Abstract
Encapsulated microbubbles have been developed over the past two decades to provide both improvements in imaging as well as new therapeutic applications. Microbubble contrast agents are used currently for clinical imaging where increased sensitivity to blood flow is required, such as echocardiography. These compressible spheres oscillate in an acoustic field, producing nonlinear responses which can be uniquely distinguished from surrounding tissue, resulting in substantial enhancements in imaging signal-to-noise ratio. Furthermore, with sufficient acoustic energy the oscillation of microbubbles can mediate localized biological effects in tissue including the enhancement of membrane permeability or increased thermal energy deposition. Structurally, microbubbles are comprised of two principal components – an encapsulating shell and an inner gas core. This configuration enables microbubbles to be loaded with drugs or genes for additional therapeutic effect. Application of sufficient ultrasound energy can release this payload, resulting in site-specific delivery. Extensive pre-clinical studies illustrate that combining microbubbles and ultrasound can result in enhanced drug delivery or gene expression at spatially selective sites. Thus, microbbubles can be used for imaging, for therapy, or for both simultaneously. In this sense, microbubbles combined with acoustics may be one of the most universal theranostic tools.
Introduction
The combination of ultrasound and microbubbles has several unique advantages unavailable to most other imaging modalities. Microbubbles provide inherently high contrast for ultrasound imaging – yet can also act as a potent mediator for ultrasound therapeutics. The physical interaction between microbubbles and acoustic energy allows modulation of local permeability of both the microvasculature and cell membranes. Microbubbles can be physically moved and concentrated using acoustic radiation force and can be fragmented using ultrasound energy. Additionally, they can enhance conversion of acoustic to thermal energy, promoting selective tissue ablation. Furthermore, the microbubble structure enables drug and gene loading, as well as the inclusion of ligands able to target to specific tissues and/or biomarkers. Few other combinations of technology enable such varied and potent diagnostic and therapeutic tools from the same platform.
Theranostics refers to the combination of diagnostic and therapeutic approaches, typically for assessing response to therapy. Although it is possible for an agent to be used simultaneously for imaging and therapy, by definition, theranostics is fundamentally the combination of a diagnostic test with a therapeutic approach. Thus, theranostics can involve either application of a diagnostic technique followed by a therapeutic, or vice versa.1 Theranostic approaches are a key aspect in the drive toward personalized medicine, where the tailoring of decisions and treatment practices for individual patients is based on diagnostic information specifically from that patient. In this review, we illustrate how microbbubles can be used for imaging, for therapy, or for both simultaneously. Because of this broad application, microbubbles combined with acoustics may be one of the most universal theranostic tools.
BACKGROUND
Advantages of Ultrasound
Ultrasound is already one of the most widespread diagnostic modalities, routinely used in cardiology and obstetrics, but also commonly used for imaging other soft tissues.2 The utility of the ultrasound platform is further enabled by recent advances in electronics. Rapidly shrinking and more powerful application-specific integrated circuits have led to ultrasound being one of the most portable imaging modalities. Many ultrasound manufacturers now offer imaging systems which are the size of laptop computers, and some of the latest devices are only slightly larger than smart phones. In contrast, the sizes of other clinical imaging systems such as MRI, CT, SPECT, and PET are substantially larger and therefore remain less portable and accessible. Although ultrasound does not provide the large-region volumetric imaging that is provided by these other modalities, ultrasound image acquisition rates for small regions of interest exceed that of MRI and nuclear imaging technologies by several fold. This high-frame rate capability provides an advantage in therapeutic applications with real-time feedback, which can be cumbersome with other imaging techniques. Another advantage of ultrasound is the significant soft-tissue contrast obtained in ultrasound imaging, although tissue attenuation severely limits the resolution and contrast of ultrasound for deep tissue applications. Furthermore, ultrasound imaging delivers no ionizing radiation, and thus is considered one of the safest imaging modalities.3 Although the safety of ultrasound contrast agents still needs to be examined for many applications, initial evaluations of contrast ultrasound in cardiology suggest that adverse effects resulting from contrast use are very rare.4–6 Furthermore, ultrasound contrast may play an important role in patient populations where MRI and CT contrast agents are contraindicated (such as renally compromised patients).7 Along with the inherent safety and portability of ultrasound imaging, the equipment cost is significantly lower in comparison to other imaging modalities. With these advantages, the future role of the microbubble platform in both diagnostics and therapeutics is highly promising.
Advent of microbubbles for medical imaging
Ultrasound image formation relies on the reception and interpretation of acoustic reflections scattered by blood and tissue. However, the scattering components associated with blood are weak, and thus blood flow in tissues and small vessels is challenging to image. Gramiak and Shah are credited with the first publication of the contrast ultrasound technique as they described the application of bubbles produced by rapid intracardiac saline injections to enhance delineation of aortic blood flow.8 It was not until over two decades later, with the marketing of Albunex® (Mallinckrodt Medical, Inc., St. Louis, MO, USA), that microbubbles became commercially available for use as an ultrasound contrast agent.9
MICROBUBBLE COMPOSITION
Encapsulating shell
Initial studies of gas bubbles for acoustic enhancement utilized un-encapsulated microbubbles that were generated in situ. Due to the solubility of air in blood, un-encapsulated microbubbles dissolved in seconds,10 and could not traverse the pulmonary vasculature.11, 12 First generation contrast agents utilized a stabilizing albumin shell which improved circulation time substantially over un-encapsulated microbubbles. The shell reduces the rate at which the gaseous core diffuses into the surrounding media.13 Microbubbles are normally injected intravenously as suspensions meaning they are exposed to aqueous solutions during clinical use. As such, shells composed of amphiphilic molecules help ensure microbubbles can achieve thermodynamic stability with a hydrophobic gas core. Various compositions have since been used to provide an encapsulating shell, including proteins, lipids, and polymers. The commercially produced contrast agents Optison™ (Mallinckrodt, San Diego, CA, USA) and Definity® (DuPont Pharmaceuticals Co., North Billerica, MA, USA), which are currently the only two FDA-approved agents in the US still in production, utilize albumin and phospholipid encapsulation, respectively.14
The diversity of encapsulating material composition, thickness, stiffness, charge, and surface area enables tailoring of bubble design for custom applications.15 Shell material choice is important in microbubble formulation and design since the shell functions as a scaffold for ligand binding, prevents core gases from diffusing, and influences biocompatibility. Phospholipid shells are widely used because they are easily modifiable for ligand binding and targeting applications. The polar heads of these phospholipids are frequently the site of conjugation to make targeted microbubbles.16 Direct modifications of the microbubble shells have been used in specialized applications to explore novel methods of enhancing microbubble function.16–21 Polyethylene glycol (PEG) chains of varying length attached to the shell of the microbubble act as steric brushes, reducing microbubble aggregation and immune recognition while also providing an attachment point for bioconjugates.16, 22, 23
Gas Core
The composition of the gas core also determines the stability of the microbubble. Commercial formulations of the inner gas core have included air (Albunex®), sulfur hexafluoride (Sonovue®, Bracco, SpA, Milan, Italy), or perfluorocarbons (Definity®, Lantheus Medical Imaging, North Billerica, MA, USA). The transition from air to high molecular weight fluorinated gas cores has improved microbubble stability. Gases with lower blood solubility and higher molecular weight take longer to diffuse across the microbubble shell allowing for longer persistence times.24
Microbubble Size Control
Most lipid and protein encapsulated microbubbles are formed through either mechanical agitation or sonication to produce a suspension of encapsulated bubbles. These methods result in microbubble populations that are highly polydisperse. The acoustic response of microbubbles varies with respect to many parameters but the largest contributor is diameter.25, 26 Acoustic scattering cross section is a function of size, and microbubbles of decreasing diameter naturally oscillate at higher resonant frequencies. By tailoring microbubble size distribution, imaging sensitivity can be optimized.27–30 Microbubble size also plays a significant role in therapeutic effects, as size is proportional to drug loading, and smaller microbubbles require lower pressure magnitudes to cause shell rupture.31 Microbubble size is also related to cavitation magnitude and corresponding changes in blood brain barrier permeability during microbubble-enhanced focused ultrasound treatment.32, 33
Microbubble preparations with custom/narrowed size distribution are generally obtained in one of four ways: microfluidics,34, 35 electrohydrodynamic atomization,36 filtering,37, or differential centrifugation28, or In microfluidics and electrohydrodynamic atomization, microbubbles are produced with uniform diameters by precisely controlling the flow rates of the encapsulating (usually lipid) phase that sheaths around the inner (usually high molecular weight gas) phase. Microfluidics uses flow-focusing devices driven by pressure along with advantageous channel geometries to direct and control flow while electrohydrodynamic atomization utilizes electric fields to pull droplets from a fluid cone. In contrast to the previous two techniques, differential centrifugation and filtering do not create microbubbles at a given size but instead refine a polydisperse population into sorted samples. Both methods are generally higher throughput than either microfluidic or electrohydrodynamic techniques. Some groups have utilized filtering to refine microbubble populations, although this method can be challenging due to the fact that most microbubbles are very susceptible to destruction at high shear rates or pressure changes,38 and soft shelled microbubbles are deformable and thus may not be efficiently sorted by specific pore sizes. Centrifugal sorting is commonly used in microbubble size refinement since it can performed on large batches of microbubbles using common laboratory equipment.
