Abstract
The effect of leucine on glucose-stimulated insulin secretion (GSIS) in pancreatic β-cells is quite controversial, and mechanism involved in the effect has not been elucidated yet. Consequently, we aimed to investigate effect of leucine on GSIS and its mechanism focusing on contribution of AMP-activated protein kinase (AMPK) and pancreatic/duodenal homeobox-1 (PDX-1). Rat insulinoma β-cells (INS-1, RIN m5F, DN-PDX-1#28 and PDX-1#6) were cultured with or without leucine, AICAR (AMPK agonist) or compound C (AMPK antagonist) for 48 hrs. In contrast to control, AICAR treatment decreased GSIS at high glucose and insulin content, also impaired protein and mRNA expression of PDX-1 and its downstream targets, glucokinase (GCK) and glucose transporter 2 (GLUT2). Compound C treatment had the opposite effects. We observed that neither AICAR nor compound C could affect expression of GCK and GLUT2 when PDX-1 expression was absent. Chronic leucine exposure inhibited GSIS at high glucose and insulin content in a dose-dependent manner, concomitant with an increase in AMPK and a decrease in PDX-1, GCK and GLUT2. The inhibitory effects of leucine was potentiated by AICAR treatment and rescued by compound C treatment. Finally, the inhibition of PDX-1 could potentiate the impaired effects induced by leucine whereas overexpression of PDX-1 could protect the cell from impairment induced by leucine. The study indicated that chronic leucine might result in an increase in AMPK and then a decrease in PDX-l, in turn to depress GCK and GLUT2 resulting in decreased GSIS at high glucose and insulin content.
Keywords: leucine, AMP-activated protein kinase, pancreatic/duodenal homeobox-1, glucokinase, GLUT2
Introduction
Recently, studies have attached great importance for the effect of leucine on glucose-stimulated insulin secretion (GSIS) and intracellular insulin content in pancreatic β-cells [1–3]. However, up to now, the results from different research groups have been quite controversial. Yang and his colleagues demonstrated that leucine was able to enhance GSIS in pancreatic β-cells [1, 2]. However Anello et al. reported that chronic leucine exposure impaired GSIS in a dose-dependent manner [3]. Moreover, the mechanism of leucine affecting insulin secretion and content has not been elucidated yet. Consequently, we aimed to investigate the effects of leucine on insulin secretion and content, also to explore the mechanism involved in the effects in rat insulinoma β-cells.
AMP-activated protein kinase (AMPK) acts as a cellular energy regulator activated by increased intracellular AMP-to-ATP ratio [4, 5]. GSIS from β-cells is directly related with the generation of metabolic intermediates; therefore, AMPK is deemed as an attractive candidate for control of insulin secretion and content [6]. Many studies have reported that high glucose or fatty acid could change insulin secretion by controlling AMPK activity in pancreatic β-cells [7–10]. However, fewer studies pay attention to AMPK activity changes under chronic leucine exposure. Du and his colleagues reported that leucine stimulated mammalian target of rapamycin signalling by inhibition of AMPK activity [11], which suggested a possible association between leucine and AMPK.
Glucokinase (GCK), an enzyme phosphorylating glucose to glucose-6-phosphate, acts as a glucose sensor and regulates insulin secretion [12–14]. GLUT2 is an important component for insulin secretion as well [15, 16]. Tiedge and Lenzen reported in their studies that the concordant regulation of GCK and GLUT2 genes might represent the basis regulation of GSIS [17]. In 2006, Yang et al. firstly reported that leucine culture altered GCK expression in INS-1 cells, rat islets and human islets, moreover, GCK contributed tight control of insulin secretion [1].
Though AMPK, GCK and GLUT2 were separately reported to be associated with insulin secretion, the relationship between them under leucine exposure remains unclear. Kim et al. demonstrated that AMPK could regulate GCK and GLUT2 expression at high glucose concentration [18]. On the basis of these reports, we supposed that chronic leucine exposure might influence insulin secretion and content by altering AMPK, GCK and GLUT2 expression, and there might be a regulatory relationship between AMPK, GCK and GLUT2 at high leucine concentrations. In addition, how AMPK regulates GCK or GLUT2 was worthy of being investigated.
Numerous studies in vitro and in vivo have demonstrated that chronic exposure to glucose or fatty acid is able to suppress pancreatic/duodenal homeobox-1 (PDX-1) expression, leading to decreased insulin secretion [19–22]. The role of PDX-1 in pancreatic β-cell insulin secretion [23–25] derives from its effect on transactivating the expression of insulin and other β-cell-specific genes, such as GCK and GLUT2 [26–29]. This promoted us to speculate that PDX-1 might be the bridge between AMPK and GCK or GLUT2.
Collectively, from these findings, we suggested that there might be a regulatory link between AMPK and PDX-1 in pancreatic β-cells exposed to leucine, in turn to affect insulin secretion and content. To test the hypothesis, we firstly examined if the regulation existed under relative physiological condition. Then, we tested the effects of elevated concentrations of leucine on insulin secretion, insulin content and the protein expression of p-AMPK, PDX-1, GCK and GLUT2. Finally, we investigated if chronic leucine exposure influenced insulin secretion and content associated with the assumed regulation (AMPK regulates GCK and GLUT2 via PDX-1).
Research design and methods
Cell culture and treatment
Rat insulinoma (RIN) cell lines, INS-1 and RIN m5F cells (passage 20–40) were grown in monolayer culture in Roswell Park Memorial Institute (RPMI) 1640 medium (without glutamine) containing 11.1 mM glucose supplemented with 10 mM HEPES, 10% (v/v) foetal bovine serum (Invitrogen, NY USA), 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C in an humidified atmosphere with 5% CO2 and 95% air [28, 30]. The cells were split weekly and the medium was changed twice weekly. For the present study, INS-1 and RIN m5F cells were cultured in RPMI 1640 medium treated with either 0.5 mM AICAR [31], a widely used AMPK agonist (Toronto Research Chemicals, CA, USA), or 10 μM compound C, a AMPK antagonist (Calbiochem, San Diego, CA, USA), or supplemented with or without elevated concentrations of leucine (10, 20 or 40 mM) (Sigma, St Louis, MO, USA), for 48 hrs. Leucine was dissolved in RPMI 1640 medium and AICAR was directly dissolved in water.
INS-1 stable cell lines, DN-PDX-1#28 and PDX-1#6 cells (donated by Prof. Haiyan Wang, University Medical Center, Geneva, Switzerland) were cultured in RPMI 1640 medium supplemented with 100 μg/ml hygromycin and 100 μg/ml G418 (Sigma) [21]. The first-step stable clones were INS-1 rαβ and INS-1rβ. Plasmids used in the secondary stable transfection were constructed by subcloning the cDNAs encoding the mouse Pdx1 and its dominant-negative mutant (DN-PDX-1) into the expression vector PUHD10–3 [32]. DN-PDX-1#28 cells with 500 ng/ml doxycycline (Sigma) treatment allow absent expression of wide-type PDX-1, but they can result in inducible expression of DN-PDX-1 (lacking the N-terminal 79 amino acids); on the other hand, PDX-1#6 cells with doxycycline treatment can lead to inducible overexpression of wide-type PDX-1 [33]. In the study, the cells were firstly treated with or without 500 ng/ml doxycycline for the indicated time (24, 48 and 72 hrs). Then, the cells were treated with either 0.5 mM AICAR or 10 μM compound C for 48 hrs.
