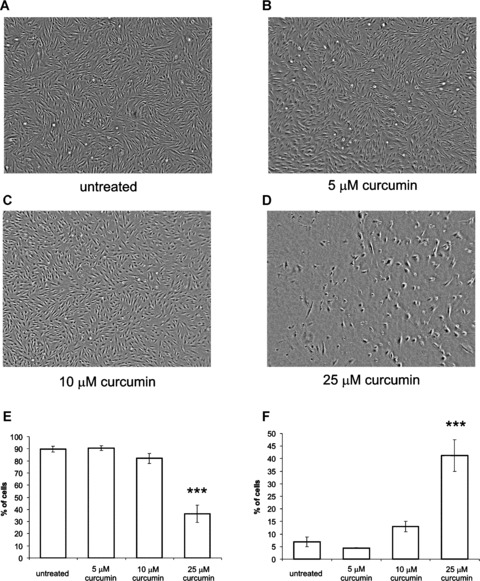
Curcumin induces fibroblast apoptosis. Fibroblast morphology (A–D). Adherent fibroblasts were not treated (A) or treated with 5 μm (B), 10 μM (C) or 25 μM (D) curcumin for 48 hrs. Images were taken using a light microscope and a digital camera. Original magnification 40×. Note rounding of fibroblasts and dead cells in the 25 μM group. FACS analysis of fibroblasts after curcumin treatment (E–F). Adherent fibroblasts were treated for 48 hrs with the indicated doses of curcumin. Afterwards, all cells were collected and stained with Annexin V-FITC and PI. Staining intensity was determined for 10,000 cells using flow cytometry and subsequent quadrant analysis. The mean ± S.D. are shown from the living (E) and early apoptotic (F) cell fractions from 5 independent experiments. ***P < 0.001 compared to untreated.
We next investigated whether this curcumin-induced cell death was a result of fibroblast necrosis or apoptosis. Using annexin-V-FITC/propidium iodide (PI) stainings and flow cytometry, we show that treatment with 25 μM curcumin for 48 hrs resulted in increased annexin-V-FITC staining (Fig. 1E). The percentage of living cells was significantly decreased from 90% in the untreated group to 54% after curcumin treatment (P < 0.001). Moreover, 25-μM curcumin caused a significant increase in the percentage of early apoptotic cells (Fig. 1F). In contrast, treatment with 5-μM curcumin for 48 hrs did not significantly change the percentage of living or early apoptotic cells compared to untreated cells. Treatment with 10-μM curcumin for 48 hrs causes a very mild increase in annexin V-FITC positive cells, although this effect was not statistically significant (Fig. 1E). Only a very small percentage stained positive for PI, indicating that the cells were not necrotic (data not shown).
Thus, high doses of curcumin induced dermal fibroblast apoptosis as indicated by annexin V-FITC staining.
Curcumin directly induces ROS formation
It has been suggested that curcumin-induced apoptosis in dermal fibroblasts was mediated by ROS. To investigate whether curcumin causes ROS formation, we used the ROS-specific labelling dye H2DCFDA. Table 1 shows that treatment with 25-μM curcumin causes significantly more ROS formation than untreated cells or cells treated with lower doses of curcumin (P < 0.001). In unlabelled cells treated with the different doses of curcumin or NAC or bilirubin no fluorescent signal could be detected (data not shown). Importantly, co-exposure of curcumin with NAC or bilirubin did not result in a significant enhanced fluorescent signal at 520 nm by 25 μM curcumin. The effect of the antioxidants was not tested on lower doses of curcumin since no significant ROS production was found using these doses. This experiment demonstrates that 25 μM of curcumin is able to directly cause ROS formation and that this can be blocked by the presence of the antioxidant NAC or bilirubin.
1.
Twenty-five μM Curcumin induces reactive oxygen species (ROS) formation which can be blocked by NAC and bilirubin
| Treatment | Curcumin | + NAC | + Bilirubin | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | Mean | S.E.M. | |||||||||||||||||||||
| Untreated | 1.00 | 0.294 | 0.99 | 0.016 | 0.95 | 0.017 | ||||||||||||||||||||
| 5 μM curcumin | 1.17 | 0.113 | n.d. | n.d. | ||||||||||||||||||||||
| 10 μM curcumin | 2.23 | 0.409 | n.d. | n.d. | ||||||||||||||||||||||
| 25 μM curcumin | 7.62* | 1.509 | 1.16 | 0.164 | 1.27 | 0.081 | ||||||||||||||||||||
Adherent cells were first labelled with the ROS-specific probe H2-DCFDA and then treated with different doses of curcumin for 90 min. in the absence or presence of 6-mM NAC or 50-μM bilirubin. Subsequently, fluorescence was determined at excitation 485 nm and emission 520 nm. The ROS level of untreated cells was stated as 1 and the other values were normalized accordingly. Shown are the mean ± S.E.M. of 3 independent experiments. *Significantly different from other treatments (P < 0.001). n.d = not determined.