MICROBUBBLE BEHAVIOR
Acoustic response
The gas core of a microbubble has a compressibility that is several orders of magnitude greater than an equivalent volume of blood. It is this combination of high compressibility coupled with the low density of the core that provides a substantial impedance mismatch between microbubbles and surrounding blood or tissue, and thus makes microbubbles excellent ultrasound contrast agents.39 When exposed to an ultrasound pulse, microbubbles oscillate in response to the acoustic pressure waves (Figure 1).10, 40 At low acoustic pressures, most microbubbles oscillate stably, scattering sound energy as they resonate. At high acoustic pressures, large cycles of expansion and contraction can result in instability that results in fragmentation of the shell and consequential microbubble destruction with diffusive loss of the gas core. Microbubble destruction can also result in the release of any incorporated payload, facilitating the use of microbubbles as a therapeutic agent.31, 41 Microbubble behavior depends on many factors, including acoustic frequency and pressure, bubble size, and physical properties of the shell and core. Environmental conditions such as hydrostatic pressure and dissolved gas saturation are also influencing factors. In general, low acoustic frequencies, high acoustic pressures, and smaller diameters increase the likelihood of microbubble destruction.31, 42
Figure 1.
High-speed optical photography of acoustically excited microbubbles illustrating microbubble fragmentation as well as stable oscillation. A) Diameter vs. time streak photography showing a 3 micron bubble in response to 2 cycle insonation at ~1.5 MHz and 1200 kPa. The microbubble is observed to expand and contract substantially and then fragment B) Standard 2-D framing photography acquired simultaneously to the diameter vs. time image presented in A). C) 20 cycle insonation of a 3.5 micron bubble at ~3.5 MHz and 200 kPa, showing stable, linear, low-amplitude oscillation. (unpublished data, courtesy of Paul Dayton)
The phenomenon of acoustic radiation force provides an additional method to increase theranostic utility of microbubbles. Primary radiation force displaces bubbles along the direction of acoustic wave propagation. This force can physically displace microbubbles from the center of flow of a vessel and concentrate them against the endothelium.43 Secondary radiation force can cause groups of microbubbles to aggregate together and is most significant when microbubbles are in close proximity (several bubble diameters) of one another.44 Radiation force has been shown to both enhance the in vivo retention of molecularly targeted contrast agents,5, 45, 46 as well as increase the local delivery of drug-carrying microbubble vehicles.47–49
In vivo behaviour
During a contrast imaging exam, a solution of microbubbles is injected through a peripheral vein. Typically, circulation half-life is fairly short, and microbubbles remain in circulation on the order of several minutes.50 Gamma scintigraphy of commercially available Quantison™ (Quadrant Healthcare Ltd, Nottingham, UK) suggests the liver and spleen as the final destination of most microbubble shell components.51 These observations have been corroborated more recently by PET imaging with lipid shelled microbubbles.52 Inert gases from defunct microbubbles are rapidly cleared through exhalation from the lungs
MICROBUBBLE ALTERNATIVES
Although microbubble research occupies the majority of publications on ultrasound contrast and therapeutic agents, several alternative agents exist that enable unique theranostic approaches. Several groups have explored echogenic liposomes (or acoustically-active liposomes) which contain small pockets of gas and therefore can provide image contrast and/or can be triggered to release therapeutic contents by the influence of ultrasound.46, 53–55 Others have developed targeted perfluorocarbon nanodroplets that increase in echogenicity as they accumulate in sites of atherosclerosis and angiogenesis and can be made to carry therapeutic payloads.56, 57 Many recent studies have focused on phase change contrast agents (PCCAs), which combine the functional similarity of microbubbles with unique delivery and activation possibilities. PCCAs are encapsulated droplets with liquid perfluorocarbon cores capable of being triggered to the gaseous phase when stimulated with acoustic energy from an ultrasound transducer.58 Once vaporized, PCCAs expand to form bubbles on the order of 5–6 times larger than the precursor droplets.59 As a result, the advantages of each state (i.e. stability and small size in liquid state, echogenicity and ultrasound interaction in gas state) can be utilized efficiently. PCCAs have been produced by several groups with mean diameters both in the microscale and nanoscale for a variety of unique approaches not possible with standard microbubble agents. This includes microvascular occlusion, cavitation/thermal ablation enhancement, and both vascular and extravascular drug delivery and imaging. A full discussion on the nature, history, and design considerations of PCCAs are reviewed elsewhere.59–61
DIAGNOSTIC APPLICATIONS
Imaging Approaches
There are two primary categories of methods for detecting microbubbles that enable contrast specific imaging. First, the oscillation of microbubbles in an acoustic field results in scattered broadband acoustic energy with a frequency range much greater than that produced by tissues. This signal is separated from tissue response via high or low pass filtering of the backscattered signal. Techniques include subharmonic imaging,62–64 the detection of microbubble signal at frequencies half of the imaging frequency; harmonic imaging,65, 66 the detection of microbubble signal at twice the imaging frequency; and superharmonic imaging,67, 68 the detection of higher harmonic multiples of the imaging frequency. A high-frequency contrast imaging technique called broadband or transient imaging is similar to harmonic and superharmonic imaging in that it involves low-frequency excitation and high-frequency detection of the broadband response from microbubbles well above that which is scattered from tissue.69–71 The isolation of the microbubble-specific signal enables visualization of contrast agents without the high background signal from tissue which could otherwise obscure the region of interest. The main advantage of these techniques is that they require only a single pulse of ultrasound for each line of sight to create image data and therefore are not susceptible to tissue motion artifacts and can operate at high frame rates. The second method of isolating microbubble signals relies on their nonlinear response to consecutive acoustic pulses, as opposed to linear responses from tissue. In order to separate microbubbles from tissue echoes, these nonlinear imaging strategies excite microbubbles with two or more pulses of varying amplitude or phase and then combine the resulting response across multiple pulses.72–75 Nonlinear components of the received signal, such as those from the echoes of microbubbles, will remain after the images are combined, while linear components such as echoes from most tissues, will be reduced.
Perfusion Imaging
Ultrasound contrast-enhanced imaging can be used to monitor tissue or organ perfusion rates by measuring the transit time of microbubbles into or out of tissue. This is accomplished through observation of a single contrast agent bolus, or by modulating contrast agent wash-in acoustically. The latter is achieved by sending a short, high intensity ultrasound pulse that fragments microbubbles in the field of view. After this clearance pulse, the returning microbubble signal wash-in rate is measured and can be correlated to microvascular flow. This method is typically performed under constant infusion of contrast agents, and imaging is performed using low intensity contrast-specific ultrasound imaging in order to preserve the microbubble population during the measurement phase.76, 77 This method, often referred to as “destruction-reperfusion”, “flash-replenishment”, or “clearance-refill” imaging, can detect changes in very small flow rates below the tissue motion noise level that limits conventional color Doppler velocity estimates.78 Use of perfusion imaging has been demonstrated to be effective in assessing blood flow in the kidneys79–81 and capillary beds of tumors,82–86 among others.
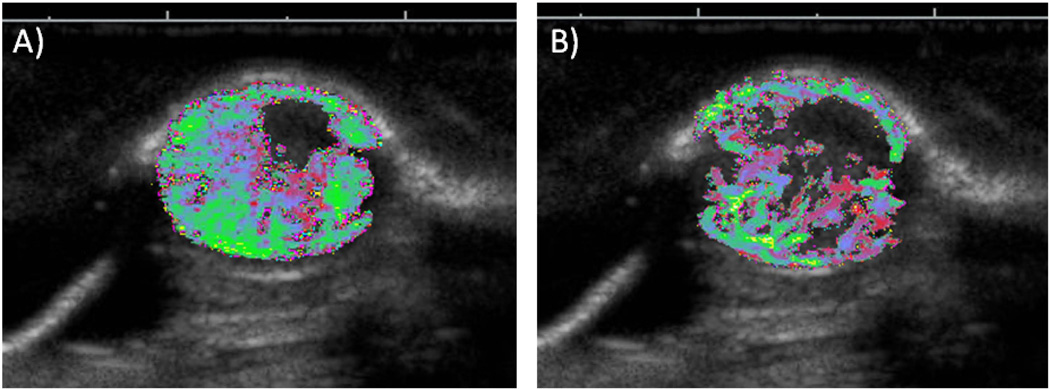
The ability of contrast ultrasound perfusion imaging to detect small changes in blood flow conditions, such as those found in capillary beds, suggests substantial potential for assessing response to therapeutic approaches that affect tissue blood flow (such as anti-angiogenic therapies – Figure 2).85, 87–92 Anti-angiogenic treatment of tumors attempts to decrease tumor viability by inhibiting vascular function and development in order to curb tumor growth and cause eventual ischemic and necrotic conditions within the tumor.93 Initial studies focusing on anti-angiogenic therapy response in tumors using perfusion imaging have reported ease of use, low cost, and high spatial resolution.85 Similarly, contrast ultrasound perfusion imaging has been utilized as an immediate means to assess clinical response to high intensity focused ultrasound ablation.94, 95
Figure 2.
