Abstract
Tendon injuries cause considerable morbidity in the general adult population. The tenocytes within the tendon have the full capacity to heal the tendon intrinsically. Activated protein C (APC) plays an important role in coagulation and inflammation and more recently has been shown to promote cutaneous wound healing. In this study we examined whether APC can induce a wound healing phenotype in tenocytes. Sheep tenocytes were treated with APC, endothelial protein C receptor (EPCR) blocking antibody (RCR252) and/or EPCR small interfering (si)RNA. Cell proliferation and migration were measured by crystal violet assay and a scratch wounding assay, respectively. The expression of EPCR, matrix metalloproteinase (MMP)-2, type I collagen and MAP kinase activity were detected by real time PCR, zymography, immunofluorescence, immunohistochemistry and Western blotting. APC stimulated proliferation, MMP-2 activity and type I collagen deposition in a dose-dependent manner and promoted migration of cultured tenocytes. APC dose-dependently stimulated phosphorylated (P)-ERK2 and inhibited P-p38. Interestingly, tenocytes expressed EPCR protein, which was up-regulated by APC. When tenocytes were pre-treated with RCR252 or EPCR siRNA the effect of APC on proliferation, MMP-2 and type 1 collagen synthesis and MAP kinases was blocked. APC promotes the growth, MMP-2 activity, type I collagen deposition and migration of tenocytes. Furthermore, EPCR is expressed by tenocytes and mediates the actions of APC, at least partly by signalling through selective MAP kinases. These data implicate APC as a potential healing agent for injured tendons.
Keywords: tenocytes, activated protein C, healing, endothelial protein C receptor
Introduction
Acute tendon rupture is a common tendinopathy often associated with disordered healing. Tendon injuries account for considerable morbidity and often prove disabling for several months, despite receiving appropriate management [1]. Acute injuries heal by a sequence of overlapping phases involving inflammation, granulation tissue formation and remodelling. Healing can occur by invasion of cells from the surrounding sheath and synovium, although, the cells within the tendon, known as tenocytes, have the full capacity to heal tendon intrinsically [1]. Within the extracellular matrix network, tenoblasts and tenocytes constitute about 90–95% of the cellular elements of tendons [1, 2]. Tenoblasts are immature tendon cells which become elongated over time and transform into tenocytes [2].
Protein C (PC), a vitamin K-dependent zymogen, is converted to activated protein C (APC) on the cell surface when thrombin binds to thrombomodulin [3, 4]. The activation of PC is augmented by its specific receptor, endothelial protein C receptor (EPCR) [3–5]. APC plays a key role in the regulation of blood coagulation and also has significant anti-inflammatory properties associated with inhibition of pro-inflammatory cytokines and a reduction of leucocyte recruitment [6–10]. The effectiveness of APC as an anticoagulant and anti-inflammatory agent is demonstrated by its efficacy as a treatment for patients with severe sepsis [11].
APC can activate endothelial matrix metalloproteinase (MMP)-2 [12], a member of the MMP family of zinc-dependent endopepti-dases that plays a vital role in the tissue repair process by remodelling the extracellular matrix [13]. In cultured human skin keratinocytes, APC enhances cell proliferation, migration and MMP-2 activity [14]. Recently, a novel function of APC as a promoter of cutaneous wound healing was identified. In a rat-healing model APC accelerates full thickness wound closure by stimulating re-epithelialization, promoting angiogenesis and preventing inflammation [15]. In pilot human trials APC is proving to be effective in healing chronic leg ulcers [16]. Many of the healing properties of APC are mediated through EPCR [14, 15, 17, 18], a 46-kD type I transmembrane glycoprotein predominantly expressed by endothelial cells, mainly of larger blood vessels [19]. Recent reports have shown this receptor to be present also on some leucocytes of the innate immune system and keratinocytes [20–23].
Whether APC regulates healing in tendons has not previously been investigated. The current study shows that APC can promote growth, migration, MMP-2 activity and collagen production in sheep tenocytes. Furthermore, EPCR is expressed by these cells and mediates the actions of APC via MAP kinases.
Materials and methods
Tenocyte isolation, culture and reagents
A segment of the superficial digital tendon was isolated from an adult sheep (about 10 g of tissue) immediately after slaughter and cut into small fragments. The tissue was digested in 25 ml of phosphate buffered saline (PBS) containing collagenase type I (1 mg/ml) and 15 ml of trypsin (25 mg/ml) for 6 hrs at 37°C with continuous stirring. Tissue debris were removed by filtration on nylon gauze and the enzymes were inactivated by the addition of 3 ml foetal calf serum (FCS). After centrifugation (350g, 10 min.) the pellet was resuspended in DMEM supplemented with 10% FCS (V/V) and the cells were seeded onto cell culture flasks. The cells were incubated at 37°C, in a 95% humiditified atmosphere with 5% CO2. The medium was replaced after 48 hrs and then every 3 days. The tenocytes employed for all tests were used at three to five passages. After confluency, cells were trypsinised and seeded into either 24-well culture plates at 2 × 105 cells per well or eight-well perm anox™ slides (Nalge Nunc International Corp., Rochester, NY USA) and incubated for 12 hrs to allow for adhesion. The confluent cells were then treated with recombinant APC (Xigris, Eli Lilly, Indianapolis, IN USA), and/or EPCR blocking antibody RCR252, EPCR non-blocking antibody RCR92 (gift from Professor Fukudome, Department of Immunology Saga Medical School, Nabeshima, Saga, Japan). Cells and culture supernatants were collected for detection of mRNA and protein expression.