Curcumin-induced ROS formation causes apoptosis
To prove that curcumin-induced ROS formation caused fibroblast apoptosis, we incubated fibroblasts with curcumin in the presence or absence of the known antioxidants NAC, biliverdin or bilirubin. Figure 2 clearly shows that 6-mM NAC completely prevented curcumin-induced apoptosis and that the percentage of living and early apoptotic cells returned to normal values. The antioxidants biliverdin and bilirubin also significantly inhibited curcumin-induced apoptosis when administered in a concentration of 1 μM (P < 0.001). These results strongly suggest that ROS are involved in curcumin-induced apoptosis in fibroblasts.
2.
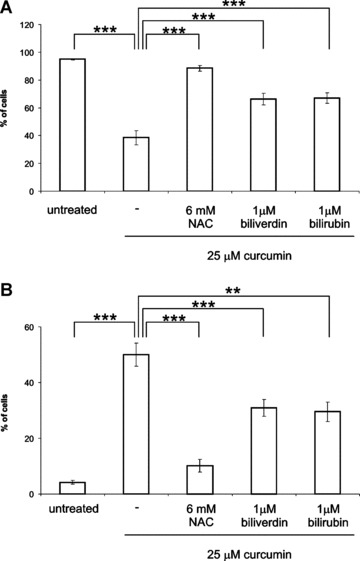
Curcumin-induced ROS formation causes apoptosis. Adherent fibroblasts were treated for 48 hrs with 6 mM NAC, 1 μM biliverdin or 1 μM bilirubin in the presence or absence of 25 μM curcumin. As a negative control, cells were not treated. Afterwards, all cells were collected and stained with Annexin V-FITC and PI and quadrant analysis after flow cytometry was performed. The mean ± S.D. are shown from the living (A) and early apoptotic (B) cell fractions from 5 independent experiments. **P < 0.01 and ***P < 0.001 compared to μM curcumin.
Glutathione and curcumin
Besides having antioxidant properties, NAC can serve as a cysteine donor, causing increased levels of the cytoprotective agent glutathione (GSH) and thus protect cells from cellular stress such as ROS. In order to analyse possible effects of GSH on curcumin-induced apoptosis, we determined total GSH and oxidized GSH content after curcumin or curcumin + NAC treatment. Our results indicate that treatment with 25-μM curcumin for 24 hrs does not alter total GSH content or the GSH/GSSG balance, compared to untreated cells (Table 2). Also at other time points (6 or 48 hrs), no effect of 25-μM curcumin on the GSH content was observed (data not shown). Unexpectedly, we did not observe an increase in GSH content after treatment with the GSH precursor NAC (6–48 hrs treatment, Table 2 and data not shown). However, the combination of NAC and curcumin resulted in a significant 2-fold increase in total GSH content but this effect was accompanied by a 3-fold increase in GSSG level, compared to untreated cells. As a result, the net balance of GSH/GSSG, a measure for cellular stress, was worse after curcumin-NAC treatment than in the untreated situation or after treatment with curcumin. Taken together, curcumin does not seem to have a direct effect on the GSH/GSSG content but it induces cellular stress that will lead to increased GSH synthesis in the presence of a cysteine donor such as NAC.
2.
Determination of total glutathione (GSH) and oxidized glutathione (GSSG)
| Treatment | Total GSH (μM) | GSSG (μM) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | ||||||||||
| Untreated | 18.6 | 1.9 | 2.6 | 1.7 | |||||||||
| 6 μM NAC | 18.9 | 1.3 | 3.5 | 1.4 | |||||||||
| 25 μM curcumin | 18.9 | 5.0 | 2.9 | 1.9 | |||||||||
| 25 μM curcumin + 6 mM NAC | 36.5** | 3.1 | 7.9* | 1.8 | |||||||||
GSH levels were determined in cell lysates of treated cells using the reaction of GSH with DTNB, which results in a yellow product. To determine GSSG levels only, 2-vinylpyridine was added to the cell lysate, which prevents reduction of GSSG to GSH by GSH-reductase. Concentrations were determined from GSH standards. Shown are mean ± S.E.M. of GSH and GSSG concentrations of 3 independent experiments. * and ***= significantly different from other treatments (P < 0.05 and P < 0.001, respectively).
Curcumin dose-dependently induces HO-1 protein expression and activity
Since biliverdin and bilirubin are down-stream products of heme degradation by the rate-limiting enzyme HO, we investigated whether pre-induction of HO-1 could protect against curcumin-induced apoptosis in fibroblasts. Therefore, we first examined whether doses of curcumin, not affecting fibroblast viability, could induce HO-1 expression in fibroblasts. Figure 3 demonstrates that, as expected, both 10-μM heme and 10-μM CoPP cause a clear induction in the expression of HO-1. Differential effects of curcumin (0–27.5 μM) on HO-1 expression are clearly visible; from 5 μM up to a dose of 17.5 μM, a significant induction of HO-1 protein can be observed, whereas from 20 to 27.5 μM no significant difference from basal levels could be detected. The maximal induction of ∼50-fold compared to untreated cells was observed between 10 and 15 μM curcumin (P < 0.001). HO-1 expression was at basal levels after treatment with 25-μM curcumin.