Example of contrast enhanced destruction-reperfusion imaging in a rodent tumor xenograft model, A) prior to and B) 48 hrs post anti-angiogenic administration, illustrating reduction in tumoral blood flow in response to therapy. Grayscale indicates anatomical orientation, the colormap indicates blood flow, where red is slower flow and green is faster flow. (unpublished data, courtesy of Steven Feingold)
Molecular Imaging
Molecular imaging enables contrast ultrasound to progress beyond anatomical imaging and provide information about changes in physiology on the molecular level.96–98 Molecularly targeted contrast agents are formulated by incorporation of targeting ligands, such as peptides, antibodies, or other adhesive molecules specific for disease biomarkers, into the contrast agent shell. Typical microbubble sizes are large enough to stay within the vascular lumen without extravasating to surrounding tissues, and thus are well equipped to target endothelial markers of disease. Upon injection, circulating targeted microbubbles adhere to endothelium presenting the appropriate receptor. After natural clearance of unbound circulating microbubbles, retained molecularly targeted agents can be imaged using nondestructive microbubble imaging methods, providing information as to extent and degree of biomarker expression. Since ultrasound cannot directly assess molecular changes, the assumption that targeted microbubble retention on diseased endothelium is proportional to biomarker expression is a crucial tenet to this method. Commonly imaged biomarkers include the αvβ3 integrin, associated with angiogenesis, which can be targeted through cyclic RGD peptides, as well as inflammatory markers such as ICAM-1 and VEGFR2, often targeted directly with microbubbles bearing specific antibodies. Intriguing applications of ultrasound molecular imaging include but are not limited to assessment of atherosclerosis predisposition or progression,99 thrombus,100 ischemic damage,101, 102 inflammatory diseases,103 and tumor-related angiogenesis.104–106
As any physical change in biological tissue is likely preceded by changes at the molecular level, the ability to assess molecular markers enables a method of predicting forthcoming biological response to treatment. Through the use of microbubbles targeted to angiogenesis markers, researchers have observed changes in biomarker expression in response to therapy (Figure 3).107 More recently, ultrasound molecular imaging has been shown to classify tumors as non-responder or responder earlier than standard tumor size measurement techniques.87, 108
Figure 3.
Ultrasound molecular imaging used to evaluate the efficacy of an aurora kinase inhibitor in treating human pancreatic adenocarcinoma xenografts in a pre-clinical model. Data illustrate that molecular imaging indicates response to therapy prior to tumor size changes. A) Ultrasound images of a representative treated and a representative untreated tumor, which were each acquired before and after treatment. The green color overlay illustrates contrast agent targeted to αvβ3, an angiogenic biomarker. The brightness of the green image overlay is assumed to be correlated with the degree of molecular marker expression. B) 3-D ultrasound rendering of a treated pancreatic adenocarcinoma tumor on day 0. The green overlay represents the contrast agent targeting to αvβ3. A section is removed to illustrate the spatial variability of contrast targeting to αvβ3 biomarker expression. C) Percent increase or decrease in volumetric contrast targeting before and after therapy (Untreated - N=5, Treated - N=5). * p < 0.05 compared to untreated tumors on day 2. D) Percent increase or decrease in volume as measured by regions of interest from brightness mode ultrasound images taken at known distances across the tumor (Untreated - N=5, Treated - N=5). (unpublished data, provided by Jason Streeter, Gabriela Herrera, and J.J. Yeh)
Acoustic Angiography
When combined with an imaging mode that provides both high resolution and tissue signal rejection, microbubbles (inherently limited to the vascular space) can provide traces of detailed vascular structure (Figure 4). Several studies reported in the literature over the last decade have utilized contrast ultrasound as a means of assessing vascular architecture with the goal of either determining tumor presence or tumor malignancy. Contrast-enhanced acoustic angiography has been demonstrated in multiple cancer types, including breast109, liver110, 111, and thyroid.112 More recently, high-frequency acoustic angiography has enabled analysis of vessel tortuosity in a preclinical model that uses vessel morphology to distinguish between healthy and tumor tissue.113 While various segmentation approaches have been implemented to extract and categorize vascular features from image data, the diagnostic utility of acoustic angiography is encouraging. Contrast-enhanced acoustic angiography could be utilized to assess vascular changes in response to therapy, based on the current understanding that vascular abnormalities re-normalize in response to treatment.114–116
Figure 4.
Two 3D images from the same sample volume acquired of a rat fibrosarcoma tumor model using traditional 30 MHz b-mode imaging (left) and contrast enhanced acoustic angiography (right). Scale bars below the images indicate 1 cm. Cartoon in the center illustrates the approximate location of the tumor denoted by the red circle (unpublished data, courtesy of Ryan Gessner)
THERAPEUTIC APPLICATIONS
The behavior of the oscillating bubble in an acoustic field lends itself not only to conventional imaging enhancement, but also to therapeutic applications which could be performed during the same sonography session. These applications range from slight physical modifications of the tissue itself – such as reversible changes in vascular or cellular permeability – to more aggressive disruption, such as clot fragmentation. The mechanical action of oscillating microbubbles also enhances conversion from acoustic to thermal energy, resulting in tissue ablation at lower acoustic energy than that required for similar effect without microbubbles. Other applications involve secondary interactions such as the local delivery and/or release of a therapeutic compound mediated by acoustically driven microbubbles. Guidance can be provided by ultrasound imaging,117 magnetic-resonance imaging,118 or other modalities to help direct the treatment to the desired location, or to monitor the therapeutic effects of microbubble mediated therapies.
Modulation of vascular and cellular permeability
The mechanical oscillations of acoustically stimulated microbubbles have been shown to increase local vascular permeability.119, 120 Effects range from mild transient changes in vascular permeability to gross vascular disruption and hemorrhage, depending on microbubble and acoustic parameters. Early in vivo studies demonstrated that insonified microbubbles were capable of small vessel rupture creating transcellular pathways for polymer microspheres (≤503 nm) and red blood cells (~7 µm) to extravasate.121 Extensive studies have shown that with appropriately chosen acoustic parameters, changes in vascular permeability due to insonified microbubbles can be mild and transient.122, 123 Thus, there is a great interest for using this approach to locally enhance extraluminal bioavailability of therapeutic materials to surrounding tissues. Studies have illustrated substantial potential for the application of acoustically excited microbubbles to locally enhance delivery to the brain,124, 125 tumors,126 and other tissues.19, 127 This is of particular interest in the brain, where the blood-brain barrier makes extravascular drug delivery challenging. There are several mechanisms hypothesized to be responsible for this permeability modulation, including disruption of the endothelium due to mechanical stresses on the vessel wall,128, 129 endothelial disruption due to liquid jetting that occurs with bubble collapse,130 and increased cellular transport (Figure 5).128, 131
Figure 5.
Illustration of three hypothesized methods of how microbubbles may cause enhanced permeability. A) An ultrasound wave places a high pressure on the microbubble to compress it and is followed by low pressure that rapidly expands it creating several microhemorrhages due to mechanical stress. B) An oscillating microbubble stimulates a cell to increase transcellular transport from the lumen of the vessel to the basal membrane. C) A microbubble oscillates nonlinearly to the point of asymmetric collapse, producing a powerful microjet that breaches the endothelium.
Similar to the effect on vascular permeability, acoustically activated microbubbles have also been shown to enhance the permeability of individual cell membranes. This phenomenon, termed “sonoporation”, can result in the internalization of compounds into a cell which otherwise would not cross the cellular membrane. The mechanisms causing sonoporation are still being studied; however, common mechanistic hypotheses include mechanical stresses, the formation of pores due to liquid jetting,132 or microstreaming,133, 134 as well as active transport processes.135 Sonoporation can be transient or irreversible, the latter often associated with cell death, depending on the acoustic parameters.122, 136, 137
The magnitudes of changes in both vascular and endothelial permeability are related to ultrasound frequency, pressure, and microbubble parameters. In general, lower acoustic frequencies and increased pressures result in greater permeability. Identifying and characterizing which parameters can be used to modify vessel or cellular permeability, the mechanisms involved, and to what extent permeability can be modulated is an active area of study in microbubble acoustics.138
Drug Delivery
Many anticancer drugs suffer from a low therapeutic index, where the systemic dose becomes toxic at only slightly higher doses than may be effective in treating the disease. Thus, any means to significantly increase drug delivery at the disease site while reducing dose to healthy tissue is of great clinical interest. One approach is to spatially distribute the drug concentration unevenly such that the local dose to the disease site is elevated while the systematic dose remains low. Microbubbles can behave as a targeted delivery system by loading them with drugs and selectively lysing (fragmenting) them in regions where the drug delivery is desired.139–141
Examples of drug loading techniques include the incorporation of therapeutic agents into the microbubble shell,142, 143 the formulation of multi-layer vehicles containing drugs in a layer separate from the shell or gas core,144, 145 or conjugation of drug-loaded liposomes or nanoparticles directly to the microbubble surface (Figure 6).48, 49 Several drugs which have been evaluated for microbubble delivery to date include, but are not limited to, paclitaxel,146, 147 doxorubicin,144, 148 rapamycin,143 and 10-hydroxycamptothecin.149
Figure 6.