Small interfering (si) RNA preparation and nucleofection
siRNA duplex oligonucleotides were purchased from Proligo (Sigma-Proligo, St. Louis, MO, USA). The designed siRNA for EPCR was: sense 5′ GUGGACGGCGAUGUUAAUUAC, antisense UCCACCUGCCGCUACAAUUAA-5′. A scrambled form of EPCR siRNA was used as a negative control. Tenocytes were adjusted to 1.5 × 105 cells/ml in growth medium and subjected to nucleofection using the siPORTs™ NeoFX™ according to the manufacturer's instructions (Amaxa Biosystems, Cologne, Germany). Transfected cells were allowed to attach overnight, trypsinised and then seeded into either 24-well plates (1 × 105 cells/well), 8-well permanox™ slides (Nalge Nunc International Corp.) or 96-well plates (2 × 103 cells/well) and incubated for a further 24, 48 and 72 hrs. The specificity of EPCR siRNA (10 nM) was confirmed by EPCR blocking antibody RCR252.
RNA extraction and reverse-transcription (RT)-PCR
Total RNA was extracted from tenocytes using Tri Reagent (Sigma-Aldrich St. Louis, MO, USA) according to the manufacturer's instructions. Single stranded cDNA was syn thesized from total RNA using AMV reverse transcriptaseand Oligo (dT)15 as a primer (Promega Corp., Madison, WI, USA). The levels of mRNA were semi-quantified using real time PCR on a Rotorgene 3000A (Corbett Research, Sydney, Australia). Samples were normalized to the housekeeping gene RPL13A and results were reported for each sample relative to the control. PCR product was also separated by 2% agarose gel electrophoresis. Primers used were as follows: EPCR (91bp): Sense 5′TCCTACCTGCTCCAGTTCCA and antisense AAGATGCCTACAGCCACACC; GAPDH (139bp): sense 5′CCT GGA GAA ACC TGC CAA GTA TG and antisense 5′GGT AGA AGA GTG AGT GTC GCT GTT G.
Cell proliferation assay
Cells (2 × 103 cells/well) were seeded into a 96-well micro plate to a final volume of 200 μl, and incubated for 4 hrs to allow cells to attach. Cells were then treated with APC at 0.01, 0.1, 1, 10 (μg/ml). After incubation for 72 hrs, culture medium was removed and cells were stained with 1 μg/ml crystal violet (Sigma, Aldrich) dissolved in PBS. The unbound dye was removed by washing with tap water and cells were left to completely dry overnight. Bound crystal violet was solubilized with 1% SDS in PBS. The optical density of each well was determined at a wavelength of 550 nm. Results were expressed as percentages of controls.
Migration assay
Cells were seeded into 24-well plates and cultured to confluence. Cell monolayers were then scratched with a 1000 μl blue plastic pipette tip, creating a cell-free area approximately 2 mm in width. ‘Wounded’ monolayers were washed three times with PBS to remove loose cell debris, and a defined area of the wound was photographed under phase-contrast microscopy. To standardize the position of the wound when photographing small indents were made in the plastic well using a sterile 31G needle. To prevent cell proliferation, cells were pre-treated with mitomycin C (10 μg/ml, Sigma, Aldrich), which was applied to the cells 2 hrs before wounding and removed with three PBS washes. Cells were then incubated in growth medium for 24 hrs with or without APC (10 μg/ml) treatment. Cell migration was determined after 24 hrs by counting the cells that had moved into the wounded area, and the percentage of cell migration was calculated as (number of migrated cells treated with agents / number of migrated control cells) × 100.
Western blotting
Tenocytes were washed three times with PBS then lysis buffer (0.15 M NaCl, 0.01 mM PMSF, 1% NP-40, 0.02 M Tris, 6 M urea/H2O) was added. Cell lysates were sonicated for 10 sec. and centrifuged at 10,000 g for 15 min. Clear supernatants were separated by 10% SDS-polyacrylamide-gel electrophoresis and transferred to a PDVF membrane. The primary antibodies used were rabbit anti-human EPCR antibody (1:500 dilution, Invitrogen Corporation, Carlsbad, CA, USA); rabbit anti-human phosphorylated forms of p38 and ERK2 (1:1000 dilutions, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), rabbit anti-human MMP-2 (R&D systems, Minneapolis, MN, USA) and mouse-anti-human collagen type I antibody (MP Biomedicals, Inc., Aurora, OH, USA). Immunoreactivity was detected using the ECL detection system (Amersham, Piscat away, NJ, USA). Anti-human β-actin antibody was included to normalize against unequal loading.