Insulin secretion and insulin content assays
INS-1 cells (1.5 × 105/well) were firstly pre-cultured in 24-well plates in standard medium for 24 hrs, and then were treated with either 0.5 mM AICAR or 10 μM compound C, supplemented with or without elevated concentrations of leucine (10, 20 or 40 mM) for 48 hrs. Secondly, all plates of cells were gently washed twice with pre-warmed phosphate-buffered saline (PBS) and incubated in pre-warmed Krebs-Ringer bicarbonate buffer (KRB) containing 3 mM glucose for 20 min. at 37°C. Thirdly, the buffer was removed and half of the plates (in each group) were cultured in KRB containing 3 mM glucose and the left half were incubated in KRB containing 27.8 mM glucose. After an additional 20 min. incubation at 37°C, aliquots of the media were collected from each well and stored at −20°C for subsequent insulin secretion test with an insulin radioimmunoassay kit (Beijing Atom HighTech Co. Ltd., Beijing, China). To measure total protein content, the cells were treated with lysis buffer containing 1 × PBS, 1% NP40, 0.1% SDS, 5 mM ethylenediaminetetraacetic acid, 0.5% sodium deoxycholate and 1 mM sodium orthovanadate (Shenneng Bo Cai Co. Ltd, Shanghai, China). Intracellular total protein concentration was determined with a bicinchoninic acid (BCA) protein assay kit (Bio-Rad, Hercules, CA, USA). Insulin secretion was normalized based on the corresponding protein in each group.
In addition, intracellular insulin contents were tested in INS-1 and RIN m5F cells, respectively. The cells were treated the same as in the insulin secretion section described. Five hundred microlitres of acid/ethanol (75% v/v ethanol, 1.5% v/v concentrated HCl) was added in each plate and incubated overnight [34]. The acid ethanol aliquots were used to measure insulin content with radioimmunoassay as described above. Total protein content was determined as described above. Insulin content was normalized based on the respective cellular protein in each group.
Cell counting kit-8 (CCK-8)
INS-1, RIN m5F, DN-PDX-1#28 and PDX-1#6 cells were seeded in 96-well plates at a density of 104 cells/well for 24 hrs. Then, they were treated with elevated concentrations of leucine (10, 20 or 40 mM) for 48 hrs. After that, 10 μl CCK-8 (Dojindo, Kumamoto, Japan) were added into each well and incubated for 4 hrs at 37°C in an humidified atmosphere with 5% CO2 and 95% air [35, 36] The wavelength to measure absorbance of the formazan product was 450 nm and the reference wavelength was 600 nm.
Protein analysis by Western blotting
The cultured INS-1, RIN m5F and DN-PDX-1#28 cells were lysed using lysis buffer supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF). Then protein extracts (60 μg total protein for GCK/GLUT2 and 40 μg protein for PDX-1) were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Millipore, Billerica, MA, USA). Membranes were gently shaked overnight at 4°C with 1:1000 p-AMPK antibody (Cell Signalling, Danvers, MA, USA), 1:10,000 PDX-1 antibody (Chemicon, Billerica, MA, USA), 1:200 GCK antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or 1:200 GLUT2 antibody (Santa Cruz Biotechnology). After the membranes were incubated with the corresponding second antibody at room temperature for 1 hr, proteins were visualized by enhanced chemiluminescence (Amersham Biosciences UK Limited, Little Chalfont, UK). The same membrane was re-blocked with 5% non-fat milk and incubated with mouse 1:10,000 β-actin monoclonal antibody (Abcam, Cambridge, UK). Immunoreactive bands were quantified by image analyzer Alphaimager 2200 (Alpha Innotech, San Leandro, CA, USA).
RNA isolation and real-time PCR
Total RNA was isolated from INS-1, RIN m5F, DN-PDX-1#28 and PDX-1#6 cells (1 × 106 cells/ well) with TRIzol (Invitrogen Corp., Carlsbad, CA, USA) method. In brief, 3 μg RNA isolated from each sample was used as the template in each reverse transcription reaction to generate first-strand cDNA using oligo (dT)18 as primer according to Fermentas RT kit (Fermentas, Glen Burnie, MD, USA). The cDNA was amplified by real-time PCR [37] using a QuantiTect SYBR Green kit (TaKaRa, Otsu, Shiga, Japan) and the ABI 7500 Prism real-time PCR instrument and software (Prism 7500; Applied Biosystems Inc., Foster city, CA, USA). All quantifications were performed with rat β-actin as an internal. Primer sequences used in the PCR are provided in Table 1. The PCR was performed for 40 cycles at 95°C for 15 sec., 60°C for 30 sec. and 72°C for 34 sec. The relative quantification of gene expression was analyzed by the 2−ΔΔCt method [38] and the results were expressed as extent of change with respect to control values.
1.
Sequence information on the primers used for real-time PCR
| Genes | Sequences | Products size (bp) | Annealing temperature (C) | Gene bank |
|---|---|---|---|---|
| PDX-1 | 5′-AAACGCCACACACCAAGGAGAA-3′ 5′-AGACCTGGCGGTTCACATG-3′ | 150 | 60 | NM_022852 |
| GCK | 5′-GCTTTTGAGACCCGTTTCGT-3′ 5′-CGCACAATGTCGCAGTCG-3′ | 119 | 60 | NMJD12565 |
| GLUT2 | 5′-CAGCTGTCTCTGTGCTGCTTGT-3′ 5′-GCCGTCATGCTCACATAACTCA-3′ | 150 | 60 | NM_012879 |
| ACTIN | 5′-CGCATCCTCTTCCTCCCTG-3′ 5′-GCCACAGGATTCCATACCCA-3′ | 130 | 60 | NM_031144 |
Statistical analysis
All of the experiments were repeated at least three independent times. All values are given as mean ± S.D. Data were analyzed using SPSS 11.5 software (SPSS, Inc., Chicago, IL, USA). Statistical significance was assessed by one-way anova. The difference was considered significant if the P-value was less than 0.05.
Results
The effects of AICAR or compound C in INS-1 and RIN m5F cells
To test the regulatory role of AMPK on PDX-1, GCK and GLUT2, INS-1 and RIN m5F cells were cultured with or without 0.5 mM AICAR or 10 μM compound C for 48 hrs. We firstly performed radioimmunoassay for insulin secretion and content detection. The results showed that in contrast to control, AICAR treatment decreased GSIS at high glucose by 29% (Fig. 1A, P < 0.05) and reduced the intracellular insulin content by 30% (Fig. 1B, P < 0.05) in INS-1 cells. In RIN m5F cells, after 48 hrs of AICAR incubation, there is a 34% decrease in the intracellular content compared with control (Fig. 1B, P < 0.05). When AMPK activity was inhibited by compound C, the results were quite opposite. In comparison with the corresponding control, compound C treatment was able to enhance GSIS at high glucose by 17% in INS-1 cells (Fig. 1A, P < 0.05) and the intracellular insulin content by 19% in INS-1 cells (Fig. 1B, P < 0.05) and 25% in RIN m5F (Fig. 1B, P < 0.05). However, neither AICAR nor compound C could affect the insulin secretion level at low glucose (3 mM) stimulation (Fig. 1A, P > 0.05) in INS-1 cells. The results revealed that AMPK played an important role in manipulating high glucose-induced insulin secretion and intracellular insulin content.
1.