3.
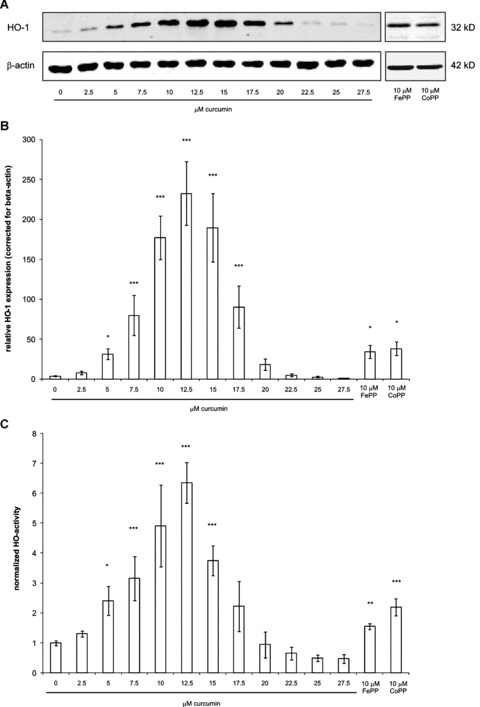
Dose-dependent induction of HO-1 protein expression and HO-activity by curcumin. HO-1 protein expression was studied in fibroblasts treated for 24 hrs with different doses of curcumin. As a positive control, cells were treated with 10-μM FePP or CoPP. Cell lysates were prepared and proteins were separated by SDS-PAGE and subsequently blotted onto a nitrocellulose membrane. HO-1 protein and beta-actin (as a loading control) were stained with specific antibodies. Detection was done with the appropriate fluorescently labelled secondary antibodies and the Odyssey Infrared Imaging System. (A) Results of the Western blot showing bands corresponding to HO-1 and beta-actin protein. (B) Relative quantification of HO-1 proteins bands corrected for beta-actin from 5 independent experiments (mean ± S.D.) (C) Determination of HO-activity by quantifying bilirubin levels via HPLC. For details on the HO-activity assay, see ‘materials and methods’ section. The mean ± S.D. of the area under the curve of the peak are shown corresponding to bilirubin of 5 independent experiments. The bilirubin peak in the untreated group was set at 1 and the bilirubin peaks in the other groups were normalized accordingly. *P < 0.05, **P < 0.01 and **P < 0.001 compared to the untreated group.
To determine if curcumin-induced HO is enzymatically active, we performed an HO-activity assay based on the detection of bilirubin using HPLC. In Figure 3C, a clear dose response of curcumin on HO-activity is seen with a maximal induction of ∼6-fold at a dose of 10–15 μM curcumin, compared to untreated cells (P < 0.001). However, already with a low dose of 5-μM curcumin a significant increase of 2.5-fold in HO-activity was observed (P < 0.001). FePP and CoPP (10 μM) induced HO-activity similar to 5-μM curcumin. Treatment with 25-μM curcumin did not lead to HO-activity, which is in line with the HO-1 protein expression patterns as observed by Western blotting.
NAC-mediated protection against curcumin-induced apoptosis involves HO-1
We further investigated how NAC prevented curcumin-induced fibroblast apoptosis and studied HO-1 expression and HO-activity. Figure 4A and B shows that either 25-μM curcumin or 6-mM NAC did not induce HO-1 protein expression or activity. Interestingly, the combination of curcumin with NAC strongly induced HO-1 expression, and, furthermore, the induced HO-1 was shown to be active. These results suggest a role for HO-1 in the protection against curcumin-induced apoptosis by NAC. Indeed when we blocked HO-activity using the specific HO-activity inhibitor QC-15 in the presence of the curcumin-NAC combination, we observed a significant inhibition of the rescuing effects of NAC on curcumin-induced apoptosis (Fig. 5A–C). Combined treatment of curcumin and NAC increased the fraction of living cells by 35% compared to curcumin alone (P < 0.001). When we inhibited HO-1-activity in the curcumin-NAC group by QC-15, the fraction of living cells was significantly decreased by 17% (P < 0.01). Moreover, application of the HO-activity inhibitor together with curcumin and NAC significantly increased the fraction of early apoptotic cells by 59%, compared to treatment with the curcumin-NAC combination (P < 0.05). Similarly, also the fraction of late apoptotic cells is significantly increased in the curcumin-NAC-QC group, 229 ± 1.3% compared to a normalized fraction of 100 ± 10.4% in the curcumin-NAC treated group (P < 0.001). These results show that HO-activity, at least partially, mediates the rescuing effects of NAC on curcumin-induced apoptosis.