Schematic of commonly used drug attachment strategies in microbubble mediated drug delivery. A) Drugs can be dissolved in a secondary oil layer using a multi-layer microbubble construction. B) Therapeutic agents can be seeded within the thin encapsulating shell C) Nanoparticles or other therapeutics can be attached to the outside of the shell, such as tethered to PEG chains. (image courtesy of Paul Sheeran)
Although there is a broad range of drug loading methods and capacities, several studies have shown that certain types of drug carrier vehicles retain the capability for substantial acoustic response, indicating that these vehicles could be fully capable of both acting as imaging agents as well as therapeutic carriers.150, 151
Gene Delivery
While the application of ultrasound alone has increased transfection rates compared to systemic delivery during gene therapy, the presence of acoustically-stimulated microbubbles results in additional benefit. The results of several microbubble-mediated gene therapy studies indicate that gene transfection rates are highest when ultrasound, microbubbles, and genes are simultaneously delivered to the same target location.17, 152, 153,127 Although the mechanisms for enhanced transfection are still being assessed, permeability changes in cellular membranes due to the oscillating microbubbles (as described above) likely play a role.135 Because of the strongly anionic backbone of plasmids and other gene transfection agents, cationic microbubbles can be used to electrostatically attach genetic material to microbubble shells in a similar manner first developed for liposomes.154 Positively charged microbubbles bound with genetic material can use lower systemic concentration of plasmids because they have higher transfection efficiencies compared to neutral microbubbles.155 Cationic binding of genetic materials likely increases transfection rates due to the increased proximity between genetic material and cell membranes which promotes intracellular flow of the genetic material.156 Thicker shelled microbubbles are an attractive option in targeted gene delivery since they are less likely to prematurely rupture, can load more genetic material, and can protect genetic material from nuclease activity if bound within the shell.157, 158
Sonothrombolysis
Sonothrombolysis is the acoustically-mediated disruption of blood clots. The addition of microbubbles further enhances the thrombolytic effect,159 both with and without the combination of thrombolytic agents. The rate of clot dissolution increases and clots degrade more fully when treated directly with ultrasound and microbubbles in comparison to administration of thrombolytic agents alone.160, 161 This technique has been explored for stroke,162 venous thrombosis,163 and myocardial infarction.164 Ultrasound-assisted thrombolysis is one of the truly combinational theranostic procedures since real-time ultrasound measurements of clot occlusion and therapeutic recanalization have been performed simultaneously with microbubbles and ultrasound.164
Tissue Ablation enhancement
Acoustic ablation involves focusing high intensity ultrasound to heat and destroy abnormal tissue. The conversion of acoustic energy into thermal energy causes a rapid rise in temperature at the target site resulting in localized tissue destruction and necrosis. One of the current challenges of high intensity focus ultrasound (HIFU) ablation is to heat only target tissue without damaging healthy tissues. HIFU ablation transmits wide beams of low acoustic energy through tissue which is then focused into a tighter beam of high acoustic energy to be delivered at depth to the target site. Microbubbles can be advantageous for HIFU ablation because they can enhance the conversion of acoustic energy to thermal energy, therefore making this approach more efficient and reducing the likelihood of thermal damage to healthy tissues.165–169 Early in vivo studies examining the effect microbubbles have on ablation enhancement during HIFU was demonstrated in canines170 and has been clinically evaluated in humans for uterine fibroid ablation.171
Current Challenges
Microbubbles are a unique platform that can unite established diagnostic ultrasound with relatively nascent therapeutic ultrasound. The largest challenges faced by microbubble technologies are government approval and physician acceptance. Microbubbles are still used clinically only for imaging applications. Even then, as an imaging agent – microbubbles are underutilized. Although microbubbles are used for several clinical imaging applications in Europe and Asia, FDA approved microbubble use in the United States is still limited only to enhancement of the left ventricle during echocardiography. A third major challenge involves industrial interest and support and production of ultrasound systems optimized for use with microbubbles, as well as in design and manufacture of the microbubbles themselves. Largely due to the lack of clinical use, industry interest in supporting microbubble imaging and therapeutic approaches on commercial systems has been a low priority. Similarly, there are only a handful of companies involved in the development and sales of microbubble products, likely due to the same reason.
Nevertheless, an active push by both academia and industry is currently underway to extend the use of microbubbles for imaging outside the heart as well as for therapeutic applications. Numerous studies, such as the ones discussed here, have demonstrated the potential uses of microbubbles beyond their current scope. Regulatory agency approval must be met both in the US and in other countries for both additional diagnostic applications, as well as any therapeutic applications of microbubbles, before their use will expand.
CONCLUSION
The microbubble platform is unique in that it presents a wide variety of strategies for ultrasound mediated diagnostics as well as therapeutics – thereby acting as a definitive theranostic agent. Because of the relatively low cost, portability, and safety of ultrasound, these techniques have advantages for reduced healthcare costs, bedside support, repeated imaging or treatment studies, and access for rural or underserved populations. While there is extensive use of microbubbles in pre-clinical studies, clinical trials are still only in early stages for molecular imaging as well as for many therapeutic approaches. However, pre-clinical data are highly encouraging for the future use of microbubbles in theranostic medicine.
Acknowledgements
The Dayton lab acknowledges financial support from the National Institutes of Health, grants R01EB008733, R01EB009066, and R21EB011704, as well as support from the University Cancer Research Fund and the Carolina Center for Cancer Nanotechnology Excellence. The images provided in figure 1 were captured with the Imacon 468 Ultra High Speed Imaging system in the laboratory of Katherine Ferrara. The authors appreciate proofreading efforts from Paul Sheeran, Jason Streeter, and Linsey Phillips.
References
- 1.Del Vecchio S, Zannetti A, Fonti R, Pace L, Salvatore M. Nuclear imaging in cancer theranostics. Q J Nucl Med Mol Imaging. 2007;51:152–163. [PubMed] [Google Scholar]
- 2.Kiessling F, Fokong S, Koczera P, Lederle W, Lammers T. Ultrasound microbubbles for molecular diagnosis, therapy, and theranostics. J Nucl Med. 2012;53:345–348. doi: 10.2967/jnumed.111.099754. [DOI] [PubMed] [Google Scholar]
- 3.Pomper MG, Gelovani JG. Molecular Imaging in Oncology. New York: Informa Healthcare USA, Inc.; 2008. [Google Scholar]
- 4.Abdelmoneim SS, Bernier M, Scott CG, Dhoble A, Ness SA, Hagen ME, Moir S, McCully RB, Pellikka PA, Mulvagh SL. Safety of contrast agent use during stress echocardiography: a 4-year experience from a single-center cohort study of 26,774 patients. JACC Cardiovasc Imaging. 2009;2:1048–1056. doi: 10.1016/j.jcmg.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Dolan MS, Gala SS, Dodla S, Abdelmoneim SS, Xie F, Cloutier D, Bierig M, Mulvagh SL, Porter TR, Labovitz AJ. Safety and efficacy of commercially available ultrasound contrast agents for rest and stress echocardiography a multicenter experience. J Am Coll Cardiol. 2009;53:32–38. doi: 10.1016/j.jacc.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh K, Chang SM, Peterson L, Rosendahl-Garcia K, Quinones MA, Nagueh SF, Kurrelmeyer K, Zoghbi WA. Safety of contrast administration for endocardial enhancement during stress echocardiography compared with noncontrast stress. Am J Cardiol. 2008;102:1444–1450. doi: 10.1016/j.amjcard.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Tay KJ, Ho HS, Low AS, Cheng CW. Is contrast enhanced ultrasound a valid alternative diagnostic modality for renal cell carcinoma in patients with renal impairment? Ann Acad Med Singapore. 41:127–122. [PubMed] [Google Scholar]
- 8.Gramiak R, Shah PM. Echocardiagraphy of the aortic root. Investigative Radiology. 1968;3:356–366. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Keller MW, Glasheen W, Kaul S. Albunex: a safe and effective commercially produced agent for myocardial contrast echocardiography. J Am Soc Echocardiogr. 1989;2:48–52. doi: 10.1016/s0894-7317(89)80028-8. [DOI] [PubMed] [Google Scholar]
- 10.Dayton PA, Morgan KE, Klibanov AL, Brandenburger GH, Ferrara KW. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 1999;46:220–232. doi: 10.1109/58.741536. [DOI] [PubMed] [Google Scholar]
- 11.Mayer S, Grayburn PA. Myocardial contrast agents: recent advances and future directions. Prog Cardiovasc Dis. 2001;44:33–44. doi: 10.1053/pcad.2001.26438. [DOI] [PubMed] [Google Scholar]