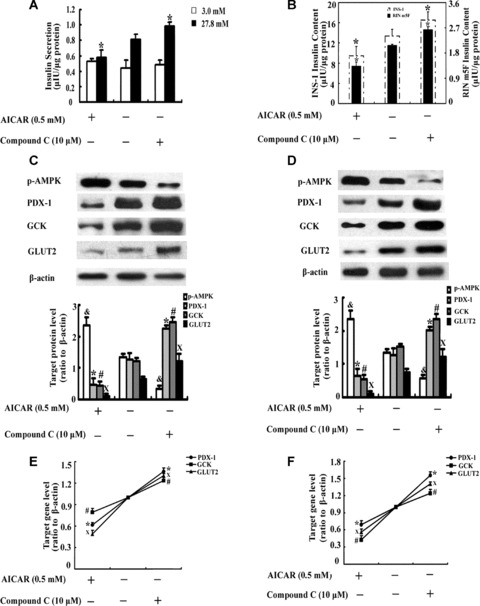
The effects of AICAR or compound C in β-cells. INS-1 and RIN m5F cells were cultured in RPMI 1640 with or without 0.5 mM AICAR or 10μM compound C for 48 hrs. Insulin secretion (A) at 3 mM glucose (□) and 27.8 mM glucose (▪) in INS-1 cells and intracellular insulin content (B) in INS-1 cells and RIN m5F cells were determined by radioimmunoas-say. The values were normalized by the intracellular protein content. The effects of AICAR or compound C on PDX-1, GCK and GLUT2 protein expression was tested by Western blotting in INS-1 cells (C) and RIN m5F cells (D). The mRNA expression of PDX-1, GCK and GLUT2 in INS-1 cells (E) and RIN m5F cells (F) was tested by real-time PCR. The data are the means ± S.D. All the values were collected from at least three separate experiments. *, #, X and &, P< 0.05, versus the corresponding control group.
Then, Western blotting and real-time PCR were used for p-AMPK, PDX-1, GCK and GLUT2 examination. In contrast to control, AICAR significantly strengthened the band of p-AMPK and weakened the bands of PDX-1 and its downstream targets, GCK and GLUT2 in INS-1 (Fig. 1C, P < 0.05) and RIN m5F cells (Fig. 1D, P < 0.05). Compared to the corresponding control, compound C obviously decreased AMPK activity and showed enhanced bands of PDX-1, GCK and GLUT2 in INS-1 (Fig. 1C, P < 0.05) and RIN m5F cells (Fig. 1D, P < 0.05). The results were confirmed by real-time PCR as well. Compared to the corresponding control, AICAR decreased PDX-1 mRNA levels by 38% and 31%, GCK mRNA levels by 21% and 57%, GLUT2 mRNA levels by 51% and 44% in INS-1 cells (Fig. 1E, P < 0.05) and RIN m5F cells (Fig. 1F, P < 0.05), respectively. In contrast to the corresponding control, compound C increased PDX-1 mRNA levels by 36% and 55%, GCK mRNA levels by 23% and 24%, GLUT2 mRNA levels by 30% and 40% in INS-1 (Fig. 1E, P < 0.05) and RIN m5F cells (Fig. 1F, P < 0.05), respectively.
The effects of AICAR or compound C in DN-PDX-1#28 cells
When DN-PDX-1#28 cells were treated with or without 500 ng/ml doxycycline for 24, 48 and 72 hrs, our results revealed a time-dependent expression of PDX-1 protein in DN-PDX-1#28 cells. Under non-induced condition (without 500 ng/ml doxycycline treatment), the PDX-1 protein expression showed a stained band and there was no significant difference between 24 and 48 or 72 hrs (Fig. 2A, P > 0.05). Under induced condition (with 500 ng/ml doxycycline treatment), PDX-1 protein band became weaker with time extension; furthermore, the weakest bands occurred in cells treated with doxycycline for 72 hrs (Fig. 2A, P < 0.05). Consistent with this, GLUT2 and GCK expression had the similar diminishing consequence with PDX-1 described above. Under non-induced condition for 24, 48 and 72 hrs, protein bands intensity of both GCK and GLUT2 presented no change (Fig. 2B, P > 0.05). Under induced condition for 24, 48 and 72 hrs, there was parallel decrease in the protein expression of GCK and GLUT2 (Fig. 2B, P < 0.05). The results suggested that PDX-1 performed its function well in the regulation of GCK and GLUT2.
2.
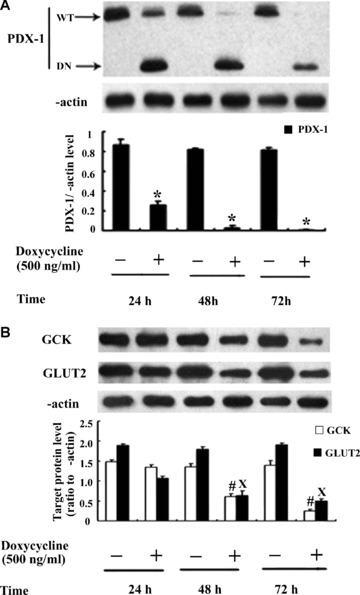
The effects of AICAR or compound C in DN-PDX-1#28 cells. DN-PDX-1#28 cells were treated with or without 500 ng/ml doxycycline for 24, 48 and 72 hrs. Western blotting was performed to detect the protein expression of PDX-1 (A), GCK and GLUT2 (B). The results were obtained from at least three independent experiments. *, # and X, P< 0.05, versus the corresponding control group.
In order to confirm the function of PDX-1 in the process of AMPK regulating GCK and GLUT2, DN-PDX-1#28 cells were pre-treated with or without 500 ng/ml doxycycline for 24 hrs prior to treatment with or without AICAR or compound C for a further 48 hrs. Radioimmunoassay, Western blotting and real-time PCR were performed for the detection. In comparison with control, doxycycline alone treatment obviously decreased high glucose-induced insulin secretion by 88% (Fig. 3A1, P < 0.05), insulin content by 82% (Fig. 3A2, P < 0.05), and compound C alone treatment significantly increased high glucose-induced insulin secretion by 17% (Fig. 3A1, P < 0.05) and insulin content by 19% (Fig. 3A2, P < 0.05). However, compared with doxycycline alone treatment, doxycycline plus compound C treatment could not significantly affect insulin secretion or content (Fig. 3A, P > 0.05). With regard to p-AMPK, PDX-1, GCK and GLUT2 protein expression changes, under non-induced condition, AICAR or compound C had the same effects as those observed in INS-1 cells described in Fig. 1C or RIN m5F cells in Fig. 1D (Fig. 3B). However, under induced condition, p-AMPK protein expression had the same result as that under non-induced condition (Fig. 3B); with regard to PDX-1, GCK or GLUT2 protein expression, neither AICAR nor compound C could affect them (Fig. 3B, P > 0.05). The results were confirmed by real-time PCR (Fig. 3C) as well.
3.
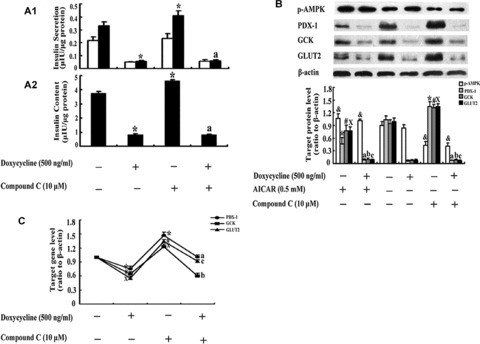
Neither AICAR nor compound C could affect GCK and GLUT2 expression when PDX-1 expression was absent. DN-PDX-1#28 cells were pre-treated with or without 500 ng/ml doxycycline for 24 hrs prior to treatment with or without 0.5 mM AICAR or 10 μM compound C for a further 48 hrs. Insulin secretion (A1) at 3 mM glucose (□) and 27.8 mM glucose (▪) and insulin content (A2) were determined by radioimmunoassay. The values were normalized by the intracellular protein content. The PDX-1, GCK and GLUT2 protein and mRNA expression were determined by Western blotting (B) and real-time PCR (C). The data are the means ± S.D. All results were collected from at least three separate experiments. *, #, X and &, P< 0.05, versus the corresponding control group. a, b and c, P > 0.05, versus the doxycycline alone treatment group.