4.
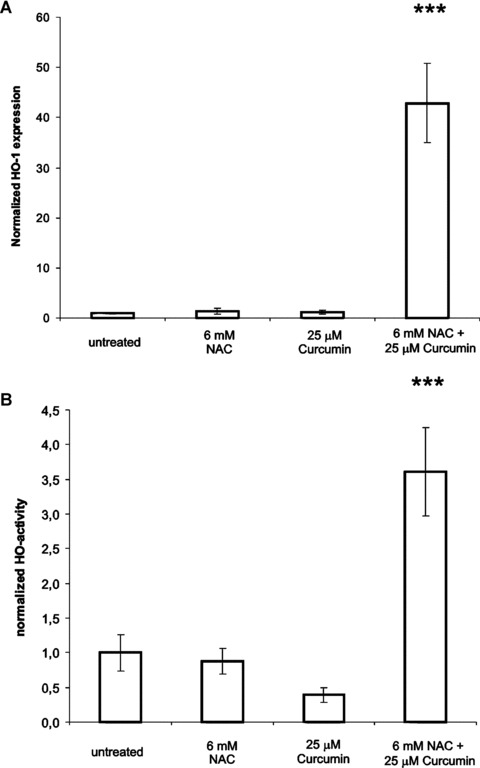
HO-1 protein expression and HO-activity in fibroblasts treated with curcumin, NAC or the combination. Adherent cells were treated with 25-μM curcumin, 6-mM NAC or the combination for 24 hrs. The HO-1 level or activity in the untreated group was set at 1. (A) Quantification of Western blot analysis for HO-1, and (B) HO-activity assay. For details on the procedures, see ‘materials and methods’ section. Shown are the mean ± S.D. from 5 independent experiments. ***P < 0.001 compared to untreated.
5.
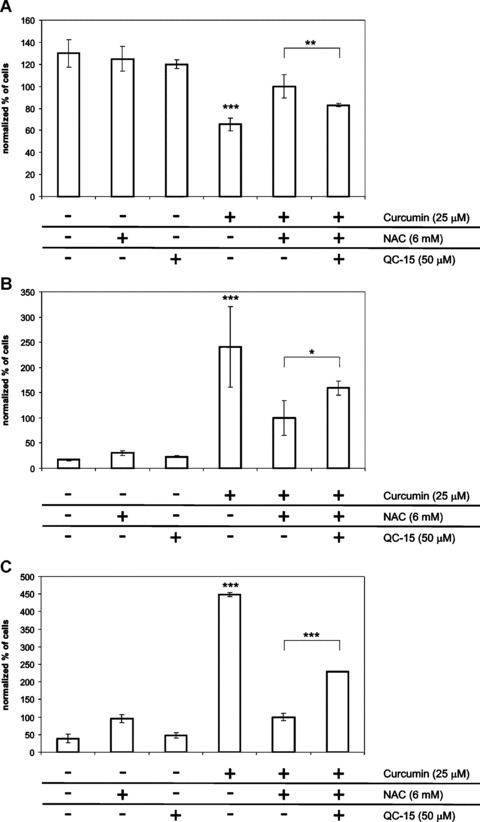
HO-activity mediates rescue of curcumin-induced apoptosis by NAC. Adherent fibroblasts were treated for 48 hrs with the combination 25 μM curcumin + 6 mM NAC, in the presence or absence of the HO- activity inhibitor QC-15 (50 μM). Negative controls included all single treatments and non-treated cells. Afterwards, all cells were collected and stained with Annexin V-FITC and PI. Subsequently, flow cytometry and quadrant analysis was performed. To compare different experiments, the percentage of cells per quadrant in the curcumin-NAC group was set at 100% and the other values were normalized accordingly. Shown are the mean ± S.D. of the living (A), early apoptotic (B) and (C) late apoptotic cell fractions of 6 independent experiments. ***P < 0.001 compared to all other treatment. Statistical significance between curcumin-NAC and curcumin-NAC-QC-15 treatments is indicated, where *P < 0.05, **P < 0.01 and ***P < 0.001.
Pre-treatment with low doses curcumin protects against 25 mM curcumin-induced apoptosis
HO has been described as an enzyme with cytoprotective functions. Therefore, we studied whether pre-induction of HO-1 would protect against apoptosis induced by 25-μM curcumin. In Figure 6, it is shown that 5-μM curcumin pre-treatment followed by 25-μM curcumin treatment indeed causes a significant increase in the percentage of living fibroblasts, compared to cells that received no pre-treatment (P < 0.001). This observation was accompanied by a significant decrease in the percentage dead cells after pre-treatment with 5-μM curcumin (P < 0.01). Pre-treatment with 2.5-μM curcumin had similar but milder effects (data not shown). Importantly, the protective effect of preconditioning was blocked significantly when co-treated with the HO-activity inhibitor SnMP (P < 0.05). Taken together, these data indicate that a modest up-regulation of HO-1 protein expression and HO-activity by preconditioning with curcumin, subsequently, had significant cytoprotective effects in fibroblasts.