Immunohistochemical staining
Tenocytes cultured in permanox slides were fixed with cold acetone for 90 sec. After quenching with 2% H2O2 in water and equilibrating in PBS, samples were incubated with mouse-anti-human collagen type 1 antibody or rabbit anti-human EPCR antibody overnight at 4°C. Cells were then processed for staining using DAKO LSAB+Systems stain kit (DAKO Corporation, Carpinteria, CA, USA) and counterstained with Haematoxylin and Scotts Blueing solution. After mounting, cells were observed under a light microscope (Nikon ECLIPSE 80i, Nikon Corporation, Shinagawa-Ku, Tokyo, Japan). For dual staining, cells were blocked by 5% horse serum in PBS and incubated with rabbit anti-human EPCR and mouse anti-human type 1 collagen antibodies for 2 days at 4°C. After washing with PBS, cells were incubated with anti-rabbit IgG conjugated with Cy3 (red) and anti-mouse IgG conjugated with FITC (green) (1:400, Sigma-Aldrich). Cells were washed with PBS, mounted with Gold anti-fade reagent with DAPI (Invitrogen Corporation) and observed under a fluorescence microscope (Nikon ECLIPSE 80i, Nikon Corporation). Images were acquired and processed using a Nikon digital camera and software (Diagnostic Instruments, Sydney, NSW, Australia) and Image J (http://rsb.info.nih.gov/ij).
Zymography
MMP-2 and MMP-9 protein secretion and activation in the culture supernatants were measured using gelatine zymography under non-reducing conditions as described previously [24].
Statistical analysis
Significance was determined using one-way anova and Student–Newman–Keuls test. P values less than 0.05 were considered statistically significant.
Results
APC stimulates tenocyte proliferation, migration and MMP-2 activity
When added to tenocytes at concentrations of 0.01, 0.1, 1 and 10 μg/ml for 72 hrs, APC accelerated cell proliferation in a dose-dependent manner (Fig. 1A). At the highest dose tested, APC enhanced the proliferation rate by 45%. The migration of APC-treated cells was measured using a scratch wounding assay. Confluent tenocytes were scratch-wounded and cell migration was evaluated 24 hrs after wounding. APC-treated cells exhibited ∼20% increase in migration into the wounded areas compared to control cells (Fig. 1B).
1.
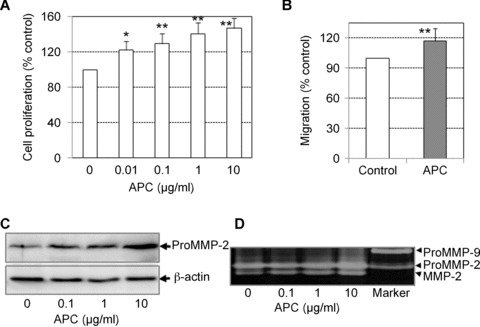
APC stimulates the growth, migration and MMP-2 activity of tenocytes. (A) Growth rate of tenocytes in response to APC treatment after 72 hrs as detected by crystal violet staining. Cell proliferation is expressed as a percentage of control (mean ± S.D.). Graphs represent one of three independent experiments *P < 0.05, **P < 0.01. (B) Cell migration detected by the scratch wounding assay described in the Materials and Methods after 24 hrs in response to APC treatment (10 μg/ml). Data are expressed as a percentage of migrated cells in control condition (mean ± S.E.M., n= 3). **P < 0.01. (C) MMP-2 detected in cell supernatants after 24 hrs treatment with APC, using Western blotting. β-actin was used as the loading control. (D) MMP-2 detected in cell supernatants after 24 hrs treatment with APC, using zymography.
MMPs, particularly MMP-2 and MMP-9, are extensively involved in tenocyte migration and wound closure. In this study, we analysed protein production of these two MMPs, using Western blotting and zymography. Under basal conditions, cultured tenocytes constitutively expressed pro-MMP-2, but not MMP-9 (Fig. 1C and D). APC up-regulated MMP-2 in a dose-dependent manner, but had no effect on MMP-9. Zymography confirmed Western blot data and revealed that APC-treatment produced a prominent band representing the activated form of MMP-2, which was also evident in the control cells (Fig. 1D).
APC up-regulates type I collagen production by tenocytes
The major matrix constituent of tendon is type I collagen, which is produced locally by tenocytes. We examined the amount of secreted and cell-associated type I collagen produced by cultured tenocytes in response to APC treatment. Using an anti-human type I collagen antibody, immunohistochemistry and immunofluores-cence showed that tenocytes produced detectable amounts of type I collagen under basal conditions (Figs 2A and 4). In response to APC, there was a marked increase in the intensity of staining for type I collagen (Figs 2A and 4). Western blotting, using same antibody, showed that under basal conditions, no type I collagen was detected in culture supernatant after 24 hrs incubation, however, APC stimulated type I collagen release, particularly when used at 10 μg/ml (Fig. 2B).