- 12.Ten Cate FJ, Feinstein S, Zwehl W, Meerbaum S, Fishbein M, Shah PM, Corday E. Two-dimensional contrast echocardiography. II. Transpulmonary studies. J Am Coll Cardiol. 1984;3:21–27. doi: 10.1016/s0735-1097(84)80425-8. [DOI] [PubMed] [Google Scholar]
- 13.Klibanov AL. Microbubble contrast agents - Targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Investigative Radiology. 2006;41:354–362. doi: 10.1097/01.rli.0000199292.88189.0f. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg BB, Raichlen JS, Forsberg F. Ultrasound Contrast Agents: Basic principals and clinical applications. London: Martin Dunitz; 2001. [Google Scholar]
- 15.Sirsi S, Borden MA. Microbubble compositions, properties and biomedical applications. Bubble Science, Engineering, and Technology. 2009;1:3–17. doi: 10.1179/175889709X446507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klibanov AL. Ligand-carrying gas-filled microbubbles: Ultrasound contrast agents for targeted molecular imaging. Bioconjugate Chemistry. 2005;16:9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 17.Christiansen JP, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound in Medicine and Biology. 2003;29:1759–1767. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 18.Fisher NG, Christiansen JP, Klibanov A, Taylor RP, Kaul S, Lindner JR. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. Journal of the American College of Cardiology. 2002;40:811–819. doi: 10.1016/s0735-1097(02)02038-7. [DOI] [PubMed] [Google Scholar]
- 19.Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- 20.Rychak JJ, Lindner JR, Ley K, Klibanov AL. Deformable gas-filled microbubbles targeted to P-selectin. Journal Of Controlled Release. 2006;114:288–299. doi: 10.1016/j.jconrel.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Unger EC, Hersh E, Vannan M, Matsunaga TO, McCreery T. Local drug and gene delivery through microbubbles. Prog Cardiovasc Dis. 2001;44:45–54. doi: 10.1053/pcad.2001.26443. [DOI] [PubMed] [Google Scholar]
- 22.Chen CC, Borden MA. The role of poly(ethylene glycol) brush architecture in complement activation on targeted microbubble surfaces. Biomaterials. 2011;32:6579–6587. doi: 10.1016/j.biomaterials.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borden MA, Sarantos MR, Stieger SM, Simon SI, Ferrara KW, Dayton PA. Ultrasound Radiation Force Modulates Ligand Availability on Targeted Contrast Agents. Mol Imaging. 2006;5:139–147. [PMC free article] [PubMed] [Google Scholar]
- 24.Wible J, Jr, Wojdyla J, Bugaj J, Brandenburger G. Effects of inhaled gases on the ultrasound contrast produced by microspheres containing air or perfluoropropane in anesthetized dogs. Invest Radiol. 1998;33:871–879. doi: 10.1097/00004424-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Qin SP, Ferrara KW. The natural frequency of nonlinear oscillation of ultrasound contrast agents in microvessels. Ultrasound in Medicine and Biology. 2007;33:1140–1148. doi: 10.1016/j.ultrasmedbio.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan KE, Allen JS, Dayton PA, Chomas JE, Klibaov AL, Ferrara KW. Experimental and theoretical evaluation of microbubble behavior: effect of transmitted phase and bubble size. IEEE Trans Ultrason Ferroelectr Freq Control. 2000;47:1494–1509. doi: 10.1109/58.883539. [DOI] [PubMed] [Google Scholar]
- 27.Kaya M, Feingold S, Streeter JE, Hettiarachchi K, Lee AP, Dayton PA. Acoustic responses of monodisperse lipid-encapsulated microbubble contrast agents produced by flow focusing. Bubble Science, Engineering, and Technology. 2:33–40. doi: 10.1179/175889610x12779105661532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feshitan JA, Chen CC, Kwan JJ, Borden MA. Microbubble size isolation by differential centrifugation. J Colloid Interface Sci. 2009;329:316–324. doi: 10.1016/j.jcis.2008.09.066. [DOI] [PubMed] [Google Scholar]
- 29.Streeter JE, Gessner R, Miles I, Dayton PA. Improving sensitivity in ultrasound molecular imaging by tailoring contrast agent size distribution: in vivo studies. Mol Imaging. 2010;9:87–95. [PMC free article] [PubMed] [Google Scholar]
- 30.Talu E, Hettiarachchi K, Zhao S, Powell RL, Lee AP, Longo ML, Dayton PA. Tailoring the size distribution of ultrasound contrast agents: possible method for improving sensitivity in molecular imaging. Mol Imaging. 2007;6:384–392. [PMC free article] [PubMed] [Google Scholar]
- 31.Chomas JE, Dayton P, May D, Ferrara K. Threshold of fragmentation for ultrasonic contrast agents. Journal of Biomedical Optics. 2001;6:141–150. doi: 10.1117/1.1352752. [DOI] [PubMed] [Google Scholar]
- 32.Choi JJ, Feshitan JA, Baseri B, Wang S, Tung YS, Borden MA, Konofagou EE. Microbubble-size dependence of focused ultrasound-induced blood-brain barrier opening in mice in vivo. IEEE Trans Biomed Eng. 2010;57:145–154. doi: 10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samiotaki G, Vlachos F, Tung YS, Konofagou EE. A quantitative pressure and microbubble-size dependence study of focused ultrasound-induced blood-brain barrier opening reversibility in vivo using MRI. Magn Reson Med. 2012;67:769–777. doi: 10.1002/mrm.23063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talu E, Lozano MM, Powell RL, Dayton PA, Longo ML. Long-term stability by lipid coating monodisperse microbubbles formed by a flow-focusing device. Langmuir. 2006;22:9487–9490. doi: 10.1021/la062095+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hettiarachchi K, Talu E, Longo ML, Dayton PA, Lee AP. On-chip generation of microbubbles as a practical technology for manufacturing contrast agents for ultrasonic imaging. Lab Chip. 2007;7:463–468. doi: 10.1039/b701481n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farook U, Zhang HB, Edirisinghe MJ, Stride E, Saffari N. Preparation of microbubble suspensions by co-axial electrohydrodynamic atomization. Medical Engineering & Physics. 2007;29:749–754. doi: 10.1016/j.medengphy.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Goertz DE, Frijlink ME, Voormolen MM, de Jong N, van der Steen AF. High frequency attenuation measurements of lipid encapsulated contrast agents. Ultrasonics. 2006;44(Suppl 1):e131–e134. doi: 10.1016/j.ultras.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Talu E, Powell RL, Longo ML, Dayton PA. Needle size and injection rate impact microbubble contrast agent population. Ultrasound Med Biol. 2008;34:1182–1185. doi: 10.1016/j.ultrasmedbio.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jong N, Hoff L, Skotland T, Bom N. Absorption and scatter of encapsulated gas filled microspheres: theoretical considerations and some measurements. Ultrasonics. 1992;31:175–181. doi: 10.1016/0041-624x(92)90041-j. [DOI] [PubMed] [Google Scholar]
- 40.de Jong N, Frinking PJA, Bouakaz A, Goorden M, Schourmans T, Xu JP, Mastik F. Optical imaging of contrast agent microbubbles in an ultrasound field with a 100-MHz camera. Ultrasound in Medicine and Biology. 2000;26:487–492. doi: 10.1016/s0301-5629(99)00159-3. [DOI] [PubMed] [Google Scholar]
- 41.Chomas J, Dayton P, May D, Allen J, Klibanov A, Ferrara K. Optical observation of contrast agent destruction. Appl Phys Lett. 2000;77:1056–1058. [Google Scholar]
- 42.Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. Mechanisms of contrast agent destruction. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2001;48:232–248. doi: 10.1109/58.896136. [DOI] [PubMed] [Google Scholar]
- 43.Dayton PA, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–1201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 44.Dayton PA, Morgan KE, Klibanov ALS, Brandenburger G, Nightingale KR, Ferrara KW. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 1997;44:1264–1277. [Google Scholar]
- 45.Gessner RC, Streeter JE, Kothadia R, Feingold S, Dayton PA. An in vivo validation of the application of acoustic radiation force to enhance the diagnostic utility of molecular imaging using 3-d ultrasound. Ultrasound Med Biol. 2012;38:651–660. doi: 10.1016/j.ultrasmedbio.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rychak JJ, Klibanov AL, Ley KF, Hossack JA. Enhanced targeting of ultrasound contrast agents using acoustic radiation force. Ultrasound in Medicine and Biology. 2007;33:1132–1139. doi: 10.1016/j.ultrasmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. A method for radiation-force localized drug delivery using gas-filled lipospheres. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:822–831. doi: 10.1109/tuffc.2004.1320741. [DOI] [PubMed] [Google Scholar]
- 48.Kheirolomoom A, Dayton PA, Lum AFH, Little E, Paoli EE, Zheng HR, Ferrara KW. Acoustically-active microbubbles conjugated to liposomes: Characterization of a proposed drug delivery vehicle. Journal of Controlled Release. 2007;118:275–284. doi: 10.1016/j.jconrel.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lum AFH, Borden MA, Dayton PA, Kruse DE, Simon SI, Ferrara KW. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. Journal Of Controlled Release. 2006;111:128–134. doi: 10.1016/j.jconrel.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mullin L, Gessner R, Kwan J, Kaya M, Borden MA, Dayton PA. Effect of anesthesia carrier gas on in vivo circulation times of ultrasound microbubble contrast agents in rats. Contrast Media Mol Imaging. 2011;6:126–131. doi: 10.1002/cmmi.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perkins AC, Frier M, Hindle AJ, Blackshaw PE, Bailey SE, Hebden JM, Middleton SM, Wastie ML. Human biodistribution of an ultrasound contrast agent (Quantison) by radiolabelling and gamma scintigraphy. Br J Radiol. 1997;70:603–611. doi: 10.1259/bjr.70.834.9227254. [DOI] [PubMed] [Google Scholar]
