Abstract
The structural and functional integrity of the retinal pigment epithelium (RPE) is fundamental for maintaining the function of the neuroretina. These specialized cells form a polarized monolayer that acts as the retinal–blood barrier, separating two distinct environments with highly specialized functions: photoreceptors of the neuroretina at the apical side and Bruch's membrane/highly vascularized choriocapillaris at the basal side. The polarized nature of the RPE is essential for the health of these two regions, not only in nutrient and waste transport but also in the synthesis and directional secretion of proteins required in maintaining retinal homoeostasis and function. Although multiple malfunctions within the RPE cells have been associated with development of age-related macular degeneration (AMD), the leading cause of legal blindness, clear causative processes have not yet been conclusively characterized at the molecular and cellular level. This article focuses on the involvement of directionally secreted RPE proteins in normal functioning of the retina and on the potential association of incorrect RPE protein secretion with development of AMD. Understanding the importance of RPE polarity and the correct secretion of essential structural and regulatory components emerge as critical factors for the development of novel therapeutic strategies targeting AMD.
Keywords: retina, retinal pigment epithelium, protein secretion, polarity, age-related macular degeneration
Introduction
RPE polarity
-
Apical secretion from the RPE
– Matrix Metalloproteinase 2 (MMP-2) and Tissue Inhibitor of Matrix Metalloproteinase 1 (TIMP-1)
– Hyaluronan
– αB Crystallin
– Pigment Epithelium-Derived Factor (PEDF)
-
Basolateral secretion from the RPE
– Fibroblast Growth Factor 5 (FGF-5)
– Endothelin I
– Vascular Endothelial Growth Factor (VEGF)
– Cystatin C
-
Mechanisms of protein secretion/polarized secretion
– Basolateral sorting signals
– Apical sorting signals
– Consequences of impaired RPE protein secretion
Concluding remarks
Introduction
The RPE consists of a monolayer of cells that form the retinal–blood barrier (RBB). On either side of this cellular monolayer lie two contrasting environments that are critical for the correct functioning of the neuroretina. Immediately on the apical side of the RPE is a thin matrix known as the interphotoreceptor matrix (IPM) 1, 2. Embedded in this matrix are the highly specialized photoreceptor cells. On the basal side of the RPE lies another unique cellular support structure, the Bruch's membrane (an elastogenesis product of the RPE/choroid) as well as the fenestrated epithelium of the choriocapillaris. The role the RPE plays in separating neural and vascular tissues is similar to that of the blood–brain barrier (BBB); the RBB, however, is unique in that the RPE acts as the impermeable barrier to nutrient/waste movement, as opposed to the endothelial cells in the vessel walls, as is the case for the BBB 3.
One of the main functions of the RPE is in the delivery of nutrients from the choroid to the photoreceptor cells, whilst transporting metabolic end products, ions and excess water in the opposite direction 4–6. This function alone renders RPE a critical role in maintaining the retinal homoeostasis. However, the RPE also carries out other essential functions in visual cycle, phagocytosis of spent photoreceptor outer segments 7, 8, light absorption 7, and the expression and secretion of retinal proteins 9. Failure of the RPE to conduct any of these processes efficiently can lead to retinal degeneration, and bring about diseases such as AMD – the leading cause of legal blindness in the Western world 10. Although multiple malfunctions within the specialized cells in the retina, most importantly in the supportive RPE, have been associated with development of this disease of multifactorial origin, clear causative processes have not yet been conclusively established.
Maintenance of the structure and function of the microenvironments on either side of the RPE via protein secretion is the focus of this article, with specific emphasis on the importance of directional, targeted secretion of trophic/growth factors and structural/structure-related proteins.
RPE polarity
The RPE displays many similarities to other epithelial layers, including a hexagonal ‘cobblestone’ appearance, organization as a single monolayer, tight junctions between cells and a highly polarized nature. Morphologically, RPE cells display polarity with apical microvilli, pigment granules and well-developed tight junctions located on the apical side of the cell, as well as basally located nuclei and membrane infolding 11, 12 (Fig. 1). A feature that distinguishes RPE from other epithelia is the fact that its apical surface does not face an acellular lumen. Instead, it is immediately adjacent to a layer of highly specialized cells, the photoreceptors.
Fig. 1.
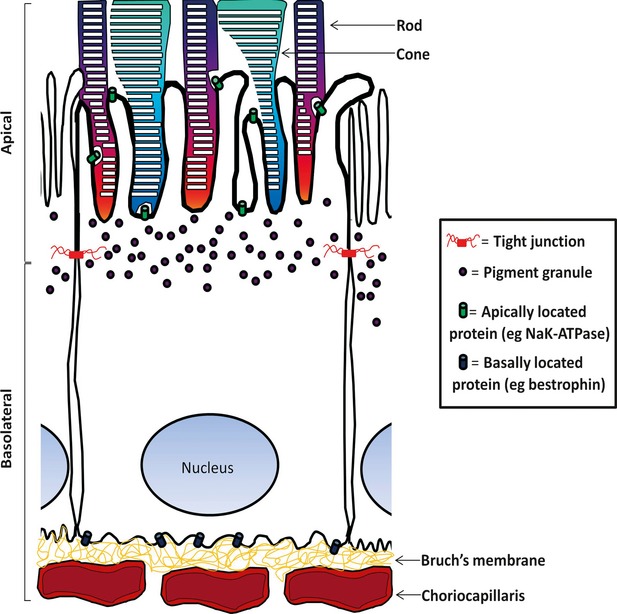
The highly polarized retinal pigment epithelium (RPE) at the interface between the retina and choroid. The organization of cellular structures into apical and basolateral domains is highlighted. At the apical surface, the microvilli of the RPE closely interact with the photoreceptor outer segments. On the basal side, the RPE are supported by the Bruch's membrane, beneath which lies the choroidal blood supply. This highly polarized arrangement ensures that the RPE remains a selectively permeable barrier between these two contrasting tissues.
Proteins expressed by RPE cells can also be localized to either apical or basal plasma membrane (PM) (Fig. 1), such as the apical cell membrane protein NaK-ATPase 11–13, and the basally located anion channel, bestrophin 14. The localization of such proteins can also distinguish RPE cells from other epithelia, as for example, NaK-ATPase is localized to the basal membrane in other epithelial cells 15.
The polarized organization of the RPE is crucial for its interaction with both its apical and basal side, as well as in the directionality of its protein secretion. It has been demonstrated that attainment of polarity in vitro increases the overall levels of growth factor secretion 16. Mechanisms by which cell polarization occur and is maintained, as well as the consequences of altered polarity and trafficking in disease states have been reviewed elsewhere 17. In the case of RPE, it is also therefore likely that factors altering the polarity of the monolayer may play an important role in the development of diseases such as AMD.
Retinal pigment epithelium cells secrete a host of growth factors and structural/structure-related proteins 9 (Table 1), and there is no doubting the importance of such secretion in supporting photoreceptor survival, as well as in maintenance of the retinal blood supply. However, the importance of directional protein secretion can often be overlooked, despite the fact it has been demonstrated that many proteins are secreted preferentially by either the apical, or basolateral PM.
Table 1.