2.
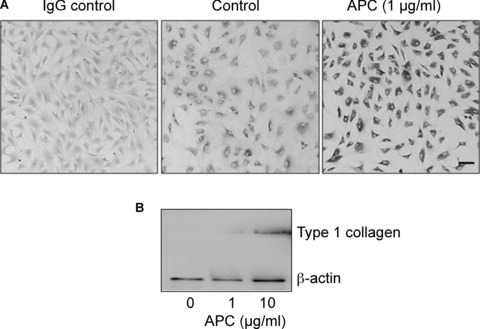
APC stimulates type I collagen in tenocytes. (A) Tenocytes were incubated for 24 hrs in the presence or absence of APC. Cells were subjected to immuno-histochemical staining using an antibody to type I collagen. IgG was used as the isotype control. Bar = 20 μm. (B) Type I collagen in the cell supernatants in response to APC treatment for 24 hrs and detected by Western blot. β-actin was used as the loading control.
4.
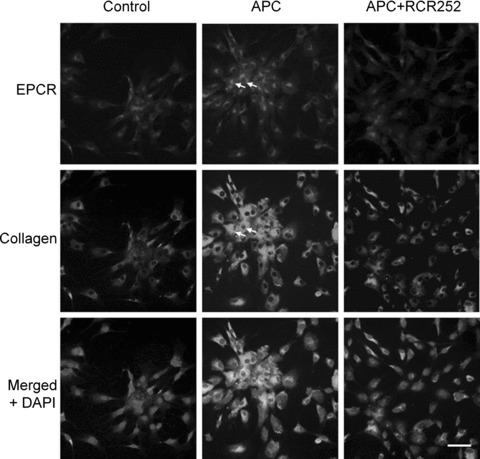
Co-localization of EPCR and type I collagen. Immunofluoresecnt staining for EPCR (red) and type I collagen (green) and nucleus using DAPI (blue) of tenocytes without (Control) or with APC (1 μg/ml) in the presence or absence of RCR252 (APC + RCR252) treatment for 24 hrs. When the frames are merged, yellow indicates co-expression. In response to APC, cells expressing high levels of EPCR also expressed high levels of type I collagen (arrows). (Bar = 20 μm).
EPCR is expressed by tenocytes and mediates the protective effects of APC
EPCR, the receptor for PC/APC, is thought to be present only on a few cell types and not known to be expressed by tenocytes. To investigate whether tenocytes produce EPCR, mRNA from unstimulated or 24-hr APC-stimulated (1 μg/ml) cells were subjected to RT-PCR. EPCR mRNA was present in cells under basal conditions and was enhanced by APC (Fig. 3A). Western blotting confirmed that EPCR protein was present in tenocytes and increased in response to APC treatment (Fig. 3B). In addition, immunohistochemical and immunofluorescent staining were performed after 24 hrs of treatment with or without APC. Under basal conditions, greater than 90% of the untreated cells stained weakly positive for EPCR (Figs. 3C and 4). All cells responded to APC with a strong increase in EPCR expression (Figs. 3C and 4). There was variability in the staining intensity between cells and, interestingly, cells which stained with the highest intensity for EPCR also stained strongest for type I collagen, as evidenced by immunofluorescence (Fig. 4, arrows). When cells were pre-treated with RCR252, the APC-induced increase in type I collagen was abolished (Fig. 4).
3.
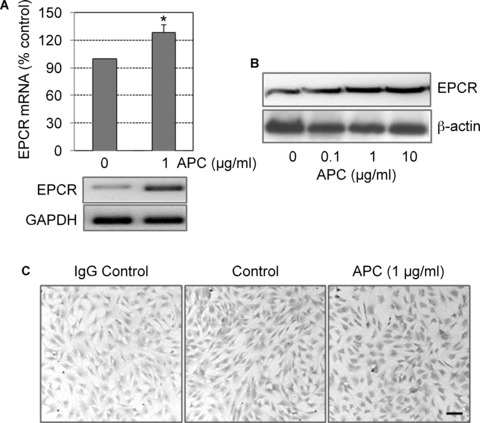
EPCR expression by cultured human tenocytes. Cells were treated with APC and incubated for 24 hrs. Expression of EPCR was detected by (A) Real time RT-PCR (graph) and PCR product was detected by 2% agarose gel with housekeeping gene GAPDH as an internal control (gel below graph) and (B) Western blot analysis, using cell lysates with β-actin used as the loading control and (C) immunohistochemical staining for EPCR in tenocytes (Bar = 20 μm).