- 52.Tartis MS, Kruse DE, Zheng H, Zhang H, Kheirolomoom A, Marik J, Ferrara KW. Dynamic microPET imaging of ultrasound contrast agents and lipid delivery. J Control Release. 2008;131:160–166. doi: 10.1016/j.jconrel.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laing ST, Moody MR, Kim H, Smulevitz B, Huang SL, Holland CK, McPherson DD, Klegerman ME. Thrombolytic efficacy of tissue plasminogen activator-loaded echogenic liposomes in a rabbit thrombus model. Thromb Res. 2012;130:629–635. doi: 10.1016/j.thromres.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nahire R, Paul S, Scott MD, Singh RK, Muhonen WW, Shabb J, Gange KN, Srivastava DK, Sarkar K, Mallik S. Ultrasound enhanced matrix metalloproteinase-9 triggered release of contents from echogenic liposomes. Mol Pharm. 2012;9:2554–2564. doi: 10.1021/mp300165s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radhakrishnan K, Haworth KJ, Huang SL, Klegerman ME, McPherson DD, Holland CK. Stability of echogenic liposomes as a blood pool ultrasound contrast agent in a physiologic flow phantom. Ultrasound Med Biol. 2012;38:1970–1981. doi: 10.1016/j.ultrasmedbio.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanza GM, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy. Curr Probl Cardiol. 2003;28:625–653. doi: 10.1016/j.cpcardiol.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 57.Lanza GM, Winter PM, Caruthers SD, Hughes MS, Hu G, Schmieder AH, Wickline SA. Theragnostics for tumor and plaque angiogenesis with perfluorocarbon nanoemulsions. Angiogenesis. 2010;13:189–202. doi: 10.1007/s10456-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kripfgans OD, Fowlkes JB, Miller DL, Eldevik OP, Carson PL. Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound in Medicine and Biology. 2000;26:1177–1189. doi: 10.1016/s0301-5629(00)00262-3. [DOI] [PubMed] [Google Scholar]
- 59.Sheeran PS, Dayton PA. Phase-change contrast agents for imaging and therapy. Curr Pharm Des. 2012;18:2152–2165. doi: 10.2174/138161212800099883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Neill BE, Rapoport N. Phase-shift, stimuli-responsive drug carriers for targeted delivery. Ther Deliv. 2011;2:1165–1187. doi: 10.4155/tde.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rapoport N. Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:492–510. doi: 10.1002/wnan.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shankar PM, Dala Krishna P, Newhouse VL. Advantages of subharmonic over second harmonic backscatter for contrast-to-tissue echo enhancement. Ultrasound Med Biol. 1998;24:395–399. doi: 10.1016/s0301-5629(97)00262-7. [DOI] [PubMed] [Google Scholar]
- 63.Shi WT, Forsberg F, Hall AL, Chiao RY, Liu JB, Miller S, Thomenius KE, Wheatley MA, Goldberg BB. Subharmonic imaging with microbubble contrast agents: initial results. Ultrason Imaging. 1999;21:79–94. doi: 10.1177/016173469902100201. [DOI] [PubMed] [Google Scholar]
- 64.Chomas J, Dayton P, May D, Ferrara K. Nondestructive subharmonic imaging. IEEE Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2002;49:883–892. doi: 10.1109/tuffc.2002.1020158. [DOI] [PubMed] [Google Scholar]
- 65.Burns PN. Harmonic imaging with ultrasound contrast agents. Clinical Radiology. 1996;51:50–55. [PubMed] [Google Scholar]
- 66.Schwarz KQ, Chen X, Steinmetz S, Phillips D. Harmonic imaging with Levovist. J Am Soc Echocardiogr. 1997;10:1–10. doi: 10.1016/s0894-7317(97)80027-2. [DOI] [PubMed] [Google Scholar]
- 67.Bouakaz A, Frigstad S, Ten Cate FJ, de Jong N. Super harmonic imaging: a new imaging technique for improved contrast detection. Ultrasound Med Biol. 2002;28:59–68. doi: 10.1016/s0301-5629(01)00460-4. [DOI] [PubMed] [Google Scholar]
- 68.van Neer PL, Danilouchkine MG, Verweij MD, Demi L, Voormolen MM, van der Steen AF, de Jong N. Comparison of fundamental, second harmonic, and superharmonic imaging: a simulation study. J Acoust Soc Am. 2011;130:3148–3157. doi: 10.1121/1.3643815. [DOI] [PubMed] [Google Scholar]
- 69.Kruse DE, Ferrara KW. A new imaging strategy using wideband transient response of ultrasound contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:1320–1329. doi: 10.1109/tuffc.2005.1509790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao S, Kruse DE, Ferrara KW, Dayton PA. Selective imaging of adherent targeted ultrasound contrast agents. Phys Med Biol. 2007;52:2055–2072. doi: 10.1088/0031-9155/52/8/002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gessner R, Lukacs M, Lee M, Cherin EF, Foster S, Dayton PA. High-resolution, high-contrast ultrasound imaging using a prototype dual-frequency transducer: in-vitro and in-vivo studies. Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 2010 doi: 10.1109/TUFFC.2010.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harvey CJ, Blomley MJ, Eckersley RJ, Heckemann RA, Butler-Barnes J, Cosgrove DO. Pulse-inversion mode imaging of liver specific microbubbles: improved detection of subcentimetre metastases. Lancet. 2000;355:807–808. doi: 10.1016/S0140-6736(99)04545-6. [DOI] [PubMed] [Google Scholar]
- 73.Phillips P, Gardner E. Contrast-agent detection and quantification. Eur Radiol. 2004;14(Suppl 8):P4–P10. [PubMed] [Google Scholar]
- 74.Simpson DH, Chin CT, Burns PN. Pulse inversion Doppler: A new method for detecting nonlinear echoes from microbubble contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1999;46:372–382. doi: 10.1109/58.753026. [DOI] [PubMed] [Google Scholar]
- 75.Verbeek XA, Ledoux LA, Willigers JM, Brands PJ, Hoeks AP. Experimental investigation of the pulse inversion technique for imaging ultrasound contrast agents. J Acoust Soc Am. 2000;107:2281–2290. doi: 10.1121/1.428508. [DOI] [PubMed] [Google Scholar]
- 76.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation. 1998;97:473–483. doi: 10.1161/01.cir.97.5.473. [DOI] [PubMed] [Google Scholar]
- 77.Cosgrove D, Eckersley R, Blomley M, Harvey C. Quantification of blood flow. Eur Radiol. 2001;11:1338–1344. doi: 10.1007/s003300100985. [DOI] [PubMed] [Google Scholar]
- 78.Krix M, Kiessling F, Vosseler S, Farhan N, Mueller MM, Bohlen P, Fusenig NE, Delorme S. Sensitive noninvasive monitoring of tumor perfusion during antiangiogenic therapy by intermittent bolus-contrast power Doppler sonography. Cancer Research. 2003;63:8264–8270. [PubMed] [Google Scholar]
- 79.Kogan P, Johnson KA, Feingold S, Garrett N, Guracar I, Arendshorst WJ, Dayton PA. Validation of dynamic contrast-enhanced ultrasound in rodent kidneys as an absolute quantitative method for measuring blood perfusion. Ultrasound Med Biol. 2010;37:900–908. doi: 10.1016/j.ultrasmedbio.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pollard RE, Dayton PA, Watson KD, Hu X, Guracar IM, Ferrara KW. Motion corrected cadence CPS ultrasound for quantifying response to vasoactive drugs in a rat kidney model. Urology. 2009;74:675–681. doi: 10.1016/j.urology.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–1140. doi: 10.1016/s0735-1097(00)01210-9. [DOI] [PubMed] [Google Scholar]
- 82.Chomas JE, Pollard RE, Sadlowski AR, Griffey SM, Wisner ER, Ferrara KW. Contrast-enhanced US of microcirculation of superficially implanted tumors in rats. Radiology. 2003;229:439–446. doi: 10.1148/radiol.2292020536. [DOI] [PubMed] [Google Scholar]
- 83.Foster FS, Burns PN, Simpson DH, Wilson SR, Christopher DA, Goertz DE. Ultrasound for the visualization and quantification of tumor microcirculation. Cancer Metastasis Rev. 2000;19:131–138. doi: 10.1023/a:1026541510549. [DOI] [PubMed] [Google Scholar]
- 84.Lassau N, Chami L, Chebil M, Benatsou B, Bidault S, Girard E, Abboud G, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US) and anti-angiogenic treatments. Discov Med. 2011;11:18–24. [PubMed] [Google Scholar]
- 85.Pollard RE, Broumas AR, Wisner ER, Vekich SV, Ferrara KW. Quantitative contrast enhanced ultrasound and CT assessment of tumor response to antiangiogenic therapy in rats. Ultrasound Med Biol. 2007;33:235–245. doi: 10.1016/j.ultrasmedbio.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 86.Pollard RE, Sadlowski AR, Bloch SH, Murray L, Wisner ER, Griffey S, Ferrara KW. Contrast-assisted destruction-replenishment ultrasound for the assessment of tumor microvasculature in a rat model. Technol Cancer Res Treat. 2002;1:459–470. doi: 10.1177/153303460200100606. [DOI] [PubMed] [Google Scholar]
- 87.Sirsi SR, Flexman ML, Vlachos F, Huang J, Hernandez SL, Kim HK, Johung TB, Gander JW, Reichstein AR, Lampl BS, et al. Contrast ultrasound imaging for identification of early responder tumor models to anti-angiogenic therapy. Ultrasound Med Biol. 2012;38:1019–1029. doi: 10.1016/j.ultrasmedbio.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watson KD, Hu X, Lai CY, Lindfors HA, Hu-Lowe DD, Tuthill TA, Shalinsky DR, Ferrara KW. Novel ultrasound and DCE-MRI analyses after antiangiogenic treatment with a selective VEGF receptor inhibitor. Ultrasound Med Biol. 2011;37:909–921. doi: 10.1016/j.ultrasmedbio.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Averkiou M, Lampaskis M, Kyriakopoulou K, Skarlos D, Klouvas G, Strouthos C, Leen E. Quantification of tumor microvascularity with respiratory gated contrast enhanced ultrasound for monitoring therapy. Ultrasound Med Biol. 2009;36:68–77. doi: 10.1016/j.ultrasmedbio.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 90.Lassau N, Koscielny S, Albiges L, Chami L, Benatsou B, Chebil M, Roche A, Escudier BJ. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res. 2010;16:1216–1225. doi: 10.1158/1078-0432.CCR-09-2175. [DOI] [PubMed] [Google Scholar]
- 91.Lavisse S, Lejeune P, Rouffiac V, Elie N, Bribes E, Demers B, Vrignaud P, Bissery MC, Brule A, Koscielny S, et al. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008;43:100–111. doi: 10.1097/RLI.0b013e3181577cfc. [DOI] [PubMed] [Google Scholar]
