Abstract
Objective
To examine the relationship between measures of sleep quality and cognitive performance in HIV+ individuals stable on combination anti-retroviral therapy.
Design
Multi-method assessments of sleep quality, patterns, and cognitive performance were assessed in a predominantly Black HIV+ cohort.
Methods
Sleep quality and patterns were characterized in 36 subjects by: polysomnogram, two-week actigraphy monitoring, and validated sleep questionnaires. Cognitive performance was assessed with a battery of neuropsychological tests.
Results
The majority of participants were cognitively impaired [based on Frascati (75%) criteria]. Self-reported mean scores on the Pittsburgh Sleep Quality Index and the Insomnia Severity Scale suggested poor sleep quality. Better cognitive performance, particularly on tasks of attention, frontal/executive function, and psychomotor/motor speed, was associated with polysomnogram sleep indices (i.e., reduced wake-after-sleep (WASO), greater sleep efficiency, greater sleep latency, and greater total sleep time). Thirty-seven percent of participants had sleep patterns suggestive of chronic partial sleep deprivation which was associated with significantly worse performance on the Digit Symbol test (p = 0.006), non-dominant pegboard (p = 0.043), and verbal fluency tests (p = 0.044).
Conclusion
Our results suggest that compromised sleep quality and duration may have a significant impact on cognitive performance in HIV+ individuals. Future studies are warranted to determine the utility of sleep quality and quantity indices as potential predictive biomarkers for development and progression of future HIV-associated neurocognitive disorder.
Keywords: HIV, sleep, sleep disorders, cognition, quality of life
Introduction
Combination anti-retroviral therapy (cART) has led to dramatic increases in the survival rate of individuals infected with human immunodeficiency virus (HIV). Although significant strides have been made in managing several acute and life threatening HIV-related conditions, significant challenges remain in managing the chronic HIV-specific and non-specific conditions facing an aging HIV patient population. HIV-associated neurocognitive disorder (HAND) prevalence remains as high as 50%,1-3 even amongst individuals with optimally-controlled viral loads and immune function due to cART. The importance of identifying predictive biomarkers of HAND now parallels the importance placed on identifying predictive markers of dementia within the general population.1
Sleep disorders have also been recently identified as a common and debilitating condition for HIV+ individuals in the post-cART era with a reported prevalence ranging from 30% to 73%,4 as compared to 10%5 prevalence in the general population. These may be among the earliest complications for all seropositive patients, both symptomatic and asymptomatic.6-8 In addition to sleep complaints, neurocognitive deficits, affective disorders, and substance use/abuse have also been identified as common co-morbid conditions amongst seropositive HIV individuals. Studies in the general population suggest a potential correlation between poor sleep quality and/or quantity with reduced neurocognitive performance,9, 10 ranging from global measures of overall cognition and mood to more specific cognitive tasks related to attention, vigilance, and decision-making skills (including risk-taking behaviors, such as gambling, aggressive driving, and recreational drug use and relapse rate).11-15 Studies specifically looking at this inter-relationship in the HIV+ subpopulation are timely and may help to shed additional insight on the complex interplay between these variables.
Studies in the general population have also shown a great deal of intra- and inter-subject variability in sleep-wake patterns and vulnerability to sleep loss.16 Moreover, sleep characteristics also appear to be affected by cultural, gender, and socioeconomic demographics. For this reason, we designed a prospective study utilizing multi-method sleep assessments and observations across several time points. To our knowledge, this investigation represents the most comprehensive evaluation to-date of sleep-wake patterns in an HIV+ population stable on cART. We hypothesized that sleep disruption severity would be associated with reduced performance on tasks of executive function.
Methods
Thirty-six HIV+ participants stable on cART (with the exception of efavirenz due to its potential sleep-altering effects)17 were recruited at Johns Hopkins Medical Institutions (JHMI) to participate in this IRB-approved study. A full medical evaluation was conducted to ensure that the participant was medically (including a demonstration of HIV viral loads of ≤ 1,000 copies/ml), cognitively, and psychologically (per physician interview or response on validated mood questionnaires) stable to participate. Participants were excluded if currently prescribed opiate medications or if any changes had been made to their pharmacotherapeutic regimen in the last 60 days. Participants were also dropped from the study if they screened positive for recreational drug use at study entry, midpoint, or exit interview. Participants received a validated clinical sleep interview along with validated sleep questionnaires, including the Insomnia Severity Index (ISI)18 and the Epworth Sleepiness Scale (ESS).19 A polysomnogram (PSG) was conducted in the Johns Hopkins Clinical Research Unit followed by 2-week in-home functional assessments with questionnaires and actigraphy monitoring of their sleep and wake activity.