We next investigated whether APC stimulation of cell proliferation and MMP-2 was mediated through EPCR. Specific siRNA was used to inhibit endogenous EPCR expression. The efficacy of EPCR siRNA was detected by Western blot, which showed >90% knockdown of protein expression in cell lysates treated for 24 hrs (Fig. 5B). EPCR siRNA treatment for 72 hrs inhibited cell proliferation by more than 20%, which suggests that either EPCR itself can stimulate proliferation or endogenously derived PC can bind to EPCR and enhance cell growth. This inhibition could not be recovered by addition of the exogenous APC (Fig. 5B), indicating that APC requires EPCR to augment proliferation. This was further confirmed by adding EPCR blocking antibody, RCR252, which abolished the stimulatory effect of APC on cell proliferation (Fig. 5B). Similarly, MMP-2 production was reduced by more than 50% after tenocytes were transfected with EPCR siRNA for 48 hrs as detected by zymography (Fig. 5C). This result was confirmed by pre-treating APC-stimulated cells with RCR 252 for 24 hrs and detected by Western blot (Fig. 5D).
5.
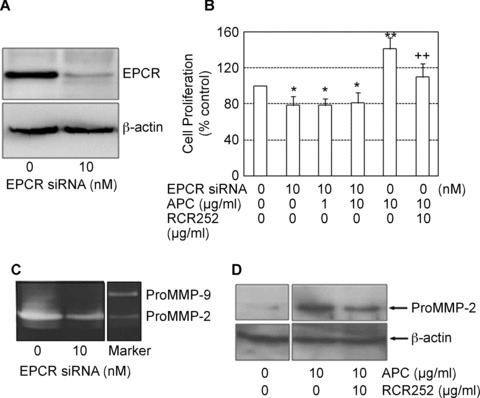
Blocking EPCR prevents APC-induced MMP-2 synthesis and proliferation. (A) ECPR protein expression in response to EPCR siRNA treatment at 48 hrs detected by Western blot using whole cell lysates. β-actin was used as the loading control. (B) Cell proliferation was measured after cells were treated with combinations of APC, siRNA and RCR252 for 72 hrs. Cell proliferation is expressed as a percentage of control (mean ± S.D., n= 3 experiments). *P < 0.05 and **P < 0.01 compared to negative control (first bar), ++P < 0.01 compared to APC (fourth bar). Images of gels represent one of three independent experiments. (C) MMP-2 was measured in supernatants of cells trans-fected with either control siRNA (0) or EPCR siRNA for 72 hrs, using zymography. (D) MMP-2 was measured in supernatants of cells treated with APC alone or APC plus RCR252 for 24 hrs using Western blot analysis. β-actin used as the loading control.
APC regulates the activation of MAP kinases via EPCR
APC-induced cell proliferation is controlled through the activation of MAP kinases in various cell types [14, 25]. In this study, the phosphorylated (P) forms of ERK2, p38 and c-Jun were measured by Western blotting in response to APC and EPCR blocking agents. The P-ERK2 level was very low in untreated tenocytes after 24 hrs incubation. The addition of APC for 24 hrs dose-dependently and markedly elevated P-ERK2 (Fig. 6). In contrast, high levels of P-p38 were present under basal conditions and the addition of APC inhibited the activity of this MAP kinase (Fig. 6). No phosporylated c-jun was detected either in control or following APC treatment (data not shown). Blocking EPCR with RCR252 (10 μg/ml) resulted in a reversal of the effects of APC, with a decrease in P-ERK2 and increase in P-p38, whereas the non-blocking antibody, RCR92, had little effect. Treatment of cells with EPCR siRNA resulted in similar effects on the activity of ERK and p38 at 72 hrs (Fig. 6).
6.
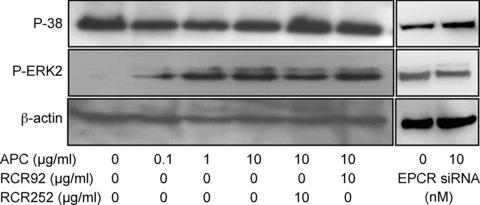
PC/APC regulates the activation of MAP kinases. Tenocytes were treated with APC in the presence or absence of RCR252 or RCR92 (non-blocking control antibody) for 24 hrs or treated with EPCR siRNA for 72 hrs and cells were collected for Western analysis. β-actin was used as the loading control. Images represent one of three independent experiments.
Discussion
Tenocytes and their precursors, tenoblasts, account for up to 95% of the cellular component of tendons [1]. These cells play a vital role during the repair of tendons by migrating to the injury site, proliferating and synthesizing collagen. They secrete MMPs to degrade the matrix for cell migration and also for the final remodelling phase. Within 24 hrs of tendon injury, surrounding tenocytes from the wound margins begin to migrate and invade the wound bed, where they proliferate, produce new collagen and remodel the matrix. The biochemical and mechanical properties of healed tendon tissue never match those of intact tendon, which is at least partly due to the length of time it takes for a tendon to heal.