The effects of elevated concentrations of leucine in INS-1 and RIN m5F cells
To obtain insight into the functional changes when exposed to elevated concentrations of leucine, INS-1 and RIN m5F cells were cultured with or without elevated levels of leucine for 48 hrs. The cells were then used to determine insulin secretion as well as intracellular insulin content. As shown in Fig. 4A and B, our results demonstrated that a 48-hr incubation with elevated concentrations of leucine led to a dose-dependent decrease of GSIS at high glucose in INS-1 cells and insulin content in both INS-1 and RIN m5F cells. In INS-1 cells, in comparison with control, 40 mM leucine exposure significantly decreased high glucose-induced insulin secretion by 34% (Fig. 4A, P < 0.05) and diminished the intracellular insulin content by 24% (Fig. 4B, P < 0.05), respectively, and there was not apparent change in insulin secretion induced by low glucose stimulation in INS-1 cells (Fig. 4A, P > 0.05). There was no difference in insulin secretion at both low and high glucose stimulation between control and either 10 mM or 20 mM leucine treatment (Fig. 4A, P > 0.05), and the same results occurred in insulin content as well in INS-1 cells (Fig. 4B, P > 0.05). With regard to RIN m5F cells, the insulin content presented the same trend as in INS-1 cells, 40 mM leucine diminished the intracellular insulin content by 24% (Fig. 4B, P < 0.05) in contrast to its control. Western blotting and real-time PCR were performed for p-AMPK, PDX-1, GCK and GLUT2 detection. In contrast to control, 40 mM leucine exposure significantly enhanced p-AMPK protein expression in INS-1 cells (Fig. 4C, P < 0.05) and RIN m5F cells (Fig. 4D, P < 0.05). The protein levels of PDX-1 and its downstream targets, GCK and GLUT2 in 40 mM leucine-treated cells were much lower than those in cells that were cultured in leucine-absent media in INS-1 cells (Fig. 4C, P < 0.05) and RIN m5F cells (Fig. 4D, P < 0.05). Compared with control, neither 10 mM nor 20 mM leucine culture could statistically change the expression of those proteins described above in INS-1 cells (Fig. 4C, P > 0.05) and RIN m5F cells (Fig. 4D, P > 0.05). The results above were confirmed by real-time PCR (Fig. 4E and F) as well.
4.
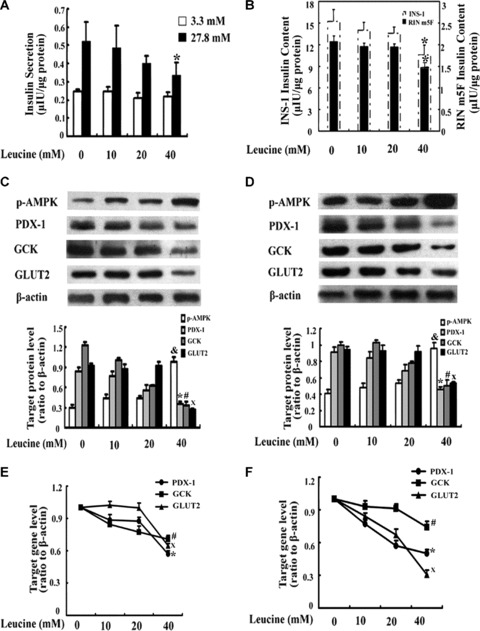
The effects of elevated concentrations of leucine in β-cells. INS-1 and RIN m5F cells were treated with or without elevated concentrations of leucine (10, 20 or 40 mM) for 48 hrs. Insulin secretion (A) at 3 mM glucose (□) and 27.8 mM glucose (▪) in INS-1 cells and intracellular insulin content in INS-1 (B) and RIN m5F cells (B) were determined by radioimmunoassay. The values were adjusted by the intracellular protein content. The protein expression of p-AMPK, PDX-1, GCK and GLUT2 in INS-1 cells (C) and RIN m5F cells (D) was detected by Western blotting. The mRNA expression of PDX-1, GCK and GLUT2 in INS-1 cells (E) and RIN m5F cells (F) was tested using real-time PCR. The data are the means ± S.D. The results were based from at least three separate experiments. *, #, X and &, P< 0.05, versus the corresponding control group.
Leucine cytotoxicity detection on pancreatic β-cell lines
In order to further testify that 40 mM leucine was appropriate for the experiment research, INS-1, RIN m5F, DN-PDX-1#28 and PDX-1#6 cells were firstly treated with or without elevated concentrations of leucine and then tested the cell viability by CCK-8 method, respectively. The results showed that in INS-1, RIN m5F, DN-PDX-1#28 and PDX-1#6 cells, compared with their corresponding controls, the cell viability of 10 mM leucine was 99% (Fig. 5A, P > 0.05), 97% (Fig. 5B, P > 0.05), 98% (Fig. 5C, P > 0.05), 98% (Fig. 5D, P > 0.05), the cell viability of 20 mM leucine was 98% (Fig. 5A, P > 0.05), 97% (Fig. 5B, P > 0.05), 98% (Fig. 5C, P > 0.05), 99% (Fig. 5D, P > 0.05), the cell viability of 40 mM leucine was 97% (Fig. 5A, P > 0.05), 97% (Fig. 5B, P > 0.05), 96% (Fig. 5C, P > 0.05), 95% (Fig. 5D, P > 0.05). The results testified that neither 10, nor 20 nor 40 mM leucine had cytotoxicity on pancreatic β-cell lines.
5.
Leucine cytotoxicity detection on pancreatic β-cell lines. INS-1, RIN m5F, DN-PDX-1#28 and PDX-1#6 cells were treated with or without elevated concentrations of leucine (10 mM, 20 mM, 40 mM) for 48 hrs. Then, the cell viability in INS-1 (A), RIN m5F (B), DN-PDX-1#28 (C) and PDX-1#6 cells (D) was evaluated using CCK-8. The data are the means ± S.D. All the results were collected from at least three separate experiments.
The effects of AICAR or compound C in INS-1 and RIN m5F cells with chronic leucine treatment
INS-1 and RIN m5F cells were incubated in RPMI 1640 media supplemented with or without 40 mM leucine, in the presence or absence of AICAR or compound C for 48 hrs. In INS-1 and RIN m5F cells, either leucine alone, AICAR alone or compound C alone treatment induced the same results as the data from Figs. 1 and 4. Moreover, in comparison with leucine treatment, leucine plus AICAR co-treatment diminished high glucose-induced insulin secretion by 21% in INS-1 cells (Fig. 6A1, P < 0.05) and intracellular insulin content by 23% in INS-1 cells (Fig. 6A2, P < 0.05), and 26% in RIN m5F cells (Fig. 6B, P < 0.05). Furthermore, the reduced insulin secretion and content caused by chronic high leucine treatment were significantly recovered by leucine plus compound C co-treatment. In contrast to leucine treatment, leucine plus compound C co-treatment increased high glucose-induced insulin secretion by 33% in INS-1 cells (Fig. 6A1, P < 0.05) and intracellular insulin content by 24% in INS-1 cells (Fig. 6A2, P < 0.05) and 29% in RIN m5F cells (Fig. 6B, P < 0.05), respectively. Neither AICAR nor compound C produced significant difference at low glucose stimulation in comparison with control in INS-1 cells (Fig. 6A1, P > 0.05).