Proteins secreted by the RPE and their putative functions in relation to AMD
| Protein | Main function/s | Links/potential links to AMD | Polarity# | References |
|---|---|---|---|---|
| αB Crystallin | Molecular chaperone, cytoprotection | Possible AMD biomarker, maybe involved in Drusen formation | Apical | 18–20 |
| BDNF | Neurotrophic growth factor | Protective role for the photoreceptors possibly lost during AMD | Unknown | 21, 22 |
| CFH | Inhibitor of the complement pathway | Gene variants implicated as a major AMD risk factor | Unknown | 23 |
| CNTF | Neurotrophic growth factor | May provide protection against neurodegenerative diseases, such as AMD | Unknown | 21, 24 |
| Cystatin C | Cysteine protease inhibitor | Variant B isoform associated with increased risk of “wet” AMD | Basal | 25, 26 |
| Endothelin I | Vasoconstriction/vasodilation | Mis-signalling associated with retinopathies associated with breakdown of blood–retinal barrier | Basal | 27 |
| *Fibulin 3/5 | ECM protein involved in elastogenesis (fibulin 5) | Variant isoform of fibulin 5 associated with increased risk of AMD | Unknown | 28, 29 |
| FGF 2 | Growth factor involved in mitogenesis, angiogenesis and cell survival | Role in choroidal neovascularization | Unknown | 21, 30, 31 |
| FGF 5 | Growth factor involved in mitogenesis, angiogenesis and cell survival | Potential functional role in AMD pathophysiology | Basal | 32, 33 |
| HB-EGF | Mitogenic growth factor | Indirect role in choroidal neovascularization via influence on VEGF expression | Unknown | 21, 34 |
| HGF | Growth factor involved in growth, motility and morphogenesis | Provides protection to RPE cells under oxidative stress, a process frequently linked with AMD progression | Unknown | 21, 35 |
| Hyaluronan | Major component of ECM | Possible role in choroidal neovascularization via interaction with CD44 receptor | Apical | 36, 37 |
| IGF-I | Growth factor involved in growth and development | Role in choroidal neovascularization | Unknown | 38, 39 |
| LIF | Cytokine involved in differentiation | May aid photoreceptor survival during periods of stress, such as AMD | Unknown | 21, 40 |
| MMP-2 | Zinc-dependent endopeptidase involved in ECM degradation | Activity within the Bruch's membrane altered in AMD, possible contribution to progression of “wet” AMD | Apical | 41–43 |
| MMP-9 | Zinc-dependent endopeptidase involved in ECM degradation | Activity within the Bruch's membrane altered in AMD, possible contribution to progression of “wet” AMD | Unknown | 41, 42 |
| NGF | Neurotrophic growth factor | Provides protection to RPE cells under oxidative stress, a process frequently linked with AMD progression | Unknown | 21, 44 |
| PEDF | Growth factor with neurotrophic and anti-angiogenic properties | Incorrect expression/localization promotes vascularization, loss of photoreceptor support role in late-stage AMD | Apical | 11, 12, 45, 46 |
| TGF-β | Growth factor involved in proliferation and differentiation | Can cause senescence-associated changes in RPE, a process associated with early AMD | Apical | 24, 47, 48 |
| TIMP-I | Inhibitor of matrix metalloproteinases | Activity of target molecules (MMP's) altered in AMD, possible contribution to progression of “wet” AMD | Apical | 41–43 |
| Tropoelastin | Involved in formation of elastin fibres (such as in Bruch's membrane) | Potential functional role in AMD-related changes in the Bruch's membrane | Unknown | 49 |
| VEGF | Angiogenic growth factor | Incorrect expression/localization promotes vascularization role in late-stage AMD | Basal | 12, 50 |
Putative polarity of secretion is indicated based on experimental data published to date.
Fibulin 3 (EFEMP 1) is known to be secreted from RPE cells 28, but its function and possible role in AMD development have not been fully characterized. The closely related Fibulin 5 is associated with AMD development and although secretion from RPE cells has not been demonstrated experimentally, it is actively secreted by other cell lines 29.
Some examples of directional protein secretion from the RPE are discussed below. The function/dysfunction of the majority of proteins presented herein have direct links to AMD pathogenesis. For those that have not, to date, been directly linked with AMD, we hypothesize how their incorrect localization/function may lead to retinal degeneration based on their involvement in fundamental biological processes and on similarities of their mechanism of action with that of known molecular determinants of the disease.
Apical secretion from the RPE
Matrix metalloproteinase 2 (MMP-2) and tissue inhibitor of matrix metalloproteinase 1 (TIMP-1)
Matrix metalloproteinase and TIMPs are apically secreted by the RPE 43. MMPs play a crucial role in the extracellular matrix (ECM) turnover throughout the body. Their activity is normally tightly regulated at several levels, including functional inhibition by TIMPs. It has been suggested that apically secreted MMPs/TIMPs could play a crucial role in the turnover and structural maintenance of the IPM, or possibly be involved in degrading the tips of photoreceptor outer segments, signalling their readiness for phagocytosis by the RPE 43.
Matrix metalloproteinase and TIMPs (including MMP-2 and TIMP-1) are also present basally to the RPE, in the Bruch's membrane 51–53. This indicates potential basolateral secretion of MMPs 54, 55. It is possible that the RPE is able to secrete certain MMPs/TIMPs in opposite directions depending on external cues such as cytokine stimulation 55, or signals from the ECM 43. Alternatively, MMPs/TIMPs present in Bruch's membrane could be secreted by choroidal cells. MMP activity is disturbed in the Bruch's membrane in AMD 42, and the MMPs role in angiogenesis 56 could also contribute to symptoms associated with the ‘wet’ (exudative) form of the disease. Malfunctions in controlled, directional secretion of MMPs/TIMPs could disrupt the balance of proteolytic activity on either side of the RPE, contributing to age-related changes in the IPM and Bruch's membrane.
Hyaluronan
Hyaluronan is a widely distributed glycosaminoglycan, and is a major component of the ECM. It is secreted from the apical side of the RPE 37, where it is believed to function as the primary scaffold protein in the IPM 1. Maintaining the structural integrity of the IPM is a crucial function of the RPE, and mis-localization of hyaluronan could lead to its degeneration, subsequently affecting the survival of the light sensitive cells of the outer retina. Indeed, it has been demonstrated that the IPM is abnormally distributed in an animal model for retinal degeneration 57. The interaction of hyaluronan with the receptor CD44 has been associated with choroidal neovascularization, a common symptom of wet AMD 36. Such an interaction could be the result of mis-targeting of the protein to the wrong tissue compartment.
αB crystallin
αB crystallin is a molecular chaperone induced by stress stimuli. By suppressing protein aggregation and inhibiting the proteolytic activity of caspase 3, it is able to play a role in cytoprotection 58. αB Crystallin is secreted from the apical surface of the RPE, where it is taken up by the photoreceptor cells. Here, it inhibits the activity of caspase 3, activates the DNA repair and apoptosis-related Poly (ADP-ribose) Polymerase (PARP) and provides the photoreceptors with protection from oxidative stress 20. More recent data suggest that the protein is released from the cell inside exosomes, and that its secretion is independent of the endoplasmic reticulum (ER)/Golgi/secretory pathway 19. αB crystallin is a major component of the IPM 59, 60 and has also been linked to AMD pathogenesis, with increased expression being proposed as a possible biomarker for the disease 18. Under severe oxidative stress, the RPE barrier can become compromised, resulting in αB crystallin aggregating on the basal side of the RPE 20. In this case, not only is the protein less available to provide a survival advantage to the photoreceptors but it can also contribute to the formation of drusen deposits 18.