Strong evidence is now emerging that APC accelerates wound repair. In a rat healing model, APC induces angiogenesis and re-epithelialization while inhibiting inflammation to promote cutaneous wound healing [15]. In human skin keratinocytes, recombinant APC stimulates proliferation, MMP-2 activity, migration and prevents apoptosis, all vital processes of re-epithelialization [17]. In addition, APC has positive effects on endothelial cells and has potent angiogenic activity, which contributes to its wound healing ability [15, 25]. The current study extends on these findings and is the first to show that APC can stimulate a wound healing phenotype in tenocytes by stimulating their proliferation, MMP-2 activity, migration and collagen production. Overall, our data suggests that APC could stimulate an injured tendon to heal faster and thus help restore normal tendon properties.
Tendon is a highly ordered composite material consisting predominantly of collagen, with smaller amounts of various proteo-glycans and glycoproteins [26]. Type I collagen is by far the most abundant, comprising ∼95% of the total collagen content [26]. The dry mass of human tendons is approximately 30% of the total tendon mass, with type I collagen accounting for 65% to 80% of this [2, 27, 28]. Although APC is known to promote cutaneous healing, there are no reports on the effect of APC on collagen in any cell type. The current study is the first to show that APC can stimulate type I collagen protein production. An interesting observation, using immunofluorescence, is that EPCR and type I collagen appear to be co-localized (Fig. 4), with tenocytes that expressed high levels of EPCR in response to APC also showing high levels of type I collagen. This provides the first evidence that EPCR is required for collagen stimulation by APC, although further experiments are required to confirm this.
APC increased MMP-2 synthesis by tenocytes, but had no effect on MMP-9, which was not expressed by tenocytes (Fig. 1). APC also stimulated the conversion of MMP-2 to its fully active form, as previously described for endothelial cells [29]. MMPs are important regulators of matrix remodelling and their expression varies during tendon healing. Using a rat flexor tendon laceration model, Oshiro [30] found that only MMP-2, MMP-3 and MT1-MMP remained elevated throughout the collagen healing process, suggesting that these three enzymes participate in both collagen degradation and collagen remodelling. MMP-2 differs from most other MMPs in that it exerts anti-inflammatory actions [31, 32]. MMP-2 efficiently cleaves and inactivates monocyte chemoattractant protein-3, a CC chemokine that promotes leucocyte chemotaxis. Itoh et al. [33] have proposed that although MMP-2 is elevated in the arthritic joint, its matrix-degrading ability appears to have little effect on development of arthritis but instead MMP-2 prevents inflammation by cleaving pro-inflammatory factors. This action of MMP-2 is likely to assist healing, as although inflammation is required very early in the tendon healing process, it needs to cease before the granulation and remodelling phases of healing can proceed [34]. Since MMP-2 is present throughout the tendon healing process [30], it is likely that by stimulating and activating MMP-2, APC contributes to tendon healing in vitro both by remodelling matrix and suppressing excessive inflammation.
An important finding from this study is that EPCR is expressed by sheep tenocytes (Fig. 3). This receptor was first identified on endothelial cells, mainly on larger blood vessels [19] and recently has been found on leucocytes, smooth muscle cells and ker-atinocytes [17, 20–22, 35]. This is the first report to show that a fibroblast-type cell expresses EPCR. In addition, this study shows that APC's actions on tenocyte proliferation, MMP-2 and type I collagen synthesis and MAP kinase regulation, all of which contribute towards wound healing, are mediated through EPCR. Although EPCR mediates these protective effects of APC, there is no evidence that it is a signalling molecule. Instead, by binding to EPCR, APC may cleave protease-activated receptor-1 which couples to G-proteins and signals through MAP kinases. This pathway is utilized by endothelial cells, keratinocytes and some leucocytes [15, 17, 20–22, 35], but it is unknown whether APC acts through this pathway in tenocytes.
An unexpected finding was that EPCR siRNA, when used alone, inhibited tenocyte proliferation (Fig. 5D). This result was confirmed by using the RCR252 blocking antibody. There are at least two possible explanations for this endogenous effect. Firstly, EPCR itself may stimulate proliferation. Secondly, PC may be produced and secreted by tenocytes which then binds to EPCR and stimulates cell growth. This is similar to the autocrine effect of PC recently described in keratinocytes [18]. Such a mechanism requires tenocytes to possess their own PC system which can not only synthesize PC, but also activate PC to APC and mediate the function of PC/APC by its receptors. Further studies are required to elucidate this mechanism.
The MAPK pathway is a prerequisite for growth factor stimulated mitogenesis in many cell types [36]. Three major downstream MAPK cascades are mitogen-activated ERK1/2 and stress/cytokine activated p38 and c-Jun N-terminal kinases, whereas p38 MAP kinase functions to promote differentiation and apoptosis [37]. Our data suggest that ERK2 is the major MAP kinase associated with APC enhancement of cell proliferation. In contrast, p38 exhibited opposite effects to ERK2. These results are in keeping with previous studies which present evidence that p38 MAP kinase functions to promote differentiation and apoptosis while signalling through ERK2 promotes keratinocyte proliferation and survival and endothelial cell proliferation [18, 25, 37].