6.
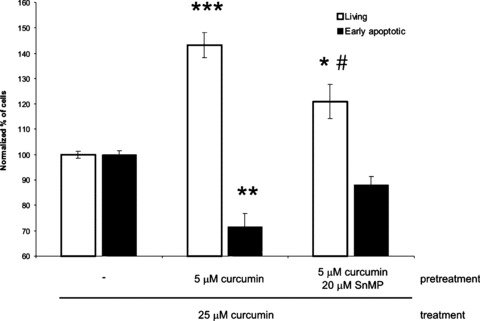
Pre-treatment with low doses of curcumin protects against apoptosis induced by 25 μM curcumin. Adherent fibroblasts were treated for 24 hrs with 5 μM curcumin alone or in combination with 20 μM SnMP. Control cells were not pre-treated. Subsequently, medium was changed and the cells were incubated with 25 μM curcumin for 48 hrs. All cells were collected and stained with Annexin V-FITC and PI. Using flow cytometry, the staining intensity of the cells for Annexin V-FITC and PI was determined. The effect of 25 μM curcumin without pre-treatment was stated as 100%. Shown is the quantification of quadrant analysis of 5 independent experiments (mean ± S.D.). Asterisks indicate statistically significant different from control cells, *P < 0.05, **P < 0.01, ***P < 0.001 and #P < 0.05 compared to 5 μM curcumin as pre-treatment.
Curcumin inhibits fibroblast-mediated collagen gel contraction
Next, we investigated if curcumin influenced fibroblast-mediated collagen gel contraction and whether this effect of curcumin could be inhibited by antioxidant treatment. As Figure 7A shows, 25-μM curcumin prevented fibroblast-mediated collagen gel contraction, whereas lower doses of curcumin or 6-mM NAC had no effect. When curcumin was removed by replacement of the medium, gel contraction resumed normally (data not shown). Interestingly, gel contraction after treatment with 25-μM curcumin in combination with 6-mM NAC returned to normal untreated levels. This rescuing effect of NAC is at least partially mediated by HO-activity since co-exposure of fibroblast-populated gels to the combination curcumin-NAC in the presence of the specific HO-inhibitor QC-15 resulted in a significantly decreased contraction, compared to treatment with curcumin-NAC alone (P < 0.01) (Fig. 7B). High curcumin (>25 μM) can inhibit in vitro wound contraction, which can be counteracted by antioxidant treatment and this is at least partially mediated by HO-activity.
7.
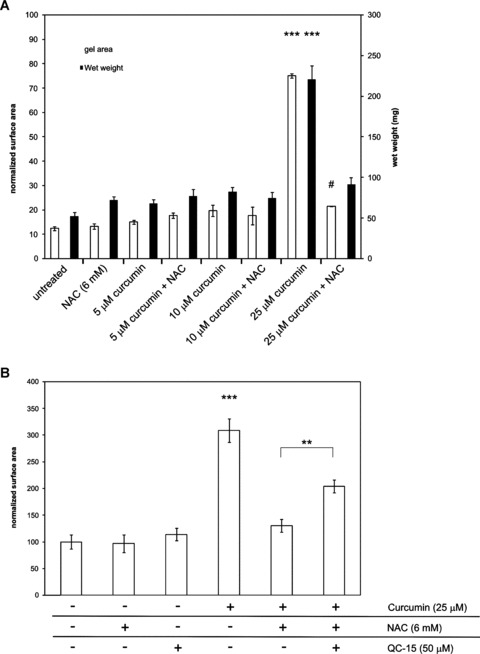
Antioxidant treatment rescues curcumin-induced inhibition of fibroblast-mediated collagen gel contraction. (A) Fibroblasts were incorporated in collagen gels and gels were treated with curcumin (5, 10 or 25 μM) in the presence or absence of 6 mM NAC. Gels were allowed to contract for 24 hrs after which the surface area (left axis) and wet weight (right axis) of the gels was determined. (B) HO-activity induced by 25-μM curcumin + NAC was blocked by QC-15 (50 μM). Shown are the mean ± S.E.M. of 3 independent experiments. ***P < 0.001 compared to all treatments and #P < 0.05 compared to the untreated group.Statistical significance between curcumin-NAC and curcumin-NAC-QC-15 treatments is also indicated (**P < 0.01).