Pigment epithelium-derived factor (PEDF)
The PEDF is highly expressed in the retina, where it serves as a neurotrophic factor 61 and angiogenesis inhibitor 45. Multiple studies have shown that PEDF is secreted preferentially from the apical surface of the RPE 11, 12, 46, where it provides such neurotrophic support to the photoreceptors, and maintains a non-angiogenic retinal environment 16. Low levels of PEDF below the basal surface of the RPE may aid in preventing vascularization in this compartment as well. Dysregulated expression of both PEDF and vascular endothelial growth factor (VEGF) (discussed below) plays a role in the pathogenesis of late-stage AMD 45. A delicate balance exists on either side of the RPE regarding the concentrations of these two growth factors. Disrupting this balance can promote vascularization of the retina, whilst simultaneously decreasing photoreceptor support 16.
Basolateral secretion from the RPE
Fibroblast growth factor 5 (FGF-5)
Fibroblast growth factor 5 is known to play a role in a range of processes including angiogenesis 62, 63, cell survival 64 and mitogenesis 65, 66. It is secreted basally from the RPE and has a possible paracrine role in choroid function, or alternatively, acts as an autocrine survival factor for RPE cells 32. Whatever its precise function in the retina, it is conceivable that its mis-regulated secretion could have detrimental effects on RPE cell survival, or vascularization, and therefore it has been suggested that it plays a role in the pathophysiology of AMD 33.
Endothelin I
Endothelin I is a protein expressed in the retina and choroid 67. It acts on two receptors located in smooth muscle cells, resulting in both vasoconstriction and vasodilation 68. It is secreted from the basal surface of the RPE, and by interacting with its receptors in the choriocapillaris, can regulate blood flow 27. It has been proposed that stresses involved in the breakdown of the blood–retinal barrier, as in AMD, can cause an increase in the expression and secretion of endothelin I, suggesting a role in wound repair via effects on proliferation and cell migration 27.
Vascular endothelial growth factor
Vascular endothelial growth factor is a pro-angiogenic growth factor that is secreted preferentially from the basal surface of the RPE 12, 50. VEGF modulates and maintains the extracellular space in and around the Bruch's membrane, and modulates the growth/density of endothelial cells in the choriocapillaris 69–71. Its secretion is normally tightly regulated, thus preventing its concentration levels from surpassing a critical threshold able to induce vascularization 16. However, considering its potent angiogenic properties, it is plausible that the incorrect localization of this growth factor can be a critical factor in late-stage exudative AMD. This theory is supported by findings that elevated local concentration of VEGF can result in the formation of abnormal blood vessels, as seen in AMD 72.
Cystatin C
Cystatin C is a reversible inhibitor of cysteine proteinases including papain and cathepsins B, H, L and S 73, 74. A polymorphism present in the genomic sequence of the cystatin C gene produces a mutant variant of the protein referred to as variant B. Homozygosity for this variant has been shown to correlate with an increased risk of developing exudative AMD, with a relatively early onset 26. It is likely that cystatin C is secreted from the basolateral side of the RPE 25 and the AMD-related variant has been shown to present a significantly reduced secretion. When considering this protein's function, its link to AMD, and the fact that the activity of its main group of substrates, the cathepsins, is altered in AMD 75–77, it is conceivable that its directional secretion could be highly important in the maintenance and turnover of the Bruch's membrane and choriocapillaris.
Mechanisms of protein secretion/polarized secretion
A large number of soluble proteins are secreted from the PM via the relatively well-characterized ‘classical’ secretion pathway. These proteins contain N-terminally located signal peptides, directing them co-translationally to the translocation apparatus of the ER 78. Following vesicular transport from the ER to the Golgi apparatus, secreted proteins are then packaged into Golgi-derived vesicles that ultimately fuse with the PM, releasing their contents into the extracellular space 79–82. Alternatively, many proteins are also secreted from the cell via other, ‘non-classical’ mechanisms. These proteins typically lack a signal peptide, and are excluded from organelles that are essential during ‘classical’ secretion, such as the ER and Golgi. These mechanisms of secretion are reviewed in detail elsewhere 83, and include import into endosomal sub-compartments, direct translocation across the PM, and membrane blebbing, releasing the secreted proteins via exosomes.
In polarized cells, secretion requires an extra level of complexity and control to ensure certain proteins are targeted to, and released from the appropriate cell surface. Mechanisms required for such control have been studied extensively within the ‘classical’ secretion pathway, and have been reviewed in detail elsewhere 84–86. Although the majority of this work has focused on the targeting of membrane-bound proteins, many secreted proteins are likely targeted via similar mechanisms. Thus, the basolateral versus apical sorting mechanisms in the secretory pathway is believed to rely on complex membrane trafficking pathways which also underpin the distinct specialization of the apical and basolateral PM domains. Targeting of proteins to a particular cell surface takes place at the level of the trans-Golgi network (TGN), following incorporation of apical and basolateral proteins into distinct vesicles 87–90, a process that usually requires the presence of directional sorting signals. More recently, it has been suggested that some targeting may actually occur earlier than the TGN 91. In addition, the endocytosis pathway itself is regulated to preserve the polarity of lipid and protein components following internalization and recycling. Some of the potential mechanisms required for directional protein secretion are discussed below and summarized in Figure 2.
Fig. 2.