APC maximally stimulated tenocyte proliferation and migration when used at 10 ug/ml. This concentration of APC is considerably higher than circulating physiological levels of ∼3 ng/ml. Nonetheless, there was a significant increase in proliferation when APC was used at 10 ng/ml, which equates to the order of magnitude of circulating PC. At sites where inflammation or healing occurs, such as in a wound, increased levels of thrombin and thrombomodulin cause increased activation of PC, which may accumulate and contribute to healing. Thus, it is feasible that endogenous levels of APC contribute to normal healing of acute tendon injuries.
In summary, the key findings of this study are that APC stimulates proliferation and migration, MMP-2 activity and type I collagen production in tenocytes, the major cell type in the tendon. Importantly, EPCR is expressed by tenocytes and mediates the protective actions of APC to promote healing. These novel findings highlight the importance of the PC pathway in tendon physiology and provide the first evidence that APC may provide a therapeutic option for tendinopathy.
Acknowledgments
This work was supported by a University of Sydney Postdoctoral Fellowship for the salary of M. Xue, and the The Lincoln Centre, Rebecca Cooper Foundation, Northern Sydney Area Health grant and the Sutton Arthritis Research Trust for consumables. We wish to thank Xiaoman Ding for technical assistance.
References
- 1.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6:181–90. [PubMed] [Google Scholar]
- 2.Kannus PJLJM. Principles and practice of orthopaedic sports medicine. In: Garrett WE Jr, editor. Basic science of tendons. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 21–37. [Google Scholar]
- 3.Fukudome K, Ye XF, Tsuneyoshi N, Tokunaga O, Sugawara K, Mizokami H, Kimoto M. Activation mechanism of anticoagulant protein C in large blood vessels involving the endothelial cell protein c receptor. J Exp Med. 1998;187:1029–35. doi: 10.1084/jem.187.7.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulinácomplex. PNAS. 1996;93:10212–6. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Esmon NL, Esmon CT. Reconstitution of the human endothelial cell protein C receptor with thrombomod-ulin in phosphatidylcholine vesicles enhances protein C activation. J Biol Chem. 1999;274:6704–10. doi: 10.1074/jbc.274.10.6704. [DOI] [PubMed] [Google Scholar]
- 6.Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein C prevents LPS-induced pulmonary vascular injury by inhibiting cytokine production. Am J Physiol. 1997;272:L197–202. doi: 10.1152/ajplung.1997.272.2.L197. [DOI] [PubMed] [Google Scholar]
- 7.Yuksel M, Okajima K, Uchiba M, Horiuchi S, Okabe H. Activated protein C inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production by inhibiting activation of both nuclear factor-kappa B and activator protein-1 in human monocytes. Thromb Haemost. 2002;88:267–73. [PubMed] [Google Scholar]
- 8.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 9.Joyce DE, Grinnell BW. Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med. 2002;30:S288–93. doi: 10.1097/00003246-200205001-00019. [DOI] [PubMed] [Google Scholar]
- 10.Cheng XW, Kuzuya M, Kanda S, Maeda K, Sasaki T, Wang QL, Tamaya-Mori N, Shibata T, Iguchi A. Epigallocatechin-3-gallate binding to MMP-2 inhibits gelati-nolytic activity without influencing the attachment to extracellular matrix proteins but enhances MMP-2 binding to TIMP-2. Arch Biochem Biophys. 2003;415:126–32. doi: 10.1016/s0003-9861(03)00221-2. [DOI] [PubMed] [Google Scholar]
- 11.Griffin JH, Zlokovic B, Fernandez JA. Activated protein C: potential therapy for severe sepsis, thrombosis, and stroke. Semin Hematol. 2002;39:197–205. doi: 10.1053/shem.2002.34093. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen M, Arkell J, Jackson CJ. Activated protein C directly activates human endothe-lial gelatinase A. J Biol Chem. 2000;275:9095–8. doi: 10.1074/jbc.275.13.9095. [DOI] [PubMed] [Google Scholar]
- 13.Ravanti L, Kahari V. Matrix metalloproteinases in wound repair (Review) Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- 14.Xue M, Thompson P, Kelso I, Jackson C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp Cell Res. 2004;299:119–27. doi: 10.1016/j.yexcr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Jackson CJ, Xue M, Thompson P, Davey RA, Whitmont K, Smith S, Buisson-Legendre N, Sztynda T, Furphy LJ, Cooper A, Sambrook P, March L. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen. 2005;13:284–94. doi: 10.1111/j.1067-1927.2005.00130311.x. [DOI] [PubMed] [Google Scholar]