Discussion
Fast wound closure and a short wound healing time have always been thought of as beneficial because of the reduced risk of infection. However, accelerated wound contraction can exacerbate scar formation [1]. Nowadays, antibiotics/antimycotics are very efficient in combating infections and consequently allow for slower wound closure. Therefore, we set out to identify possible pathways to slow down wound contraction and thereby reduce scar formation. Since regulation of fibroblast apoptosis is of great importance for controlling scar formation and because the spice curcumin has shown to induce apoptosis in a variety of cell types [6–10], we investigated the effects of curcumin on human dermal fibroblasts in detail. We show here that a high concentration of curcumin (>25 μM) can induce fibroblast apoptosis in vitro and consequently inhibit fibroblast-mediated collagen gel contraction. We show that these processes are mediated by ROS, since curcumin directly induced ROS formation and the effects of curcumin could be completely blocked by treatment with various antioxidants.
It has been demonstrated that GSH and curcumin can interact, thereby blocking the effect of curcumin [26, 27]. The inhibiting effect of the GSH-precursor NAC on curcumin-induced apoptosis could be based on a similar quenching of curcumin. However, the stability of curcumin and its subsequent activity can be strongly improved by the addition of GSH or NAC, suggesting that GSH does not interfere with curcumin activity [28]. Additionally, several studies show that curcumin treatment results in an increased GSH content [29–31]. Since we found no evidence for an interaction of curcumin with GSH or NAC, we believe that our findings concerning the effect of NAC on curcumin-induced apoptosis and inhibition of gel contraction are the result of the antioxidant properties of NAC. This is further supported by our observation that also the HO-derived antioxidants biliverdin and bilirubin very potently inhibited apoptosis caused by curcumin in a dose 6000-fold lower than NAC. This observation underscores the powerful potency of bilirubin and biliverdin as antioxidants. Moreover, this strongly suggests a role for HO-activity in the inhibition of curcumin-induced apoptosis since biliverdin and bilirubin are both downstream signalling products of heme metabolism.
There exists a clear relationship between the heme–heme oxygenase (HO) system and the early phases of wound healing. Free heme is released in large quantities following tissue damage and free heme contributes to the inflammatory reaction. In the latter stages of the inflammatory response macrophages expressing high levels of HO-1 protein, infiltrate the wound bed [32, 33]. Moreover, also infiltrating fibroblasts show elevated levels of the HO-1 protein [33]. This increase in HO-1 expression and activity has shown to be involved in the resolution of inflammation [21, 34]. However, the role of the HO system during the later stages of wound healing and scar formation is poorly understood and warrants further investigation.
Interestingly, low concentrations of curcumin (10–15 μM) strongly induced HO-1 protein expression in dermal fibroblasts in a dose-dependent fashion. Curcumin was much more potent than the known HO-inducers heme and CoPP. The mechanism by which curcumin induces HO-1 expression is complex but is most likely redox status-dependent, since NAC could almost completely attenuate HO-1 expression induced by 10 μM curcumin (data not shown). Cells can respond to curcumin-induced ROS by inducing cytoprotective genes such as HO-1 through the transcription factors AP-1, NF-κB and Nrf2 [35]. Although, a reduction of ROS causes a block in the transcription of HO-1 in several cell systems [36–38], Scapagnini et al. showed that 1-mM NAC failed to prevent HO-1 induction in astrocytes following curcumin administration, implying that ROS where not involved in HO-1 induction in this model [17]. Interestingly, although both 25-μM curcumin and 6-mM NAC alone did not induce HO-1 expression in fibroblasts, the combination resulted in a strong induction of HO-1 expression and activity, in a fashion that likely does not involve ROS. This strongly suggests that HO-induction is involved in NAC-mediated protection against apoptosis and contraction, which is supported by our finding that addition of HO-activity inhibitors partly blocks the effects of NAC.
The protective actions of HO-activity are further supported by our findings that also preconditioning of fibroblasts with low doses curcumin protected them against apoptosis induced by a high dose of curcumin. This protective preconditioning effect was likely mediated by HO-1 induction since the protective effect was attenuated in the presence of a HO-activity inhibitor. We have shown that this preconditioning increased both the expression and activity of HO, resulting in increased bilirubin production in the cell. However, we cannot rule out that also CO or ferritin up-regulation are involved in HO-mediated protection against curcumin-induced apoptosis. Ferritin is rapidly induced by free ferrous iron (as is generated by heme degradation by HO) and its anti-apoptotic function most likely depends on the prevention of ROS formation by rendering the pro-oxidative iron inactive [39, 40] CO can protect against apoptosis by the activation of the cGMP pathway by increasing sGC activity [41]. Furthermore, CO can increase anti-apoptotic pathways by modulating p38 MAPK signalling [42] and CO can induce NF-kB-dependent anti-apoptotic (cIAP2 and A1) genes that protect against TNF-α-mediated apoptosis [43]. Further research is warranted to clarify whether CO or ferritin are indeed involved in the rescue of curcumin-induced apoptosis.
The cytoprotective properties of HO-preconditioning are in line with earlier observations in different cell systems. Soares et al. showed that preinduction of HO-1 gene expression was associated with cardiac xenograft survival [44]. Furthermore, in murine fibroblasts the overexpression of HO-1 inhibited TNF-α-induced apoptosis, which was not observed in the presence of the HO-activity blockert in protoporphyrin [45].