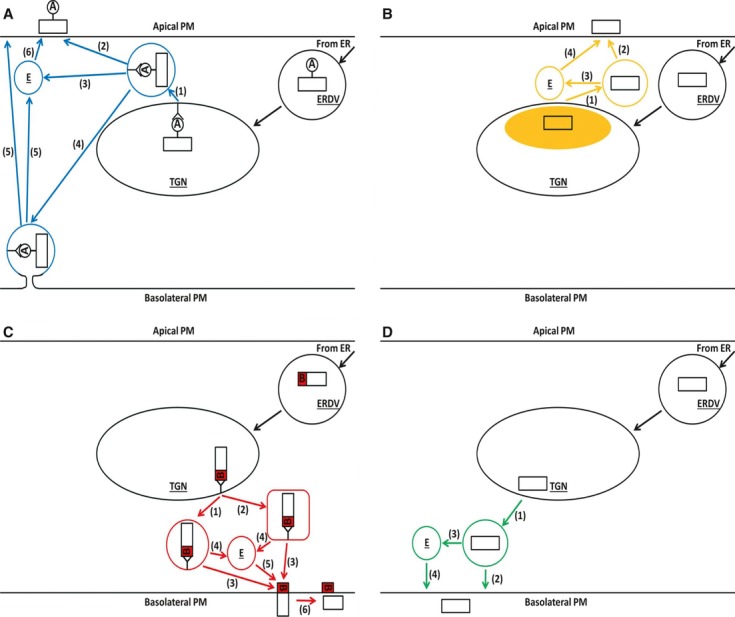
Models for targeted protein secretion from the endoplasmic reticulum (ER)-Golgi secretory pathway. Proteins containing apical sorting signals (A in white circle), basolateral sorting signals (B in red square) or no sorting signals (plain white rectangle) are delivered from the ER in ER derived vesicles (ERDV). Upon entering the Golgi apparatus, proteins progress to the trans-Golgi network (TGN) and sorting occurs. In pathway (A), apical signals (glycans, GPI linkages) interact with specific cellular machinery (e.g. apical targeting receptors, lipid rafts), resulting in packaging into apical vesicles (1). These vesicles can then traffic directly to the apical plasma membrane (PM) for extracellular release (2). Alternatively, some proteins are first trafficked to endosomes (E) (3), or to the basolateral PM (4). Basally trafficked proteins are subsequently redirected to the apical PM via transcytosis, either directly, or via endosomes (5). Proteins in the endosomal pathway are subsequently trafficked to the apical PM (6). In pathway (B), proteins that lack a typical targeting signal can undergo ‘specialized packaging’ in particular environments (yellow area). For example, proteins with an affinity for lipids can localize in areas of high lipid content. These proteins are subsequently packaged into vesicles and targeted to the apical PM directly (1, 2), or indirectly via endosomes (1, 3, 4). In pathway (C), basolateral signals interact with a specific cellular machinery (e.g. clathrin adapters), resulting in packaging into basolateral vesicles (1). It is possible that some secreted proteins are packaged into secretory vesicles (2) distinct from those used for transmembrane proteins (circular vesicle versus rectangular). Basolateral vesicles can then traffic to the basolateral PM directly (3), or via endosomes (4, 5). For some secreted proteins (e.g. TGFα), the cytoplasmic domain is cleaved, resulting in secretion of the mature form (6). Pathway (D), can act as a default for any proteins not otherwise targeted (proteins that lack a typical targeting signal), or alternatively, proteins that do not enter pathway (B) (e.g. due to lack of affinity for lipids). These proteins are subsequently packaged into vesicles and targeted to the basolateral PM directly (1, 2), or indirectly via endosomes (1, 3, 4).
Basolateral sorting signals
Basolateral sorting signals typically consist of tyrosine-based (YxxØ, FxNPxY) or di-leucine-based peptide sequences, found in the disposed, cytoplasmic portion of transmembrane proteins 84, 89, 91. Interestingly, these basolateral signals are usually dominant over their apical counterparts 92. In many cases, these signal peptides are recognized, and directly bind to heterotetrameric clathrin adapter protein complexes. This event triggers the incorporation of cargo into nascent vesicles, which are subsequently trafficked to the basolateral PM 84, 93. As these adaptor proteins are thought to mediate transport between the TGN and endosomal compartments, it is possible that some basolateral proteins traverse recycling endosomes before reaching the PM 91. Indeed, the importance of indirect sorting of proteins via such endosomal pathways has been highlighted 94–96. Alternatively, some proteins are targeted directly to the PM 97. Although there are only few examples of basolateral signals demonstrated in secreted proteins, it is likely that these are sorted in a similar fashion as transmembrane proteins.
Transforming growth factor-α (TGFα) provides one of the relatively few examples of a typical basolateral signal in a secreted protein. Its precursor form (pro-TGFα) contains a dominant basolateral sorting signal within the cytoplasmic region that is subsequently cleaved to produce the soluble, mature form 98. It has also been suggested that basolateral secretion can occur independently of any sorting signal, relying instead on a default, cell-dependent pathway that is governed by the association of particular proteins to intracellular lipids 99. The overall lipid content of the cell, as well as the lipid composition of apical and basal vesicles are therefore important factors in this process. There is also evidence of separate branches within the basolateral sorting pathway for secreted and transmembrane proteins, resulting in loading into distinct carrier vesicles 100.
Apical sorting signals
Apical sorting signals are much more diverse than basolateral signals and consist of post-translational modifications, rather than distinct peptide sequences. Perhaps the most extensively studied of these are N- and O-linked glycans, which are present on particular proteins. Two models have been proposed to explain how these glycans are involved in apical sorting 101. The first suggests that these carbohydrates are critical for the assumption of a correct conformation that is necessary to progress along the biosynthetic pathway. The second model suggests the existence of a sorting receptor that recognizes carbohydrates, or alternatively, protein conformations that are dependent upon such carbohydrates. Glycans can also cause aggregation of proteins into pre-export complexes 91. Non-glycan apical sorting signals have also been suggested, imagined as three-dimensional proteinaceous patches 101. Glycosylphosphatidylinositol (GPI) linkages enable sorting through association with apical PM-bound lipid rafts 92. Apical sorting can also occur independently of signals (glycosylation). In this case, it is hypothesized that certain cell types can provide an environment that encourages ‘specialized packaging’ of proteins destined for apical secretion 102.
The route that apical proteins take to the PM seems as equally diverse as the signals that direct them. Many transmembrane proteins destined for the apical PM are transported from the TGN in different vesicles, and are then processed via distinct subsets of endosomal compartments before reaching their final destination 103, 104. It is, however, hypothesized that soluble, secreted proteins may in fact be targeted directly to the PM, to avoid degradation within the endocytic pathway 86. RPE cells, in particular, also differ in their processing of many apical proteins, directing them first to the basolateral PM, before a process of relocation to the apical PM via transcytosis 105.
Mechanisms for targeting secreted proteins for apical PM release are similar to those for transmembrane proteins. Glycans can promote such targeting, as is the case during apical sorting of rat growth hormone 106. Glycoprotein 2 is targeted to the apical PM via a GPI anchor in its transmembrane domain. Subsequent cleavage then results in secretion of the soluble form 107 in a similar manner to the basolateral secretion of TGFα. Human growth hormone, thyroglobulin and parathyroid hormone have been shown to be secreted apically, independently of known apical signals, relying instead on particular cellular conditions 102.
Consequences of impaired RPE protein secretion
It is becoming increasingly apparent that directional protein secretion is a highly regulated and complex process, and that malfunctions occurring at any step in these pathways could lead either to increased/decreased levels of essential growth factors and structural proteins in the extracellular space or to their incorrect intracellular/extracellular localization. It is therefore possible that such malfunctions could serve as relatively overlooked mechanisms in the pathophysiology of the highly secretory RPE, with specific consequences for the development of retinal disorders such as AMD.
Indeed, such alterations in protein secretion have been implicated in the development of other diseases. Such an example is the increase in growth factor secretion from cancer cells, leading to autocrine growth stimulation and metastasis 108. Secretion of cystatin C variant B from human primary fibroblasts has also been shown to be reduced compared with the wild-type 109. It is possible that such reduced secretion of cystatin C in the brain can result in a lack of protection following toxic insult, or in stem-cell mediated regeneration, factors that may contribute to the development of Alzheimer's disease 109. A recent study has demonstrated that cells derived from patients with AMD display a striking difference in the secretion of several proteins compared with matched healthy donors. Many of these proteins are involved in angiogenesis and protein aggregation, processes that have been heavily implicated in AMD pathogenesis 110.