6.
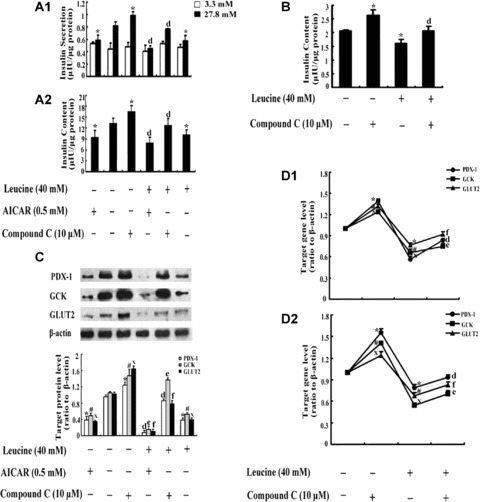
The effects of AICAR or compound C in INS-1 and RIN m5F cells with chronic leucine treatment. INS-1 and RIN m5F cells were incubated in RPMI 1640 media supplemented with or without 40 mM leucine, in the presence or absence 0.5 mM AICAR or 10 μM compound C for 48 hrs. Insulin secretion (A1) at 3 mM glucose (□) and 27.8 mM glucose (▪) in INS-1 cells and insulin content in INS-1 (A2) and RIN m5F (B) cells were determined by radioim-munoassay. The values were adjusted by the intracellular protein content. The protein expression of PDX-1, GCK and GLUT2 in INS-1 cells (C) was tested by Western blotting. The mRNA expression of PDX-1, GCK and GLUT2 in INS-1 cells (D1) and RIN m5F cells (D2) was tested by real-time PCR. The data are the means ± S.D. All the results were collected from at least three separate experiments. *, # and X, P< 0.05, versus the corresponding control group. d, e and f, P< 0.05, versus leucine alone treatment group.
In order to further determine whether AMPK regulated PDX-1 under chronic high leucine exposure, Western blotting and realtime PCR were performed in INS-1 and RIN m5F cells. The weak bands representing protein expression of PDX-1, GCK and GLUT2, respectively, appeared in 40 mM leucine alone and AICAR alone groups, and the weakest band occurred in 40 mM leucine plus AICAR co-treatment group compared with control (Fig. 6C, P < 0.05). The reduced protein expression of PDX-1, GCK and GLUT2 induced by chronic high leucine was recovered almost to normal in leucine plus compound C co-treatment group (Fig. 6C, P < 0.05). The results were confirmed by real-time PCR in both INS-1 (Fig. 6D1) and RIN m5F cells (Fig. 6D2).
The effects of leucine in DN-PDX-1#28 and PDX-1#6 β-cells
To further prove that the PDX-1 plays an important role in impaired insulin secretion and content induced by chronic leucine, DN-PDX-1#28 and PDX-1#6 cells were treated with or without 500 ng/ml doxycycline, in the presence or absence of 40 mM leucine for 48 hrs. In DN-PDX-1#28 cells, in comparison with control, leucine alone treatment and doxycycline alone treatment diminished high glucose-induced insulin secretion by 36% and 84% (Fig. 7A1, P < 0.05), decreased intracellular insulin content by 23% and 62% (Fig.7A2, P < 0.05), respectively. In comparison with leucine treatment alone group, doxycycline plus leucine treatment decreased high glucose-induced insulin secretion by 67% (Fig. 7A1, P < 0.05) and intracellular insulin content by 44% (Fig. 7A2, P < 0.05). In PDX-1#6 cells, compared with control, leucine alone treatment diminished high glucose-induced insulin secretion by 26% (Fig. 7C1, P < 0.05) and insulin content by 25% (Fig. 7C2, P < 0.05), doxycycline alone treatment increased high glucose-induced insulin secretion by 20% (Fig. 7C1, P < 0.05) and insulin content by 21% (Fig. 7C2, P < 0.05). However, relative to leucine alone treatment, doxycycline plus leucine treatment significantly increased GSIS by 36% (Fig. 7C1, P < 0.05) and insulin content by 21% (Fig. 7C2, P < 0.05). The results were confirmed by real-time in DN-PDX-1#28 cells (Fig. 7B) and PDX-1#6 cells (Fig. 7D) as well.
7.
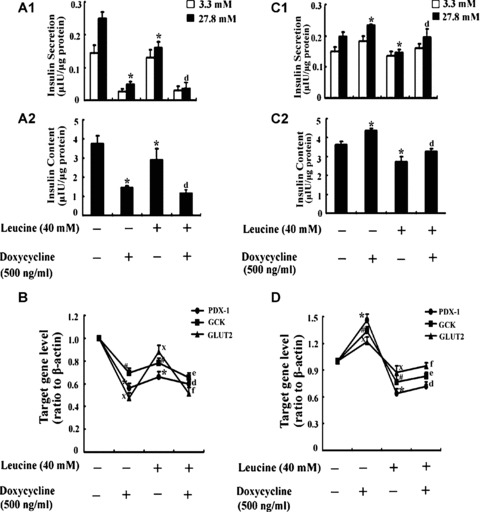
The effects of leucine in DN-PDX-1#28 and PDX-1#6 β-cells. DN-PDX-1#28 and PDX-1#6 cells were treated with or without 500 ng/ml doxycycline, in the presence or absence of 40 mM leucine for 48 hrs. Insulin secretion at 3 mM glucose (□) and 27.8 mM glucose (▪) and intracellular insulin content in DN-PDX-1#28 (A) and PDX-1#6 cells (C) were determined by radioim-munoassay. The values were adjusted for the intracellular total protein content. The mRNA expression of PDX-1, GCK and GLUT2 in DN-PDX-1#28 (B) and PDX-1#6 cells (D) was detected by realtime PCR. The data are the means ± S.D. All the results were collected from at least three separate experiments. *# and X, P< 0.05, versus the corresponding control group. d, e and f, P< 0.05, versus leucine alone treatment group.
Discussion
In the present study, we found firstly that PDX-1, which activated GCK and GLUT2, was a possible target for AMPK action in INS-1 and RIN m5F cells. AMPK activation induced by AICAR, a AMPK agonist, might result in decreased intracellular insulin content in β-cells; as a result, high glucose-induced insulin secretion decreased. We also demonstrated that AICAR enhanced chronic high leucine-induced reduction of insulin secretion and content by down-regulating PDX-1, GCK and GLUT2 expression. Finally, we reconfirmed our results in both DN-PDX-1#28 and PDX-1#6 cells that the inhibition of PDX-1 could potentiate the impaired effects induced by leucine and overexpression of PDX-1 could protect the cell from impairment induced by leucine.