Curcumin has been shown to decrease wound-healing time and increase contraction in wound-healing studies in rodents [2, 5]. Here, we show in an in vitro model that high concentrations (>25 μM) of curcumin inhibit contraction. This discrepancy between in vivo and in vitro could be the result of the curcumin dose used or the antioxidant status during in vivo wound healing. Indeed, it has been demonstrated that curcumin can increase superoxide dismutase, catalase and GSH levels in vivo[2, 4].
In summary, we have demonstrated that a concentration of 25-μM curcumin induced dermal fibroblast apoptosis and inhibit collagen gel contraction via a ROS-mediated mechanism. In addition, we showed that pre-conditioning of fibroblasts, resulting in enhanced HO-1 expression and HO-activity, protects against curcumin-induced apoptosis. Our results indicate that curcumin in high concentrations may be a therapeutic strategy to aid in the prevention or reduction of hypertrophic scar formation and that via antioxidants or through modulation of HO-activity or administration of HO-effector molecules curcumin-induced fibroblast apoptosis can be regulated.
Acknowledgments
We thank P.H.H. van den Broek for excellent technical assistance regarding the heme oxygenase activity assay. A. Scharstuhl and S.W.C Pennings are funded by a grant from the Dutch Burns Foundation (# 05.104). F.A.D.T.G. Wagener is supported by a VENI grant from the Netherlands Organization for Scientific Research and the Van Leersum Foundation.
References
- 1.Nedelec B, Shankowsky H, Scott PG, Ghahary A, Tredget EE. Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-alpha2b. Surgery. 2001;130:798–808. doi: 10.1067/msy.2001.116453. [DOI] [PubMed] [Google Scholar]
- 2.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87–96. doi: 10.1007/s11010-006-9170-2. [DOI] [PubMed] [Google Scholar]
- 3.Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999;7:362–74. doi: 10.1046/j.1524-475x.1999.00362.x. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998;6:167–77. doi: 10.1046/j.1524-475x.1998.60211.x. [DOI] [PubMed] [Google Scholar]
- 5.Jagetia GC, Rajanikant GK. Role of cur-cumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wound, in mice whole-body exposed to various doses of gamma-radiation. J Surg Res. 2004;120:127–38. doi: 10.1016/j.jss.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Atsumi T, Murakami Y, Shibuya K, Tonosaki K, Fujisawa S. Induction of cytotoxicity and apoptosis and inhibition of cyclooxygenase-2 gene expression, by curcumin and its analog, alpha-diisoeugenol. Anticancer Res. 2005;25:4029–36. [PubMed] [Google Scholar]
- 7.Chan WH, Wu HY, Chang WH. Dosage effects of curcumin on cell death types in a human osteoblast cell line. Food Chem Toxicol. 2006;44:1362–71. doi: 10.1016/j.fct.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen HW, Huang HC. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br J Pharmacol. 1998;124:1029–40. doi: 10.1038/sj.bjp.0701914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dujic J, Kippenberger S, Hoffmann S, Ramirez-Bosca A, Miquel J, Az-Alperi J, Bereiter-Hahn J, Kaufmann R, Bernd A. Low concentrations of curcumin induce growth arrest and apoptosis in skin keratinocytes only in combination with UVA or visible light. J Invest Dermatol. 2007;127:1992–2000. doi: 10.1038/sj.jid.5700801. [DOI] [PubMed] [Google Scholar]
- 10.Kuo ML, Huang TS, Lin JK. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim Biophys Acta. 1996;1317:95–100. doi: 10.1016/s0925-4439(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 11.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165–72. [PubMed] [Google Scholar]
- 12.Atsumi T, Tonosaki K, Fujisawa S. Induction of early apoptosis and ROS-generation activity in human gingival fibrob-lasts (HGF) and human submandibular gland carcinoma (HSG) cells treated with curcumin. Arch Oral Biol. 2006;51:913–21. doi: 10.1016/j.archoralbio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Ciolino HP, Daschner PJ, Wang TT, Yeh GC. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem Pharmacol. 1998;56:197–206. doi: 10.1016/s0006-2952(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 14.Piper JT, Singhal SS, Salameh MS, Torman RT, Awasthi YC, Awasthi S. Mechanisms of anticarcinogenic properties of curcumin: the effect of curcumin on glutathione linked detoxification enzymes in rat liver. Int J Biochem Cell Biol. 1998;30:445–56. doi: 10.1016/s1357-2725(98)00015-6. [DOI] [PubMed] [Google Scholar]
- 15.Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927–31. doi: 10.1097/00005373-200111000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxyge-nase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–12. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 17.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and cur-cumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–61. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 18.Tourkina E, Gooz P, Oates JC, Ludwicka-Bradley A, Silver RM, Hoffman S. Curcumin-induced apoptosis in scleroder-ma lung fibroblasts: role of protein kinase cepsilon. Am J Respir Cell Mol Biol. 2004;31:28–35. doi: 10.1165/rcmb.2003-0354OC. [DOI] [PubMed] [Google Scholar]