Extracellular mislocalization of secreted proteins can occur as a result of changed cell polarity and alterations in directional secretion. Such alterations can also have implications in disease development. The presence of the Alzheimer's-linked, ‘Swedish’ double mutation in the amyloid precursor protein results in a proportion being mis-secreted from the apical, rather than basolateral PM 111. Directional secretion can also be altered as a result of external stimulation of cells with cytokines 112, 113, the activity of which can be altered in disease states. Total secretion of important factors such as PEDF (apically or basally) from RPE cells can be dependent on the cell attaining a highly polarized configuration 16, 114, again highlighting the importance of polarity for correct protein secretion.
Some of the RPE-secreted proteins highlighted above have been shown to have altered secretion patterns during AMD development. MMP-2 is secreted at levels threefold higher in AMD RPE cells compared with healthy RPE 110. It is possible that this protease is indirectly involved in angiogenesis via its proteolytic and ECM remodelling properties 115. PEDF secretion is also, surprisingly, increased in AMD RPE cells 110. It has been suggested that this may be a compensatory response by the RPE to balance the angiogenic properties of VEGF 110. This same study 110 was also able to show an increase in clusterin secretion and a decrease in SPARC secretion in AMD cells. The precise function of clusterin has not been defined, but its presence in Drusen suggests that it may be involved in their formation 116, 117, therefore contributing to one of the greatest hallmarks of AMD. SPARC is known to have anti-angiogenic properties 118, 119, meaning reduced levels in the choroid could aid in neovascularization.
One of the most abundantly expressed and secreted proteins of the RPE is the cysteine proteinase inhibitor cystatin C 120. The role that this protein plays in the RPE has not yet been characterized, yet its function as a cysteine proteinase inhibitor together with its extracellular targeting 25 suggest an important involvement in matrix remodelling and turnover, processes that are essential for retinal homoeostasis. A signal peptide present in the 26 amino acid leader sequence 121 of cystatin C targets it to the ‘classical’ ER/Golgi secretory pathway 25. The polymorphism that results in the variant B cystatin C translates into an amino acid substitution (alanine to threonine) at the penultimate position of this leader sequence. This alteration results in a failure of cystatin C to efficiently enter the secretory pathway, leading to a diffuse intracellular distribution of the protein, an association with mitochondria, and a reduction in its secretion of ca. 50% 122, 123. Reduced hydrophobicity of the signal sequence caused by the amino acid substitution is thought to be the cause of this secretory malfunction 124.
Concluding remarks
The tissues on either side of the RPE present two different microenvironments, each placing varying demands on the RPE in terms of protein requirements. High levels of particular growth factors and structural proteins that can be beneficial in one compartment can be detrimental in the other. Maintaining the correct concentrations of particular factors, in the correct location, at the correct time is therefore of critical importance for retinal health.
This article highlights several examples of directional protein secretion by the RPE, all of which could play important roles in maintaining its surrounding extracellular environments. Malfunctions in trafficking/secretory pathways can lead to mis-localization of these proteins, which can ultimately manifest as a number of AMD symptoms.
Therapies such as RPE transplants and gene therapy could offer improved treatments for AMD in the future. The importance of polarity and directional protein secretion in maintaining RPE/retinal functioning will be key considerations in the further development of such therapies.
Acknowledgments
The authors gratefully acknowledge the support from R&D Fund of The Royal Wolverhampton NHS Trust.
Conflicts of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Hollyfield JG. Hyaluronan and the functional organization of the interphotoreceptor matrix. Invest Ophthalmol Vis Sci. 1999;40:2767–9. [PubMed] [Google Scholar]
- 2.Rohlich P. The interphotoreceptor matrix: electron microscopic and histochemical observations on the vertebrate retina. Exp Eye Res. 1970;10:80–6. doi: 10.1016/s0014-4835(70)80013-6. [DOI] [PubMed] [Google Scholar]
- 3.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Dornonville DL. Ion transport in the retinal pigment epithelium. A study with double barrelled ion-selective microelectrodes. Acta Ophthalmol Suppl. 1993;209:1–32. [PubMed] [Google Scholar]
- 5.Hamann S. Molecular mechanisms of water transport in the eye. Int Rev Cytol. 2002;215:395–431. doi: 10.1016/s0074-7696(02)15016-9. [DOI] [PubMed] [Google Scholar]
- 6.Miller SS, Edelman JL. Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol. 1990;424:283–300. doi: 10.1113/jphysiol.1990.sp018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–95. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 8.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–54. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 10.Ferris FL., III Senile macular degeneration: review of epidemiologic features. Am J Epidemiol. 1983;118:132–51. doi: 10.1093/oxfordjournals.aje.a113624. [DOI] [PubMed] [Google Scholar]
- 11.Sonoda S, Spee C, Barron E, et al. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–73. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–24. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okami T, Yamamoto A, Omori K, et al. Immunocytochemical localization of Na+, K(+)-ATPase in rat retinal pigment epithelial cells. J Histochem Cytochem. 1990;38:1267–75. doi: 10.1177/38.9.2167328. [DOI] [PubMed] [Google Scholar]
- 14.Marmorstein AD, Marmorstein LY, Rayborn M, et al. Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2000;97:12758–63. doi: 10.1073/pnas.220402097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyte J. Immunoferritin determination of the distribution of (Na+ + K+) ATPase over the plasma membranes of renal convoluted tubules. I. Distal segment. J Cell Biol. 1976;68:287–303. doi: 10.1083/jcb.68.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonoda S, Sreekumar PG, Kase S, et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging. 2010;2:28–42. doi: 10.18632/aging.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein MP, Wandinger-Ness A, Roitbak T. Altered trafficking and epithelial cell polarity in disease. Trends Cell Biol. 2002;12:374–81. doi: 10.1016/s0962-8924(02)02331-0. [DOI] [PubMed] [Google Scholar]
- 18.De S, Rabin DM, Salero E, et al. Human retinal pigment epithelium cell changes and expression of αB-crystallin: a biomarker for retinal pigment epithelium cell change in age-related macular degeneration. Arch Ophthalmol. 2007;125:641–5. doi: 10.1001/archopht.125.5.641. [DOI] [PubMed] [Google Scholar]