- 16.Whitmont K, Reid I, Tritton S, March L, Xue M, Lee M, Fulcher G, Sambrook P, Slobedman E, Cooper A, Jackson C. Treatment of chronic leg ulcers with topical activated protein C. Arch Dermatol. 2008;144:1479–83. doi: 10.1001/archderm.144.11.1479. [DOI] [PubMed] [Google Scholar]
- 17.Xue M, Campbell D, Sambrook PN, Fukudome K, Jackson CJ. Endothelial protein C receptor and protease-activated receptor-1 mediate induction of a wound-healing phenotype in human keratinocytes by activated protein C. J Invest Dermatol. 2005;125:1279–85. doi: 10.1111/j.0022-202X.2005.23952.x. [DOI] [PubMed] [Google Scholar]
- 18.Xue M, Campbell D, Jackson CJ. Protein C is an autocrine growth factor for human skin keratinocytes. J Biol Chem. 2007;282:13610–6. doi: 10.1074/jbc.M610740200. [DOI] [PubMed] [Google Scholar]
- 19.Laszik Z, Mitro A, Taylor FB, Ferrell G, Esmon CT. Human protein c receptor is present primarily on endothelium of large blood vessels – implications for the control of the protein c pathway. Circulation. 1997;96:3633–40. doi: 10.1161/01.cir.96.10.3633. [DOI] [PubMed] [Google Scholar]
- 20.Feistritzer C, Sturn DH, Kaneider NC, Djanani A, Wiedermann CJ. Endothelial protein C receptor-dependent inhibition of human eosinophil chemotaxis by protein C. J Allergy Clin Immunol. 2003;112:375–81. doi: 10.1067/mai.2003.1609. [DOI] [PubMed] [Google Scholar]
- 21.Galligan L, Livingstone W, Volkov Y, Hokamp K, Murphy C, Lawler M, Fukudome K, Smith O. Characterization of protein C receptor expression in mono-cytes. Br J Haematol. 2001;115:408–14. doi: 10.1046/j.1365-2141.2001.03187.x. [DOI] [PubMed] [Google Scholar]
- 22.Sturn DH, Kaneider NC, Feistritzer C, Djanani A, Fukudome K, Wiedermann CJ. Expression and function of the endothelial protein C receptor in human neutrophils. Blood. 2003;102:1499–505. doi: 10.1182/blood-2002-12-3880. [DOI] [PubMed] [Google Scholar]
- 23.Xue M, Le NT, Jackson CJ. Targeting matrix metalloproteases to improve cutaneous wound healing. Expert Opin Ther Targets. 2006;10:143–55. doi: 10.1517/14728222.10.1.143. [DOI] [PubMed] [Google Scholar]
- 24.Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986;261:2814–8. [PubMed] [Google Scholar]
- 25.Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, Isobe H, Suda T. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- 26.Riley G. Chronic tendon pathology: molecular basis and therapeutic implications. Expert Rev Mol Med. 2005;7:1–25. doi: 10.1017/S1462399405008963. [DOI] [PubMed] [Google Scholar]
- 27.Hess GP, Cappiello WL, Poole RM, Hunter SC. Prevention and treatment of overuse tendon injuries. Sports Med. 1989;8:371–84. doi: 10.2165/00007256-198908060-00005. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien M. Structure and metabolism of tendons. Scand J Med Sci Sports. 1997;7:55–61. doi: 10.1111/j.1600-0838.1997.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen LL, D’Amore PA. Cellular interactions in vascular growth and differentiation. Int Rev Cytol. 2001;204:1–48. doi: 10.1016/s0074-7696(01)04002-5. [DOI] [PubMed] [Google Scholar]
- 30.Oshiro W, Lou J, Xing X, Tu Y, Manske PR. Flexor tendon healing in the rat: a his-tologic and gene expression study. Am J Hand Surg. 2003;28:814–23. doi: 10.1016/s0363-5023(03)00366-6. [DOI] [PubMed] [Google Scholar]
- 31.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelati-nase A cleavage of monocyte chemoat-tractant protein-3. Science. 2000;289:1202–6. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 32.McQuibban GA, Gong JH, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–7. [PubMed] [Google Scholar]
- 33.Itoh T, Matsuda H, Tanioka M, Kuwabara K, Itohara S, Suzuki R. The role of matrix metalloproteinase-2 and matrix metallo-proteinase-9 in antibody-induced arthritis. J Immunol. 2002;169:2643–47. doi: 10.4049/jimmunol.169.5.2643. [DOI] [PubMed] [Google Scholar]
- 34.Oberyszyn TM. Inflammation and wound healing. Front Biosci. 2007;12:2993–9. doi: 10.2741/2289. [DOI] [PubMed] [Google Scholar]
- 35.Joyce DE, Nelson DR, Grinnell BW. Leukocyte and endothelial cell interactions in sepsis: Relevance of the protein C pathway. Crit Care Med. 2004;32:S280–6. doi: 10.1097/01.ccm.0000128037.72072.22. [DOI] [PubMed] [Google Scholar]
- 36.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckert RL, Efimova T, Dashti SR, Balasubramanian S, Deucher A, Crish JF. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J Investig Dermatol Symp Proc. 2002;7:36–40. doi: 10.1046/j.1523-1747.2002.19634.x. [DOI] [PubMed] [Google Scholar]