- 92.Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, Atri M, Bjarnason GA, Burns PN. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology. 2011;260:581–590. doi: 10.1148/radiol.11101893. [DOI] [PubMed] [Google Scholar]
- 93.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 94.Kennedy JE, ter Haar GR, Wu F, Gleeson FV, Roberts IS, Middleton MR, Cranston D. Contrast-enhanced ultrasound assessment of tissue response to high-intensity focused ultrasound. Ultrasound Med Biol. 2004;30:851–854. doi: 10.1016/j.ultrasmedbio.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 95.Burke CW, Klibanov AL, Sheehan JP, Price RJ. Inhibition of glioma growth by microbubble activation in a subcutaneous model using low duty cycle ultrasound without significant heating. J Neurosurg. 114:1654–1661. doi: 10.3171/2010.11.JNS101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dayton PA, Ferrara KW. Targeted imaging using ultrasound. Journal of Magnetic Resonance Imaging. 2002;16:362–377. doi: 10.1002/jmri.10173. [DOI] [PubMed] [Google Scholar]
- 97.Lanza GM, Wickline SA. Targeted ultrasonic contrast agents for molecular imaging and therapy. Progress in Cardiovascular Diseases. 2001;44:13–31. doi: 10.1053/pcad.2001.26440. [DOI] [PubMed] [Google Scholar]
- 98.Lindner JR. Molecular imaging with contrast ultrasound and targeted microbubbles. J Nucl Cardiol. 2004;11:215–221. doi: 10.1016/j.nuclcard.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 100.Lanza GM, Wallace KD, Fischer SE, Christy DH, Scott MJ, Trousil RL, Cacheris WP, Miller JG, Gaffney PJ, Wickline SA. High-frequency ultrasonic detection of thrombi with a targeted contrast system. Ultrasound Med Biol. 1997;23:863–870. doi: 10.1016/s0301-5629(97)00046-x. [DOI] [PubMed] [Google Scholar]
- 101.Villanueva FS, Lu E, Bowry S, Kilic S, Tom E, Wang J, Gretton J, Pacella JJ, Wagner WR. Myocardial ischemic memory imaging with molecular echocardiography. Circulation FIELD Full Journal Title:Circulation. 2007;115:345–352. doi: 10.1161/CIRCULATIONAHA.106.633917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104:2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 103.Deshpande N, Lutz AM, Ren Y, Foygel K, Tian L, Schneider M, Pai R, Pasricha PJ, Willmann JK. Quantification and monitoring of inflammation in murine inflammatory bowel disease with targeted contrast-enhanced US. Radiology. 2012;262:172–180. doi: 10.1148/radiol.11110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ellegala DB, Poi HL, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME, Sklenar J, Lindner JR. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta(3) Circulation. 2003;108:336–341. doi: 10.1161/01.CIR.0000080326.15367.0C. [DOI] [PubMed] [Google Scholar]
- 105.Lyshchik A, Fleischer AC, Huamani J, Hallahan DE, Brissova M, Gore JC. Molecular imaging of vascular endothelial growth factor receptor 2 expression using targeted contrast-enhanced high-frequency ultrasonography. J Ultrasound Med. 2007;26:1575–1586. doi: 10.7863/jum.2007.26.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Streeter JE, Gessner RC, Tsuruta J, Feingold S, Dayton PA. Assessment of Molecular Imaging of Angiogenesis with Three-Dimensional Ultrasonography. Mol Imaging. 2011 [PMC free article] [PubMed] [Google Scholar]
- 107.Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323–330. doi: 10.1158/1078-0432.CCR-06-1313. [DOI] [PubMed] [Google Scholar]
- 108.Streeter JE, Herrera-Loeza SG, Neel NF, Yeh JJ, Dayton PA. A Comparative Evaluation of Ultrasound Molecular imaging, Perfusion Imaging, and Volume Measurements in Evaluating Response to Therapy in Patient-Derived Xenografts. Technology for Cancer Research and Treatment. 2013 Jan 25; doi: 10.7785/tcrt.2012.500321. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eisenbrey JR, Joshi N, Dave JK, Forsberg F. Assessing algorithms for defining vascular architecture in subharmonic images of breast lesions. Phys Med Biol. 2011;56:919–930. doi: 10.1088/0031-9155/56/4/003. [DOI] [PubMed] [Google Scholar]
- 110.Shiraishi J, Sugimoto K, Moriyasu F, Kamiyama N, Doi K. Computer-aided diagnosis for the classification of focal liver lesions by use of contrast-enhanced ultrasonography. Phys Med Biol. 2008;35:1734–1746. doi: 10.1118/1.2900109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sugimoto K, Shiraishi J, Moriyasu F, Ichimura S, Metoki R, Doi K. Analysis of intrahepatic vascular morphological changes of chronic liver disease for assessment of liver fibrosis stages by micro-flow imaging with contrast-enhanced ultrasound: preliminary experience. Eur Radiology. 2010;20:2749–2757. doi: 10.1007/s00330-010-1852-1. [DOI] [PubMed] [Google Scholar]
- 112.Molinari F, Mantovani A, Deandrea M, Limone P, Garberoglio R, Suri JS. Characterization of single thyroid nodules by contrast-enhanced 3-D ultrasound. Ultrasound Med Biol. 2010;36:1616–1625. doi: 10.1016/j.ultrasmedbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 113.Gessner RC, Aylward SR, Dayton PA. Mapping microvasculature with acoustic angiography yields quantifiable differences between healthy and tumor-bearing tissue volumes in a rodent model. Radiology. 2012;264:733–740. doi: 10.1148/radiol.12112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bullitt E, Ewend M, Vredenburgh J, Friedman A, Lin W, Wilber K, Zeng D, Aylward SR, Reardon D. Computerized assessment of vessel morphological changes during treatment of glioblastoma multiforme: report of a case imaged serially by MRA over four years. Neuroimage. 2009;47(Suppl 2):T143–T151. doi: 10.1016/j.neuroimage.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bullitt E, Ewend MG, Aylward S, Lin W, Gerig G, Joshi S, Jung I, Muller K, Smith JK. Abnormal vessel tortuosity as a marker of treatment response of malignant gliomas: preliminary report. Technol Cancer Res Treat. 2004;3:577–584. doi: 10.1177/153303460400300607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 117.Seip R, Chin CT, Hall CS, Raju BI, Ghanem A, Tiemann K. Targeted ultrasound-mediated delivery of nanoparticles: on the development of a new HIFU-based therapy and imaging device. IEEE Trans Biomed Eng. 2010;57:61–70. doi: 10.1109/TBME.2009.2028874. [DOI] [PubMed] [Google Scholar]
- 118.Huang Y, Hynynen K. MR-guided focused ultrasound for brain ablation and blood-brain barrier disruption. Methods Mol Biol. 2011;711:579–593. doi: 10.1007/978-1-61737-992-5_30. [DOI] [PubMed] [Google Scholar]
- 119.Kang ST, Yeh CK. Ultrasound microbubble contrast agents for diagnostic and therapeutic applications: current status and future design. Chang Gung Med J. 2012;35:125–139. doi: 10.4103/2319-4170.106159. [DOI] [PubMed] [Google Scholar]
- 120.Geis NA, Katus HA, Bekeredjian R. Microbubbles as a vehicle for gene and drug delivery: current clinical implications and future perspectives. Curr Pharm Des. 2012;18:2166–2183. doi: 10.2174/138161212800099946. [DOI] [PubMed] [Google Scholar]
- 121.Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal, particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264–1267. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 122.Karshafian R, Bevan PD, Williams R, Samac S, Burns PN. Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol. 2009;35:847–860. doi: 10.1016/j.ultrasmedbio.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 123.Wang F, Cheng Y, Mei J, Song Y, Yang YQ, Liu Y, Wang Z. Focused ultrasound microbubble destruction-mediated changes in blood-brain barrier permeability assessed by contrast-enhanced magnetic resonance imaging. J Ultrasound Med. 2009;28:1501–1509. doi: 10.7863/jum.2009.28.11.1501. [DOI] [PubMed] [Google Scholar]
- 124.Choi JJ, Wang S, Brown TR, Small SA, Duff KE, Konofagou EE. Noninvasive and transient blood-brain barrier opening in the hippocampus of Alzheimer's double transgenic mice using focused ultrasound. Ultrason Imaging. 2008;30:189–200. doi: 10.1177/016173460803000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jordao JF, Ayala-Grosso CA, Markham K, Huang Y, Chopra R, McLaurin J, Hynynen K, Aubert I. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PLoS One. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fokong S, Theek B, Wu Z, Koczera P, Appold L, Jorge S, Resch-Genger U, van Zandvoort M, Storm G, Kiessling F, et al. Image-guided, targeted and triggered drug delivery to tumors using polymer-based microbubbles. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 127.Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA, Hohmeier HE, Newgard CB, Grayburn PA. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci U S A. 2006;103:8469–8474. doi: 10.1073/pnas.0602921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Caskey CF, Stieger SM, Qin S, Dayton PA, Ferrara KW. Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall. The Journal of the Acoustical Society of America. 2007;122:1191–1200. doi: 10.1121/1.2747204. [DOI] [PubMed] [Google Scholar]
- 129.Chen H, Kreider W, Brayman AA, Bailey MR, Matula TJ. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett. 2011;106 doi: 10.1103/PhysRevLett.106.034301. 034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ohl CD, Arora M, Ikink R, de Jong N, Versluis M, Delius M, Lohse D. Sonoporation from jetting cavitation bubbles. Biophys J. 2006;91:4285–4295. doi: 10.1529/biophysj.105.075366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stieger SM, Caskey CF, Adamson RH, Qin S, Curry FR, Wisner ER, Ferrara KW. Enhancement of vascular permeability with low-frequency contrast-enhanced ultrasound in the chorioallantoic membrane model. Radiology. 2007;243:112–121. doi: 10.1148/radiol.2431060167. [DOI] [PubMed] [Google Scholar]