- 19.Gangalum RK, Atanasov IC, Zhou ZH, et al. αB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011;286:3261–9. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sreekumar PG, Kannan R, Kitamura M, et al. αB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PLoS ONE. 2010;5:e12578. doi: 10.1371/journal.pone.0012578. Doi: 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolomeyer AM, Sugino IK, Zarbin MA. Characterization of conditioned media collected from cultured adult versus fetal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:5973–86. doi: 10.1167/iovs.10-6965. [DOI] [PubMed] [Google Scholar]
- 22.Paskowitz DM, Nune G, Yasumura D, et al. BDNF reduces the retinal toxicity of verteporfin photodynamic therapy. Invest Ophthalmol Vis Sci. 2004;45:4190–6. doi: 10.1167/iovs.04-0676. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, He S, Kase S, et al. Regulated secretion of complement factor H by RPE and its role in RPE migration. Graefes Arch Clin Exp Ophthalmol. 2009;247:651–9. doi: 10.1007/s00417-009-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li R, Wen R, Banzon T, et al. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS ONE. 2011;6:e23148. doi: 10.1371/journal.pone.0023148. Doi: 10.1371/journal.pone.0023148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paraoan L, White MR, Spiller DG, et al. Precursor cystatin C in cultured retinal pigment epithelium cells: evidence for processing through the secretory pathway. Mol Membr Biol. 2001;18:229–36. doi: 10.1080/09687680110075101. [DOI] [PubMed] [Google Scholar]
- 26.Zurdel J, Finckh U, Menzer G, et al. CST3 genotype associated with exudative age related macular degeneration. Br J Ophthalmol. 2002;86:214–9. doi: 10.1136/bjo.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayan S, Brun AM, Yorio T. Endothelin-1 distribution and basolateral secretion in the retinal pigment epithelium. Exp Eye Res. 2004;79:11–9. doi: 10.1016/j.exer.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Marmorstein LY, Munier FL, Arsenijevic Y, et al. Aberrant accumulation of EFEMP1 underlies drusen formation in Malattia Leventinese and age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:13067–72. doi: 10.1073/pnas.202491599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones RPO, Ridley C, Jowitt TA, et al. Structural effects of fibulin 5 missense mutations associated with age-related macular degeneration and cutis laxa. Invest Ophthalmol Vis Sci. 2010;51:2356–62. doi: 10.1167/iovs.09-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mousa SA, Lorelli W, Campochiaro PA. Role of hypoxia and extracellular matrix-integrin binding in the modulation of angiogenic growth factors secretion by retinal pigmented epithelial cells. J Cell Biochem. 1999;74:135–43. [PubMed] [Google Scholar]
- 31.Rosenthal R, Malek G, Salomon N, et al. The fibroblast growth factor receptors, FGFR-1 and FGFR-2, mediate two independent signalling pathways in human retinal pigment epithelial cells. Biochem Biophys Res Commun. 2005;337:241–7. doi: 10.1016/j.bbrc.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 32.Dunn KC, Marmorstein AD, Bonilha VL, et al. Use of the ARPE-19 cell line as a model of RPE polarity: basolateral secretion of FGF5. Invest Ophthalmol Vis Sci. 1998;39:2744–9. [PubMed] [Google Scholar]
- 33.Kitaoka T, Morse LS, Schneeberger S, et al. Expression of FGF5 in choroidal neovascular membranes associated with ARMD. Curr Eye Res. 1997;16:396–9. doi: 10.1076/ceyr.16.4.396.10685. [DOI] [PubMed] [Google Scholar]
- 34.Hollborn M, Iandiev I, Seifert M, et al. Expression of HB-EGF by retinal pigment epithelial cells in vitreoretinal proliferative disease. Curr Eye Res. 2006;31:863–74. doi: 10.1080/02713680600888807. [DOI] [PubMed] [Google Scholar]
- 35.Jin M, Yaung J, Kannan R, et al. Hepatocyte growth factor protects RPE cells from apoptosis induced by glutathione depletion. Invest Ophthalmol Vis Sci. 2005;46:4311–9. doi: 10.1167/iovs.05-0353. [DOI] [PubMed] [Google Scholar]
- 36.Mochimaru H, Takahashi E, Tsukamoto N, et al. Involvement of hyaluronan and its receptor CD44 with choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50:4410–5. doi: 10.1167/iovs.08-3044. [DOI] [PubMed] [Google Scholar]
- 37.Senanayake PD, Calabro A, Nishiyama K, et al. Glycosaminoglycan synthesis and secretion by the retinal pigment epithelium: polarized delivery of hyaluronan from the apical surface. J Cell Sci. 2001;114:199–205. doi: 10.1242/jcs.114.1.199. [DOI] [PubMed] [Google Scholar]
- 38.Moriarty P, Boulton M, Dickson A, et al. Production of IGF-I and IGF binding proteins by retinal cells in vitro. Br J Ophthalmol. 1994;78:638–42. doi: 10.1136/bjo.78.8.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenthal R, Wohlleben H, Malek G, et al. Insulin-like growth factor-1 contributes to neovascularization in age-related macular degeneration. Biochem Biophys Res Commun. 2004;323:1203–8. doi: 10.1016/j.bbrc.2004.08.219. [DOI] [PubMed] [Google Scholar]
- 40.Burgi S, Samardzija M, Grimm C. Endogenous leukemia inhibitory factor protects photoreceptor cells against light-induced degeneration. Mol Vis. 2009;15:1631–7. [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann S, He S, Ehren M, et al. MMP-2 and MMP-9 secretion by rpe is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina. 2006;26:454–61. doi: 10.1097/01.iae.0000238549.74626.33. [DOI] [PubMed] [Google Scholar]
- 42.Hussain AA, Lee Y, Zhang JJ, et al. Disturbed matrix metalloproteinase activity of Bruch's membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:4459–66. doi: 10.1167/iovs.10-6678. [DOI] [PubMed] [Google Scholar]
- 43.Padgett LC, Lui GM, Werb ZENA, et al. Matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-1 in the retinal pigment epithelium and inter-photoreceptor matrix: vectorial secretion and regulation. Exp Eye Res. 1997;64:927–38. doi: 10.1006/exer.1997.0287. [DOI] [PubMed] [Google Scholar]
- 44.Cao GF, Liu Y, Yang W, et al. Rapamycin sensitive mTOR activation mediates nerve growth factor (NGF) induced cell migration and pro-survival effects against hydrogen peroxide in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2011;414:499–505. doi: 10.1016/j.bbrc.2011.09.094. [DOI] [PubMed] [Google Scholar]
- 45.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 46.Becerra SP, Fariss RN, Wu YQ, et al. Pigment epithelium-derived factor in the monkey retinal pigment epithelium and interphotoreceptor matrix: apical secretion and distribution. Exp Eye Res. 2004;78:223–34. doi: 10.1016/j.exer.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Tan J, Deng ZH, Liu SZ, et al. TGF-ß2 in human retinal pigment epithelial cells: expression and secretion regulated by cholinergic signals in vitro. Curr Eye Res. 2009;35:37–44. doi: 10.3109/02713680903374190. [DOI] [PubMed] [Google Scholar]
- 48.Yu AL, Fuchshofer R, Kook D, et al. Subtoxic oxidative stress induces senescence in retinal pigment epithelial cells via TGFß release. Invest Ophthalmol Vis Sci. 2009;50:926–35. doi: 10.1167/iovs.07-1003. [DOI] [PubMed] [Google Scholar]