We did not use pancreatic islets because we encountered many difficulties, including the preparation of large numbers of viable islets and cellular heterogeneity as in the previous reports [34]. In addition, our aim in the present study focused on effects of leucine on the β-cells. Thus, we chose INS-1 and RIN m5F cells used for the experiment. Though the cell lines cannot own all the features of mature islets, their usage can avoid disadvantages of isolated primary β-cells, such as potential alterations during the isolation procedure [39] and also has its special characters. INS-1 cells are a stable and highly differentiated rat insulinoma cell line which maintains β-cell characteristics, such as glucose responsiveness, and has been used as a model for β-cell function [40–42]. RIN m5F cells can also secrete detectable amounts of insulin [34, 43]. Moreover, the two cell lines maintain a stable balance of insulin secretion under physiological condition [44]. Furthermore, the responsiveness of the cell lines is almost equal to that of islet and they can be kept with fuction in culture media for cumulative periods [34]. Insulin content is contributed to by varying rates of proinsulin synthesis and processing, insulin secretion and intracellular insulin degradation. Under normal circumstances, in primary β-cells, insulin content stays constant. Either leucine or AICAR has been reported to be able to affect insulin secretion and insulin content [1, 6, 18], which were partially in agreement with our results.
The present study reports the role of AMPK activation on insulin secretion and intracellular insulin content; moreover, it proposes that AMPK regulates GCK and GLUT2 under relative physiological condition. Recently, a number of studies have reported that AMPK activation exerts inhibitory effects on insulin secretion and content in vitro and in vivo[18, 45–47]. Consistent with the reports, our data showed that AMPK activation by AICAR significantly suppressed high glucose-induced insulin secretion and insulin content in both INS-1 and RIN m5F cells. To date, the regulatory relationship between AMPK and GCK or GLUT2 has not been confirmed, although a few reports have suggested its existence. Kim et al. found that there existed a regulatory effect of AMPK on GCK and GLUT2 expression under high glucose concentration [18]. In agreement with the observation, our results predominantly revealed that AMPK played a potent role in the down-regulation of GCK and GLUT2 as well. Our results were different from the work of Kim et al., which also examined the effects of AICAR on insulin gene expression and notably found no effect on PDX-1 expression in those cells [48]. The main reason might be due to different treatment conditions, such as AICAR concentration, treatment time and different culture condition. With regard to the AICAR concentration, we used 0.5 mM whereas Kim et al. used 0.4 mM. Most of the papers used 0.5 or 1 mM [8, 18]. Firstly, we tested 0.5 and 1 mM AICAR – there was no difference between them (both AICAR at 0.5 and 1 mM levels could change PDX-1 expression); therefore, we used 0.5 mM AICAR. Moreover, we cultured cells with AICAR for 48 hrs whereas Kim et al. cultured cells with AICAR for 12 hrs. Furthermore, the glucose concentration was different. We cultured cells with 11.1 mM glucose whereas Kim cultured cells with low glucose and glucolipotoxic condition.
Therefore, these differences might be the reasons that we obtained different results.
The creative finding in the present study is that it demonstrates firstly that AMPK regulates GCK and GLUT2 via PDX-1 under relative physiological environment. Previous studies reported that PDX-1 regulated pancreatic β-cell specific gene expression including GLUT2 and GCK [26–29]. In DN-PDX-1#28 cells, we found that PDX-1 positively regulated GCK and GLUT2, which were in accordance with the reports from the previous studies [33]. In addition, we observed that neither AICAR nor compound C could change GCK or GLUT2 protein and mRNA expression when PDX-1 expression was absent. Similarly, neither AICAR nor compound C could change insulin secretion and content at the absence of PDX-1. The results powerfully testified that PDX-1 was an essential mediate agent in the process of AMPK regulating GCK and GLUT2 under physiological conditions. Collectively, based on the present data, we obtained the preliminary conclusion that AMPK activation induced GCK and GLUT2 down-regulation by decreasing PDX-1 protein expression.
Then, we tested whether chronic leucine exposure affected insulin secretion and content associated with the regulation described above. With regard to the effects of leucine on insulin secretion and content, the related reports were quite contradictory. Yang et al. reported that 10 mM leucine was able to stimulate GSIS [1, 2]. However, Anello and his colleagues reported that leucine decreased GSIS in a dose-dependent manner, 20 mM leucine significantly reduced GSIS [3]. From these present reports, we could not get the exact leucine concentration for our study. Therefore, we redesigned our experiment to investigate the effects of elevated concentrations of leucine on insulin secretion and content. Our studies showed that 48 hrs incubation with increasing concentrations of leucine decreased high glucose-induced insulin secretion and content in a dose-dependent manner, and the difference was most significant at 40 mM leucine. The results were partially in agreement with the reports from Anello and his colleagues. The disparity appeared to be derived from different experimental conditions, such as the category of cells, duration of exposure to leucine.
Bolea et al. reported in their paper that the plasma concentrations of leucine in fed mice was less than 1mM [49], quite different from the high amounts of leucine used in our studies. In order to testify that 40 mM leucine affecting pancreatic β-cells was corr elated with changes of AMPK, PDX-1, GCK and GLUT2, not with cell apoptosis or cell death, we supplemented CCK-8 assay experiment. The results showed that 40 mM leucine did not significantly inhibit cell viability compared with corresponding controls in four pancreatic β-cell lines. Furthermore, we also found some other papers using 40 mM leucine for study research [50–52]. We understand that data with high leucine concentrations have limited physiological significance. However it can alter the pancreatic β-cell function and it may help us to dissect the signalling pathway of gene regulations in the β-cells, such as GCK and PDX-1. These genes play important roles in β-cell functions and in the development of diabetes. Bolea et al. aimed to study the acute effect of physiological amino acids on pancreatic β-cells; therefore, they used leucine at the concentration found in the plasma of fed mice. We aimed to study the chronic high leucine on pancreatic β-cells; therefore, according to the papers focusing on the effect of leucine on pancreatic β-cells as well as our results, we used 40 mM leucine for our study. Bolea et al. and us explored different leucine concentrations probably due to different aims.
With regard to the probable mechanism by which leucine affected insulin secretion and content, we speculated that it was related to AMPK, PDX-1, GCK and GLUT2 expression changes. AMPK activation was reported to be negative with insulin secretion and content [14, 18, 45–47]. In the present study, our investigation showed that 40 mM leucine significantly enhanced p-AMPK protein expression. PDX-1, GCK and GLUT2 were reported to be positively correlated with insulin secretion by different mechanisms [1, 17, 25, 26]. Our results indicated that 40 mM leucine diminished PDX-1, GCK and GLUT2 protein expression. The results were able to address the decreased insulin secretion and content induced by chronic leucine treatment, which might be due to increased AMPK protein expression or decreased PDX-1, GCK and GLUT2 protein expression. However, this cannot exclude other factors that might result in the reduction of insulin secretion and content.
Based on the data above, we reached the preliminary conclusion that chronic leucine culture impaired insulin secretion and content, suggesting the probable correlation with its effect activating AMPK and inhibiting PDX-1, GCK and GLUT2 protein expression; furthermore, we also obtained the regulatory effect of AMPK on GCK or GLUT2 via PDX-1 under relative physiological conditions. However, whether the regulation also existed equally under leucine-induced pathophysiological conditions, was still unknown. Our results revealed that chronic high leucine treatment or AICAR treatment reduced PDX-1, GCK and GLUT2 protein expression, and their protein expression was strongly abrogated by leucine plus AICAR co-treatment in INS-1 cells. Furthermore, the reduced protein expression caused by leucine alone treatment could be rescued by leucine plus compound C co-treatment in INS-1 cells. The results were also confirmed by insulin secretion, insulin content and real-time PCR in both INS-1 cells and RIN m5F cells.