- 132.van Wamel A, Kooiman K, Harteveld M, Emmer M, ten Cate FJ, Versluis M, de Jong N. Vibrating microbubbles poking individual cells: drug transfer into cells via sonoporation. J Control Release. 2006;112:149–155. doi: 10.1016/j.jconrel.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 133.Prentice P, Alfred Cuschieri, Kishan Dholakia, Mark Prausnitz, Paul Campbell. Membrane disruption by optically controlled microbubble cavitation. Nature Physics. 2005;1:107–110. [Google Scholar]
- 134.Zhou Y, Yang K, Cui J, Ye JY, Deng CX. Controlled permeation of cell membrane by single bubble acoustic cavitation. J Control Release. 2012;157:103–111. doi: 10.1016/j.jconrel.2011.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bao SP, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound in Medicine and Biology. 1997;23:953–959. doi: 10.1016/s0301-5629(97)00025-2. [DOI] [PubMed] [Google Scholar]
- 136.Miller DL, Quddus J. Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:10179–10184. doi: 10.1073/pnas.180294397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Keyhani K, Guzman HR, Parsons A, Lewis TN, Prausnitz MR. Intracellular drug delivery using low-frequency ultrasound: quantification of molecular uptake and cell viability. Pharm Res. 2001;18:1514–1520. doi: 10.1023/a:1013066027759. [DOI] [PubMed] [Google Scholar]
- 138.Forbes MM, Steinberg RL, O'Brien WD., Jr Frequency-dependent evaluation of the role of definity in producing sonoporation of Chinese hamster ovary cells. J Ultrasound Med. 2011;30:61–69. doi: 10.7863/jum.2011.30.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound microbubbles: therapeutic application in drug/gene delivery. J Control Release. 2006;114:89–99. doi: 10.1016/j.jconrel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 140.Schneider M. Molecular imaging and ultrasound-assisted drug delivery. J Endourol. 2008;22:795–802. doi: 10.1089/end.2007.9821. [DOI] [PubMed] [Google Scholar]
- 141.Xiong X, Zhao F, Shi M, Yang H, Liu Y. Polymeric microbubbles for ultrasonic molecular imaging and targeted therapeutics. J Biomater Sci Polym Ed. 2011;22:417–428. doi: 10.1163/092050610X540440. [DOI] [PubMed] [Google Scholar]
- 142.Lentacker I, De Smedt SC, Sanders NN. Drug loaded microbubble design for ultrasound triggered delivery. Soft Matter. 2009;5:2161. [Google Scholar]
- 143.Phillips LC, Klibanov AL, Wamhoff BR, Hossack JA. Localized ultrasound enhances delivery of rapamycin from microbubbles to prevent smooth muscle proliferation. J Control Release. 2011;154:42–49. doi: 10.1016/j.jconrel.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hettiarachchi K, Zhang S, Feingold S, Lee AP, Dayton PA. Controllable microfluidic synthesis of multiphase drug-carrying lipospheres for site-targeted therapy. Biotechnol Prog. 2009;25:938–945. doi: 10.1002/btpr.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu YQ. Acoustically active lipospheres containing paclitaxel - A new therapeutic ultrasound contrast agent. Investigative Radiology. 1998;33:886–892. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 146.Tartis MS, McCallan J, Lum AF, Labell R, Stieger SM, Matsunaga TO, Ferrara KW. Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol. 2006;32:1771–1780. doi: 10.1016/j.ultrasmedbio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 147.Cochran MC, Eisenbrey J, Ouma RO, Soulen M, Wheatley MA. Doxorubicin and paclitaxel loaded microbubbles for ultrasound triggered drug delivery. Int J Pharm. 414:161–170. doi: 10.1016/j.ijpharm.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Geers B, Lentacker I, Cool S, Demeester J, De Smedt SC, Sanders NN. Ultrasound responsive doxorubicin-loaded microbubbles; towards an easy applicable drug delivery platform. J Control Release. 148:e59–e60. doi: 10.1016/j.jconrel.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 149.Hou Z, Li L, Zhan C, Zhu P, Chang D, Jiang Q, Ye S, Yang X, Li Y, Xie L, et al. Preparation and in vitro evaluation of an ultrasound-triggered drug delivery system: 10-Hydroxycamptothecin loaded PLA microbubbles. Ultrasonics. doi: 10.1016/j.ultras.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 150.May DJ, Allen JS, Ferrara KW. Dynamics and fragmentation of thick-shelled microbubbles. Ieee Transactions on Ultrasonics Ferroelectrics and Frequency Control. 2002;49:1400–1410. doi: 10.1109/tuffc.2002.1041081. [DOI] [PubMed] [Google Scholar]
- 151.Borden MA, Caskey CF, Little E, Gillies RJ, Ferrara KW. DNA and polylysine adsorption and multilayer construction onto cationic lipid-coated microbubbles. Langmuir. 2007;23:9401–9408. doi: 10.1021/la7009034. [DOI] [PubMed] [Google Scholar]
- 152.Phillips LC, Klibanov AL, Bowles DK, Ragosta M, Hossack JA, Wamhoff BR. Focused in vivo delivery of plasmid DNA to the porcine vascular wall via intravascular ultrasound destruction of microbubbles. J Vasc Res. 2010;47:270–274. doi: 10.1159/000258905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–1239. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 154.Tiukinhoy SD, Mahowald ME, Shively VP, Nagaraj A, Kane BJ, Klegerman ME, MacDonald RC, McPherson DD, Matsumura JS. Development of echogenic, plasmid-incorporated, tissue-targeted cationic liposomes that can be used for directed gene delivery. Invest Radiol. 2000;35:732–738. doi: 10.1097/00004424-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 155.Tlaxca JL, Anderson CR, Klibanov AL, Lowrey B, Hossack JA, Alexander JS, Lawrence MB, Rychak JJ. Analysis of in vitro transfection by sonoporation using cationic and neutral microbubbles. Ultrasound Med Biol. 2010;36:1907–1918. doi: 10.1016/j.ultrasmedbio.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nomikou N, Tiwari P, Trehan T, Gulati K, McHale AP. Studies on neutral, cationic and biotinylated cationic microbubbles in enhancing ultrasound-mediated gene delivery in vitro and in vivo. Acta Biomater. 2012;8:1273–1280. doi: 10.1016/j.actbio.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 157.Wang D, Robinson DR, Kwon GS, Samuel J. Encapsulation of plasmid DNA in biodegradable poly(D, L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. J Control Release. 1999;57:9–18. doi: 10.1016/s0168-3659(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 158.Seemann S, Hauff P, Schultze-Mosgau M, Lehmann C, Reszka R. Pharmaceutical evaluation of gas-filled microparticles as gene delivery system. Pharm Res. 2002;19:250–257. doi: 10.1023/a:1014430631844. [DOI] [PubMed] [Google Scholar]
- 159.Xie F, Tsutsui JM, Lof J, Unger EC, Johanning J, Culp WC, Matsunaga T, Porter TR. Effectiveness of lipid microbubbles and ultrasound in declotting thrombosis. Ultrasound Med Biol. 2005;31:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 160.Perren F, Loulidi J, Poglia D, Landis T, Sztajzel R. Microbubble potentiated transcranial duplex ultrasound enhances IV thrombolysis in acute stroke. J Thromb Thrombolysis. 2008;25:219–223. doi: 10.1007/s11239-007-0044-6. [DOI] [PubMed] [Google Scholar]
- 161.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, et al. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 162.Alexandrov AV. Ultrasound enhancement of fibrinolysis. Stroke. 2009;40:S107–S110. doi: 10.1161/STROKEAHA.108.530931. [DOI] [PubMed] [Google Scholar]
- 163.Kutty S, Xie F, Gao S, Drvol LK, Lof J, Fletcher SE, Radio SJ, Danford DA, Hammel JM, Porter TR. Sonothrombolysis of intra-catheter aged venous thrombi using microbubble enhancement and guided three-dimensional ultrasound pulses. J Am Soc Echocardiogr. 2010;23:1001–1006. doi: 10.1016/j.echo.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xie F, Slikkerveer J, Gao S, Lof J, Kamp O, Unger E, Radio S, Matsunaga T, Porter TR. Coronary and microvascular thrombolysis with guided diagnostic ultrasound and microbubbles in acute ST segment elevation myocardial infarction. J Am Soc Echocardiogr. 2011;24:1400–1408. doi: 10.1016/j.echo.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen W-S, Lafon C, Matula TJ, Vaezy S, Crum LA. Mechanisms of lesion formation in high intensity focused ultrasound therapy. Acoustics Research Letters Online. 2003;4:41. [Google Scholar]
- 166.Coussios CC, Farny CH, Haar GT, Roy RA. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU) Int J Hyperthermia. 2007;23:105–120. doi: 10.1080/02656730701194131. [DOI] [PubMed] [Google Scholar]
- 167.Holt RG, Roy RA. Measurements of bubble-enhanced heating from focused, MHz-frequency ultrasound in a tissue-mimicking material. Ultrasound Med Biol. 2001;27:1399–1412. doi: 10.1016/s0301-5629(01)00438-0. [DOI] [PubMed] [Google Scholar]
- 168.Tran BC, Seo J, Hall TL, Fowlkes JB, Cain CA. Microbubble-enhanced cavitation for noninvasive ultrasound surgery. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50:1296–1304. doi: 10.1109/tuffc.2003.1244746. [DOI] [PubMed] [Google Scholar]
- 169.Tung YS, Liu HL, Wu CC, Ju KC, Chen WS, Lin WL. Contrast-agent-enhanced ultrasound thermal ablation. Ultrasound Med Biol. 2006;32:1103–1110. doi: 10.1016/j.ultrasmedbio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 170.Hynynen K. The threshold for thermally significant cavitation in dog's thigh muscle in vivo. Ultrasound Med Biol. 1991;17:157–169. doi: 10.1016/0301-5629(91)90123-e. [DOI] [PubMed] [Google Scholar]
- 171.Peng S, Xiong Y, Li K, He M, Deng Y, Chen L, Zou M, Chen W, Wang Z, He J, et al. Clinical utility of a microbubble-enhancing contrast ("SonoVue") in treatment of uterine fibroids with high intensity focused ultrasound: A retrospective study. Eur J Radiol. 2012;81:3832–3838. doi: 10.1016/j.ejrad.2012.04.030. [DOI] [PubMed] [Google Scholar]