- 49.Wachi H, Sato F, Nakazawa J, et al. Domains 16 and 17 of tropoelastin in elastic fibre formation. Biochem J. 2007;402:63–70. doi: 10.1042/BJ20061145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ablonczy Z, Dahrouj M, Tang PH, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci. 2011;52:8614–20. doi: 10.1167/iovs.11-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vranka JA, Johnson E, Zhu X, et al. Discrete expression and distribution pattern of TIMP-3 in the human retina and choroid. Curr Eye Res. 1997;16:102–10. doi: 10.1076/ceyr.16.2.102.5086. [DOI] [PubMed] [Google Scholar]
- 52.Guo L, Hussain AA, Limb GA, et al. Age-dependent variation in metalloproteinase activity of isolated human bruchs membrane and choroid. Invest Ophthalmol Vis Sci. 1999;40:2676–82. [PubMed] [Google Scholar]
- 53.Fariss RN, Apte SS, Olsen BR, et al. Tissue inhibitor of metalloproteinases-3 is a component of Bruch's membrane of the eye. Am J Pathol. 1997;150:323–8. [PMC free article] [PubMed] [Google Scholar]
- 54.Alexander JP, Bradley JM, Gabourel JD, et al. Expression of matrix metalloproteinases and inhibitor by human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1990;31:2520–8. [PubMed] [Google Scholar]
- 55.Hunt RC, Fox A, al Pakalnis V, et al. Cytokines cause cultured retinal pigment epithelial cells to secrete metalloproteinases and to contract collagen gels. Invest Ophthalmol Vis Sci. 1993;34:3179–86. [PubMed] [Google Scholar]
- 56.Jackson C. Matrix metalloproteinases and angiogenesis. Curr Opin Nephrol Hypertens. 2002;11:295–9. doi: 10.1097/00041552-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 57.LaVail MM, Pinto LH, Yasumura D. The interphotoreceptor matrix in rats with inherited retinal dystrophy. Invest Ophthalmol Vis Sci. 1981;21:658–68. [PubMed] [Google Scholar]
- 58.Horwit J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–53. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauck SM, Schoeffmann S, Deeg C, et al. Proteomic analysis of the porcine interphotoreceptor matrix. Proteomics. 2005;5:3623–36. doi: 10.1002/pmic.200401223. [DOI] [PubMed] [Google Scholar]
- 60.Steiner C, Sahel J, Hicks D. Retinoschisin forms a multi-molecular complex with extracellular matrix and cytoplasmic proteins: interactions with beta2 laminin and alphaB-crystallin. Mol Vis. 2006;12:892–901. [PubMed] [Google Scholar]
- 61.Cayouette M, Smith SB, Becerra SP, et al. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol Dis. 1999;6:523–32. doi: 10.1006/nbdi.1999.0263. [DOI] [PubMed] [Google Scholar]
- 62.Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 63.Giordano FJ, Ping P, McKirnan MD, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–9. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 64.Sensenbrenner M. The neurotrophic activity of fibroblast growth factors. Prog Neurobiol. 1993;41:683–704. doi: 10.1016/0301-0082(93)90031-m. [DOI] [PubMed] [Google Scholar]
- 65.Hoppenreijs VP, Pels E, Vrensen GF, et al. Basic fibroblast growth factor stimulates corneal endothelial cell growth and endothelial wound healing of human corneas. Invest Ophthalmol Vis Sci. 1994;35:931–44. [PubMed] [Google Scholar]
- 66.Clements DA, Wang JK, Dionne CA, et al. Activation of fibroblast growth factor (FGF) receptors by recombinant human FGF-5. Oncogene. 1993;8:1311–6. [PubMed] [Google Scholar]
- 67.Stitt AW, Chakravarthy U, Gardiner TA, et al. Endothelin-like immunoreactivity and receptor binding in the choroid and retina. Curr Eye Res. 1996;15:111–7. doi: 10.3109/02713689609017618. [DOI] [PubMed] [Google Scholar]
- 68.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu Rev Pharmacol Toxicol. 2001;41:851–76. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 69.Roberts WG, Palad GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–79. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 70.Saint-Geniez M, Kurihara T, Sekiyama E, et al. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2009;106:18751–6. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yokomori H, Oda M, Yoshimura K, et al. Vascular endothelial growth factor increases fenestral permeability in hepatic sinusoidal endothelial cells. Liver Int. 2003;23:467–75. doi: 10.1111/j.1478-3231.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- 72.Ozawa CR, Banfi A, Glazer NL, et al. Microenvironmental VEGF concentration, not total dose, determines a threshold between normal and aberrant angiogenesis. J Clin Invest. 2004;113:516–27. doi: 10.1172/JCI18420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrett AJ. The cystatins: a diverse superfamily of cysteine peptidase inhibitors. Biomed Biochim Acta. 1986;45:1363–74. [PubMed] [Google Scholar]
- 74.Turk V, Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991;285:213–9. doi: 10.1016/0014-5793(91)80804-c. [DOI] [PubMed] [Google Scholar]
- 75.Ogawa T, Boylan SA, Oltjen SL, et al. Changes in the spatial expression of genes with aging in the mouse RPE/choroid. Mol Vis. 2005;11:380–6. [PubMed] [Google Scholar]
- 76.Rakoczy PE, Zhang D, Robertson T, et al. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am J Pathol. 2002;161:1515–24. doi: 10.1016/S0002-9440(10)64427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang D, Brankov M, Makhija MT, et al. Correlation between inactive cathepsin D expression and retinal changes in mcd2/mcd2 transgenic mice. Invest Ophthalmol Vis Sci. 2005;46:3031–8. doi: 10.1167/iovs.04-1510. [DOI] [PubMed] [Google Scholar]
- 78.Walter P, Gilmore R, Blobel G. Protein translocation across the endoplasmic reticulum. Cell. 1984;38:5–8. doi: 10.1016/0092-8674(84)90520-8. [DOI] [PubMed] [Google Scholar]
- 79.Mellman I, Warren G. The road taken: past and future foundations of membrane traffic. Cell. 2000;100:99–112. doi: 10.1016/s0092-8674(00)81687-6. [DOI] [PubMed] [Google Scholar]
- 80.Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:867–86. doi: 10.1126/science.189.4206.867-b. [DOI] [PubMed] [Google Scholar]
- 81.Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–34. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 82.Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–33. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- 83.Nickel W. The mystery of nonclassical protein secretion. Eur J Biochem. 2003;270:2109–19. doi: 10.1046/j.1432-1033.2003.03577.x. [DOI] [PubMed] [Google Scholar]
- 84.Fölsch H, Mattila PE, Weisz OA. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic. 2009;10:972–81. doi: 10.1111/j.1600-0854.2009.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–57. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 86.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–66. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hunziker W, Fumey C, Honing S, et al. Trafficking of immunoglobulin receptors in epithelial cells: signals and cellular factors. Cell Biol Int. 1994;18:321–5. doi: 10.1006/cbir.1994.1081. [DOI] [PubMed] [Google Scholar]
- 88.Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–9. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- 89.Mostov KE. Regulation of protein traffic in polarized epithelial cells. Histol Histopathol. 1995;10:423–31. [PubMed] [Google Scholar]