Collectively, the results above presented that optimal level of AMPK activation was able to decrease GSIS and insulin content under high leucine exposure by regulating PDX-1, GCK and GLUT2 protein and mRNA expression. Thus, to further prove that the decrease of PDX-l plays a key role in impaired GSIS and insulin content induced by chronic leucine treatment, we reconfirmed our results with DN-PDX-1#28 and PDX-1#6 cells. The results showed that the inhibition of PDX-1 could potentiate the impaired effects induced by leucine and overexpression of PDX-1 could protect the cell from impairment induced by leucine. However, up to now, the question remains unclear whether the influence of chronic leucine exposure on AMPK and the regulation of AMPK on PDX-1 are direct or indirect. More research should be done to study the correlated association. Furthermore, we should investigate whether leucine needs to be metabolized for its effect. To solve the question, leucine should be compared with the non-metabolized leucine analogue 2-amino-2-norbornane-carboxylic acid to address this question.
At present, the environmental factors are deemed as the main causes of diabetes [53, 54]. Recently, much light has been shed on the effects and mechanisms of glucose and lipid on pancreatic β-cells; fewer studies are on the effects and mechanisms of amino acids, the third class of nutrient insulin secretagogues. Our studies show that leucine impairs pancreatic β-cells which are associated with AMPK, PDX-1, GCK and GLUT2 changes. These findings might have potential clinical implications in preventing type 2 diabetes. In addition, clinically, some patients with liver damage often received branched chain amino acids (BCAAs) treatment; however, the exact concentration and its effects on pancreatic β-cells are not clear. Therefore, the study might give a hint in supplementing the exact concentration BCAAs to patients as well.
In conclusion, the current study indicated that chronic leucine might result in an increase in AMPK expression and then a decrease in PDX-1 level, in turn to depress the activity of GCK and GLUT2; as a result, high glucose-induced insulin secretion and intracellular content decreased. We speculated that this might be one of the mechanisms of impaired GSIS and insulin content induced by chronic leucine treatment.
Acknowledgments
This research was funded by grants from the National Natural Science Foundation of China (No: 30670994). The authors thank Prof. Haiyan Wang for providing DN-PDX-1#28 and PDX-1#6 cells, Prof. Xiao Han for providing INS-1 cells and the teachers in the Scientific Center of Shandong Provincial Hospital for professional technical assistance. The authors also thank Dr. Zhiyong Gao for his valuable proposals and Dr. Yiman Zheng for his critical rectification.
References
- 1.Yang J, Wong RK, Park M, Wu J, Cook JR, York DA, Deng S, Markmann J, Naji A, WoLf BA, Gao Z. Leucine regulation of glucokinase and ATP synthase sensitizes glucose-induced insulin secretion in pancreatic beta-cells. Diabetes. 2006;55:193–201. [PubMed] [Google Scholar]
- 2.Yang J, Wong RK, Wang X, Moibi J, Hessner MJ, Greene S, Wu J, Sukumvanich S, Wolf BA, Gao Z. Leucine culture reveals that ATP synthase functions as a fuel sensor in pancreatic beta-cells. J Biol Chem. 2004;279:53915–23. doi: 10.1074/jbc.M405309200. [DOI] [PubMed] [Google Scholar]
- 3.Anello M, Ucciardello V, Piro S, Patane G, Frittitta L, Calabrese V, Giuffrida Stella AM, Vigneri R, Purrello F, Rabuazzo AM. Chronic exposure to high leucine impairs glucose-induced insulin release by lowering the ATP-to-ADP ratio. Am J Physiol Endocrinol Metab. 2001;281:E1082–7. doi: 10.1152/ajpendo.2001.281.5.E1082. [DOI] [PubMed] [Google Scholar]
- 4.Leff T. AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins. Biochem Soc Trans. 2003;31:224–7. doi: 10.1042/bst0310224. [DOI] [PubMed] [Google Scholar]
- 5.Da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–74. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–51. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 7.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335:533–9. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raile K, Klammt J, Laue S, Garten A, Bluher M, Kralisch S, Kloting N, Kiess W. Glucose concentration and AMP-dependent kinase activation regulate expression of insulin receptor family members in rat islets and INS-1E beta cells. Diabetologia. 2005;48:1798–809. doi: 10.1007/s00125-005-1860-x. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Zhou L, Li G, Luo T, Gu Y, Qian L, Fu X, Li F, Li J, Luo M. Palmitate activates AMP-activated protein kinase and regulates insulin secretion from beta cells. Biochem Biophys Res Commun. 2007;352:463–8. doi: 10.1016/j.bbrc.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Ravnskjaer K, Boergesen M, Dalgaard LT, Mandrup S. Glucose-induced repression of PPARalpha gene expression in pancreatic beta-cells involves PP2A activation and AMPK inactivation. J Mol Endocrinol. 2006;36:289–99. doi: 10.1677/jme.1.01965. [DOI] [PubMed] [Google Scholar]
- 11.Du M, Shen QW, Zhu MJ, Ford SP. Leucine stimulates mammalian target of rapamycin signaling in C2C12 myoblasts in part through inhibition of adenosine monophosphate-activated protein kinase. J Anim Sci. 2007;85:919–27. doi: 10.2527/jas.2006-342. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Herrero CM, Galan M, Vincent O, Flandez B, Gargallo M, Delgado-Alvarez E, Blazquez E, Navas MA. Functional analysis of human glucokinase gene mutations causing MODY2: exploring the regulatory mechanisms of glucokinase activity. Diabetologia. 2007;50:325–33. doi: 10.1007/s00125-006-0542-7. [DOI] [PubMed] [Google Scholar]
- 13.Roche E, Assimacopoulos-Jeannet F, Witters LA, Perruchoud B, Yaney G, Corkey B, Asfari M, Prentki M. Induction by glucose of genes coding for glycolytic enzymes in a pancreatic beta-cell line (INS-1) J Biol Chem. 1997;272:3091–8. doi: 10.1074/jbc.272.5.3091. [DOI] [PubMed] [Google Scholar]
- 14.Kim WH, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, Song JH, Gao B, Jung MH. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: downregulation of glucokinase in pancreatic beta-cells. Diabetes. 2005;54:2602–11. doi: 10.2337/diabetes.54.9.2602. [DOI] [PubMed] [Google Scholar]
- 15.Guillam MT, Dupraz P, Thorens B. Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes. 2000;49:1485–91. doi: 10.2337/diabetes.49.9.1485. [DOI] [PubMed] [Google Scholar]
- 16.Thorens B, Guillam MT, Beermann F, Burcelin R, Jaquet M. Transgenic reex-pression of GLUT1 or GLUT2 in pancreatic beta cells rescues GLUT2-null mice from early death and restores normal glucose-stimulated insulin secretion. J Biol Chem. 2000;275:23751–8. doi: 10.1074/jbc.M002908200. [DOI] [PubMed] [Google Scholar]
- 17.Tiedge M, Lenzen S. Regulation of glucokinase and GLUT-2 glucose-transporter gene expression in pancreatic B-cells. Biochem J. 1991;279:899–901. doi: 10.1042/bj2790899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WH, Lee JW, Suh YH, Lee HJ, Lee SH, Oh YK, Gao B, Jung MH. AICAR potentiates ROS production induced by chronic high glucose: roles of AMPK in pancreatic beta-cell apoptosis. Cell Signal. 2007;19:791–805. doi: 10.1016/j.cellsig.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Leahy JL, Cooper HE, Deal DA, Weir GC. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest. 1986;77:908–15. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao CQ, Deng HM, Huang Y. Effects of supraphysiologic concentration glucose on pancreatic duodenal homeobox-1 expression and insulin secretion in rats. Chin Med J. 2007;120:1020–3. [PubMed] [Google Scholar]