- 19.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–8. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 21.Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, Van Kooyk Y, De Witte T, Figdor CG. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–11. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 22.Kinobe RT, Dercho RA, Vlahakis JZ, Brien JF, Szarek WA, Nakatsu K. Inhibition of the enzymatic activity of heme oxygenases by azole-based antifungal drugs. J Pharmacol Exp Ther. 2006;319:277–84. doi: 10.1124/jpet.106.102699. [DOI] [PubMed] [Google Scholar]
- 23.Vlahakis JZ, Kinobe RT, Bowers RJ, Brien JF, Nakatsu K, Szarek WA. Synthesis and evaluation of azalanstat analogues as heme oxygenase inhibitors. Bioorg Med Chem Lett. 2005;15:1457–61. doi: 10.1016/j.bmcl.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 24.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 25.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 26.Awasthi S, Pandya U, Singhal SS, Lin JT, Thiviyanathan V, Seifert WE, Jr, Awasthi YC, Ansari GA. Curcumin-glutathione interactions and the role of human glutathione S-transferase P1–1. Chem Biol Interact. 2000;128:19–38. doi: 10.1016/s0009-2797(00)00185-x. [DOI] [PubMed] [Google Scholar]
- 27.Mathews S, Rao MNA. Interaction of curcumin with glutathione. Int J Pharmaceutics. 1991;76:257–9. [Google Scholar]
- 28.Oetari S, Sudibyo M, Commandeur JN, Samhoedi R, Vermeulen NP. Effects of curcumin on cytochrome P450 and glu-tathione S-transferase activities in rat liver. Biochem Pharmacol. 1996;51:39–45. doi: 10.1016/0006-2952(95)02113-2. [DOI] [PubMed] [Google Scholar]
- 29.Sandur SK, Ichikawa H, Pandey MK, Kunnumakkara AB, Sung B, Sethi G, Aggarwal BB. Role of pro-oxidants and antioxidants in the anti-inflammatory and apoptotic effects of curcumin (diferuloyl-methane) Free Radic Biol Med. 2007;43:568–80. doi: 10.1016/j.freeradbiomed.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–8. [PubMed] [Google Scholar]
- 31.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 32.Hanselmann C, Mauch C, Werner S. Haem oxygenase-1: a novel player in cutaneous wound repair and psoriasis? Biochem J. 2001;353:459–66. doi: 10.1042/0264-6021:3530459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagener FA, Van Beurden HE, Von Den Hoff JW, Adema GJ, Figdor CG. The heme-heme oxygenase system: a molecular switch in wound healing. Blood. 2003;102:521–8. doi: 10.1182/blood-2002-07-2248. [DOI] [PubMed] [Google Scholar]
- 34.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 35.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol. 2006;69:1033–40. doi: 10.1124/mol.105.018374. [DOI] [PubMed] [Google Scholar]
- 36.Borger DR, Essig DA. Induction of HSP 32 gene in hypoxic cardiomyocytes is attenuated by treatment with N-acetyl-L-cysteine. Am J Physiol. 1998;274:965–73. doi: 10.1152/ajpheart.1998.274.3.H965. [DOI] [PubMed] [Google Scholar]
- 37.Camhi SL, Alam J, Wiegand GW, Chin BY, Choi AM. Transcriptional activation of the HO-1 gene by lipopolysaccharide is mediated by 5′ distal enhancers: role of reactive oxygen intermediates and AP-1. Am J Respir Cell Mol Biol. 1998;18:226–34. doi: 10.1165/ajrcmb.18.2.2910. [DOI] [PubMed] [Google Scholar]
- 38.Rizzardini M, Carelli M, Cabello Porras MR, Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: synergism by glu-tathione depletion and protection by N-acetylcysteine. Biochem J. 1994;304:477–83. doi: 10.1042/bj3040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, Shen X, Busuttil RW, Yamashita K, Csizmadia E, Tyagi S, Otterbein LE, Brouard S, Tobiasch E, Bach FH, Kupiec-Weglinski JW, Soares MP. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003;17:1724–6. doi: 10.1096/fj.03-0229fje. [DOI] [PubMed] [Google Scholar]
- 40.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De SE, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–42. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 41.Liu XM, Chapman GB, Peyton KJ, Schafer Al, Durante W. Antiapoptotic action of carbon monoxide on cultured vascular smooth muscle cells. Exp Biol Med. 2003;228:572–5. doi: 10.1177/15353702-0322805-30. [DOI] [PubMed] [Google Scholar]
- 42.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–26. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–61. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 44.Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–7. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 45.Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-alpha-induced apoptosis in cultured fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2000;278:312–9. doi: 10.1152/ajplung.2000.278.2.L312. [DOI] [PubMed] [Google Scholar]