- 90.Rodriguez-Boulan E, Zurzolo C. Polarity signals in epithelial cells. J Cell Sci Suppl. 1993;17:9–12. doi: 10.1242/jcs.1993.supplement_17.2. [DOI] [PubMed] [Google Scholar]
- 91.Ellis MA, Potter BA, Cresawn KO, et al. Polarized biosynthetic traffic in renal epithelial cells: sorting, sorting, everywhere. Am J Physiol Renal Physiol. 2006;291:F707–13. doi: 10.1152/ajprenal.00161.2006. [DOI] [PubMed] [Google Scholar]
- 92.Rodriguez-Boulan E, Müsch A. Protein sorting in the Golgi complex: shifting paradigms. Biochim Biophys Acta. 2005;1744:455–64. doi: 10.1016/j.bbamcr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–37. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Aroeti B, Okhrimenko H, Reich V, et al. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta. 1998;1376:57–90. doi: 10.1016/s0304-4157(98)00005-7. [DOI] [PubMed] [Google Scholar]
- 95.Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–90. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 96.Yeaman CHAR, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 97.Gravotta D, Deora A, Perret E, et al. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci USA. 2007;104:1564–9. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dempse PJ, Meise KS, Coffey RJ. Basolateral sorting of transforming growth factorβ precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants. Exp Cell Res. 2003;285:159–74. doi: 10.1016/s0014-4827(03)00035-1. [DOI] [PubMed] [Google Scholar]
- 99.Remaley AT, Hoeg JM. Polarized secretion of apoA-I and apoA-II by transfected MDCK cells. J Lipid Res. 1995;36:407–13. [PubMed] [Google Scholar]
- 100.Boll W, Partin JS, Katz AI, et al. Distinct pathways for basolateral targeting of membrane and secretory proteins in polarized epithelial cells. Proc Natl Acad Sci USA. 1991;88:8592–6. doi: 10.1073/pnas.88.19.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodriguez-Boulan E, Gonzalez A. Glycans in post-Golgi apical targeting: sorting signals or structural props? Trends Cell Biol. 1999;9:291–4. doi: 10.1016/s0962-8924(99)01595-0. [DOI] [PubMed] [Google Scholar]
- 102.Prabakaran D, Ahima RS, Harney JW, et al. Polarized targeting of epithelial cell proteins in thyrocytes and MDCK cells. J Cell Sci. 1999;112:1247–56. doi: 10.1242/jcs.112.8.1247. [DOI] [PubMed] [Google Scholar]
- 103.Guerriero CJ, Lai Y, Weisz OA. Differential sorting and Golgi export requirements for raft-associated and raft-independent apical proteins along the biosynthetic pathway. J Biol Chem. 2008;283:18040–7. doi: 10.1074/jbc.M802048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacob R, Heine M, Alfalah M, et al. Distinct cytoskeletal tracks direct individual vesicle populations to the apical membrane of epithelial cells. Curr Biol. 2003;13:607–12. doi: 10.1016/s0960-9822(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 105.Bonilha VL, Marmorstein AD, Cohen-Gould L, et al. Apical sorting of influenza hemagglutinin by transcytosis in retinal pigment epithelium. J Cell Sci. 1997;110:1717–27. doi: 10.1242/jcs.110.15.1717. [DOI] [PubMed] [Google Scholar]
- 106.Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–8. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- 107.Fritz BA, Lowe AW. Polarized GP2 secretion in MDCK cells via GPI targeting and apical membrane-restricted proteolysis. Am J Physiol Gastrointest Liver Physiol. 1996;270:G176–83. doi: 10.1152/ajpgi.1996.270.1.G176. [DOI] [PubMed] [Google Scholar]
- 108.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 109.Benussi L, Ghidoni R, Steinhoff T, et al. Alzheimer disease-associated cystatin C variant undergoes impaired secretion. Neurobiol Dis. 2003;13:15–21. doi: 10.1016/s0969-9961(03)00012-3. [DOI] [PubMed] [Google Scholar]
- 110.An E, Lu X, Flippin J, et al. Secreted proteome profiling in human RPE cell cultures derived from donors with age related macular degeneration and age matched healthy donors. J Proteome Res. 2006;5:2599–610. doi: 10.1021/pr060121j. [DOI] [PubMed] [Google Scholar]
- 111.De Strooper B, Craessaerts K, Dewachter I, et al. Basolateral secretion of amyloid precursor protein in Madin-Darby canine kidney cells is disturbed by alterations of intracellular pH and by introducing a mutation associated with familial Alzheimers disease. J Biol Chem. 1995;270:4058–65. doi: 10.1074/jbc.270.8.4058. [DOI] [PubMed] [Google Scholar]
- 112.Zuehlke J, Ebenau A, Krueger B, et al. Vectorial secretion of CTGF as a cell-type specific response to LPA and TGF-β in human tubular epithelial cells. Cell Commun Signal. 2012;10:25. doi: 10.1186/1478-811X-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sonnier D, Bailey S, Schuster R, et al. TNF-alpha induces vectorial secretion of IL-8 in caco-2 cells. J Gastrointest Surg. 2010;14:1592–9. doi: 10.1007/s11605-010-1321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu D, Deng X, Spee C, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest Ophthalmol Vis Sci. 2011;52:1573–85. doi: 10.1167/iovs.10-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lambert V, Wielockx B, Munaut C, et al. MMP-2 and MMP-9 synergize in promoting choroidal neovascularization. FASEB J. 2003;17:2290–2. doi: 10.1096/fj.03-0113fje. [DOI] [PubMed] [Google Scholar]
- 116.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci USA. 2002;99:14682–7. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakaguchi H, Miyagi M, Shadrach KG, et al. Clusterin is present in drusen in age-related macular degeneration. Exp Eye Res. 2002;74:547–9. doi: 10.1006/exer.2002.1186. [DOI] [PubMed] [Google Scholar]
- 118.Chlenski A, Liu S, Crawford SE, et al. SPARC Is a key Schwannian-derived inhibitor controlling neuroblastoma tumor angiogenesis. Cancer Res. 2002;62:7357–63. [PubMed] [Google Scholar]
- 119.Nozaki M, Sakurai E, Raisler BJ, et al. Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest. 2006;116:422–9. doi: 10.1172/JCI26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paraoan L, Grierson I, Maden BE. Analysis of expressed sequence tags of retinal pigment epithelium: cystatin C is an abundant transcript. Int J Biochem Cell Biol. 2000;32:417–26. doi: 10.1016/s1357-2725(99)00143-0. [DOI] [PubMed] [Google Scholar]
- 121.Abrahamson M, Grubb A, Olafsson I, et al. Molecular cloning and sequence analysis of cDNA coding for the precursor of the human cysteine proteinase inhibitor cystatin C. FEBS Lett. 1987;216:229–33. doi: 10.1016/0014-5793(87)80695-6. [DOI] [PubMed] [Google Scholar]
- 122.Paraoan L, Grierson I, Maden BE. Fate of cystatin C lacking the leader sequence in RPE cells. Exp Eye Res. 2003;76:753–6. doi: 10.1016/s0014-4835(03)00061-7. [DOI] [PubMed] [Google Scholar]
- 123.Paraoan L, Ratnayaka A, Spiller DG, et al. Unexpected intracellular localization of the AMD-associated cystatin C variant. Traffic. 2004;5:884–95. doi: 10.1111/j.1600-0854.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- 124.Ratnayaka A, Paraoan L, Spiller DG, et al. A dual Golgi- and mitochondria-localised Ala25Ser precursor cystatin C: an additional tool for characterising intracellular mis-localisation leading to increased AMD susceptibility. Exp Eye Res. 2007;84:1135–9. doi: 10.1016/j.exer.2006.01.030. [DOI] [PubMed] [Google Scholar]