- 21.Sun Y, Zhang L, Gu HF, Han W, Ren M, Wang F, Gong B, Wang L, Guo H, Xin W, Zhao J, Gao L. Peroxisome proliferator-activated receptor-alpha regulates the expression of pancreatic/duodenal homeobox-1 in rat insulinoma (INS-1) cells and ameliorates glucose-induced insulin secretion impaired by palmitate. Endocrinology. 2008;149:662–71. doi: 10.1210/en.2007-1275. [DOI] [PubMed] [Google Scholar]
- 22.Gremlich S, Bonny C, Waeber G, Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem. 1997;272:30261–9. doi: 10.1074/jbc.272.48.30261. [DOI] [PubMed] [Google Scholar]
- 23.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–9. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 24.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–95. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 25.Kaneto H, Miyatsuka T, Shiraiwa T, Yamamoto K, Kato K, Fujitani Y, Matsuoka TA. Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr Med Chem. 2007;14:1745–52. doi: 10.2174/092986707781058887. [DOI] [PubMed] [Google Scholar]
- 26.Iype T, Francis J, Garmey JC, Schisler JC, Nesher R, Weir GC, Becker TC, Newgard CB, Griffen SC, Mirmira RG. Mechanism of insulin gene regulation by the pancreatic transcription factor Pdx-1: application of pre-mRNA analysis and chromatin immunoprecipitation to assess formation of functional transcriptional complexes. J Biol Chem. 2005;280:16798–807. doi: 10.1074/jbc.M414381200. [DOI] [PubMed] [Google Scholar]
- 27.Marshak S, Totary H, Cerasi E, Melloul D. Purification of the beta-cell glucose-sensitive factor that transactivates the insulin gene differentially in normal and transformed islet cells. Proc Natl Acad Sci USA. 1996;93:15057–62. doi: 10.1073/pnas.93.26.15057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 1996;10:1327–34. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- 29.Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–26. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 30.Bennett RG, Hamel FG, Duckworth WC. An insulin-degrading enzyme inhibitor decreases amylin degradation, increases amylin-induced cytotoxicity, and increases amyloid formation in insulinoma cell cultures. Diabetes. 2003;52:2315–20. doi: 10.2337/diabetes.52.9.2315. [DOI] [PubMed] [Google Scholar]
- 31.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 32.Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, Wollheim CB. Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J Biol Chem. 2001;276:25279–86. doi: 10.1074/jbc.M101233200. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Iezzi M, Theander S, Antinozzi PA, Gauthier BR, Halban PA, Wollheim CB. Suppression of Pdx-1 perturbs proinsulin processing, insulin secretion and GLP-1 signalling in INS-1 cells. Diabetologia. 2005;48:720–31. doi: 10.1007/s00125-005-1692-8. [DOI] [PubMed] [Google Scholar]
- 34.Hamid M, McCluskey JT, McClenaghan NH, Flatt PR. Comparison of the secretory properties of four insulin-secreting cell lines. Endocr Res. 2002;28:35–47. doi: 10.1081/erc-120004536. [DOI] [PubMed] [Google Scholar]
- 35.Zou C, Shen Z. An optimized in vitro assay for screening compounds that stimulate liver cell glucose utilization with low cyto-toxicity. J Pharmacol Toxicol Methods. 2007;56:58–62. doi: 10.1016/j.vascn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Jiang Y, Ahn EY, Ryu SH, Kim DK, Park JS, Yoon HJ, You S, Lee BJ, Lee DS, Jung JH. Cytotoxicity of psammaplin A from a two-sponge association may correlate with the inhibition of DNA replication. BMC Cancer. 2004;4:70. doi: 10.1186/1471-2407-4-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats’ skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–7. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 39.Bode HP, Moormann B, Dabew R, Goke B. Glucagon-like peptide 1 elevates cytosolic calcium in pancreatic beta-cells independently of protein kinase A. Endocrinology. 1999;140:3919–27. doi: 10.1210/endo.140.9.6947. [DOI] [PubMed] [Google Scholar]
- 40.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–78. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 41.Xiao J, Gregersen S, Pedersen SB, Hermansen K. Differential impact of acute and chronic lipotoxicity on gene expression in INS-1 cells. Metabolism. 2002;51:155–62. doi: 10.1053/meta.2002.29977. [DOI] [PubMed] [Google Scholar]
- 42.Alstrup KK, Brock B, Hermansen K. Long-Term exposure of INS-1 cells to cis and trans fatty acids influences insulin release and fatty acid oxidation differentially. Metabolism. 2004;53:1158–65. doi: 10.1016/j.metabol.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Hohmeier HE, Newgard CB. Cell lines derived from pancreatic islets. Mol Cell Endocrinol. 2004;228:121–8. doi: 10.1016/j.mce.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J Clin Invest. 1998;101:1094–101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 46.Riboulet-Chavey A, Diraison F, Siew LK, Wong FS, Rutter GA. Inhibition of AMP-activated protein kinase protects pancreatic beta-cells from cytokine-mediated apoptosis and CD8+ T-cell-induced cyto-toxicity. Diabetes. 2008;57:415–23. doi: 10.2337/db07-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo. J Endocrinol. 2005;187:225–35. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 48.Kim JW, Cho JH, Ko SH, Park HS, Ha J, Song KH, Son HY, Kim SS, Yoon KH, Suh-Kim H. Transcriptional mechanism of suppression of insulin gene expression by AMP-activated protein kinase activator 5-amino-4-imidazolecarboxamide riboside (AICAR) in beta-cells. Biochem Biophys Res Commun. 2008;365:614–20. doi: 10.1016/j.bbrc.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 49.Bolea S, Pertusa JA, Martin F, Sanchez-Andres JV, Soria B. Regulation of pancreatic beta-cell electrical activity and insulin release by physiological amino acid concentrations. Pflugers Arch. 1997;433:699–704. doi: 10.1007/s004240050334. [DOI] [PubMed] [Google Scholar]
- 50.Ganapathy V, Radhakrishnan AN. Interaction of amino acids with glycl-L-leucine hydrolysis and transport in monkey small intestine. Clin Sci. 1979;57:521–7. doi: 10.1042/cs0570521. [DOI] [PubMed] [Google Scholar]
- 51.Rideau N, Simon J. L-leucine or its keto acid potentiate but do not initiate insulin release in chicken. Am J Physiol. 1989;257:E15–9. doi: 10.1152/ajpendo.1989.257.1.E15. [DOI] [PubMed] [Google Scholar]
- 52.Lennernas H, Nilsson D, Aquilonius SM, Ahrenstedt O, Knutson L, Paalzow LK. The effect of L-leucine on the absorption of levodopa, studied by regional jejunal perfusion in man. Br J Clin Pharmacol. 1993;35:243–50. doi: 10.1111/j.1365-2125.1993.tb05691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dahlquist G, Bjork E, Eizirik D, Hagglof B, Kockum I, Persson LA. [Causes of diabetes in children and adolescents. A combination of heredity and environment] Lakartidningen. 1996;93:2673. [PubMed] [Google Scholar]
- 54.Ronningen KS, Stene LC, Rasmussen T, Wetlesen T, Magnus P. [Environmental causes of type 1 diabetes] Tidsskr Nor Laegeforen. 2007;127:2405–8. [PubMed] [Google Scholar]