Findings for this report were generated from a larger prospective study evaluating the relationship of sleep and pertinent measures of overall function in a seropositive HIV cohort. The overall protocol for the study is outlined in Table 1; readers can also refer to a previously published report for a more detailed description of the complete research design and sleep methods.20
Table 1.
Study Protocol
| Screen |
| Informed consent |
| Medical records review |
| Urine toxicology screen |
| Neurological exam |
| History & Physical |
| Sleep Assessment |
|
|
| Admission to General Clinical Research Unit (Day 1) |
| Urine toxicology screen |
| Admit to General Clinical Research Center |
| Neurological exam (if not done at screen) |
| History & Physical (if not done at screen) |
| Sleep assessment (if not done at screen) |
| Psychomotor Vigilance Test |
| Sleep and Functional Questionnaire Packet* |
| Polysomnogram |
|
|
| Discharge from General Clinical Research Unit (Day 2) |
| Discharge from research unit |
| Education of Actigraphy Device |
| Quantitative Sensory Testing |
| Epworth Sleepiness Scale |
| Psychomotor Vigilance Test |
|
|
| Mid-Protocol Follow up Visit (1 week after PSG) |
| Urine toxicology |
| Epworth Sleepiness Scale |
| Psychomotor Vigilance Test |
|
|
| Final Visit (2 weeks after PSG) |
| Urine toxicology |
| Neuropsych battery |
| Psychomotor Vigilance Test |
| Epworth Sleepiness Scale |
| Return actigraph |
| Beck Depression Inventory |
| Exit interview regarding results of PSG/sleep assessment |
| Sleep referral as needed |
Neuropsychological Testing
Neurocognitive testing was performed on the final day (day 14) of the protocol and consisted of a comprehensive neuropsychological battery aimed at evaluating several cognitive domains (i.e., psychomotor speed, verbal memory, visual memory, frontal/executive, and motor speed) shown to be sensitive to sleep loss in previous general population studies11, 12, 21 (Table 2).
Table 2.
Neuropsychological Battery
| Cognitive Domain | Test (subtest) |
|---|---|
|
| |
| PSYCHOMOTOR | 1. WAIS-III Digit Symbol54 |
| 2. Trail Making Test A55 | |
|
| |
| VERBAL MEMORY | 3. Hopkins Verbal Learning Test56
|
|
| |
| VISUAL MEMORY | 4. Brief Visual-spatial Learning Test-revised 57
|
|
| |
5. Figure Memory Test 55
|
|
|
| |
| FRONTAL/EXECUTIVE | 6. Wisconsin Card Sorting Test 58 |
|
| |
7. Verbal Fluency 59
|
|
|
| |
| 8. WAIS Letter-Number Sequencing Test 54 | |
|
| |
| Trail Making Test B 55 | |
|
| |
| MOTOR SPEED | 9. Grooved Pegboard 60
|
|
| |
| PREMORBID IQ | 10. National Adult Reading Test (NART) 61 |
The 10 tests above were included in generating the global composite score.
Determination of overall cognitive/neuropsychological functioning was based on a calculated global composite score. Global scores were calculated by averaging the scores from the 10 neuropsychological tests and their subtest scores (see table 2), which were each standardized using Z-scores based on normative data stratified by age and education.22, 23 Normative data, for participants with less than or equal to 12 years of education, were derived from the ALIVE (AIDS Link to Intravenous Experience) study.22-24 For participants with greater than 12 years of education, normative data were based on the Multicenter AIDS Cohort Study (MACS).24 For neuropsychological subtests not included in the ALIVE and the MACS neuropsychological batteries, normative data were based on published data for the Halstead-Reitan battery.25 Using the global neuropsychological score, participants were placed in one of several categories (i.e., normal, Asymptomatic neurocognitive impairment [ANI], Minor neurocognitive disorder [MND], and HIV associated dementia [HAD]) as defined by the Frascati criteria.
Functional measure
In an effort to capture the impact of cognitive deficits on physical functioning, the role functioning items of the Medical Outcomes Study26 were administered to all HIV-seropositive participants.
Statistical Analyses
Descriptive analyses were conducted to characterize each participant’s demographics, health, cognitive functioning, and sleep patterns. Pearson correlations explored potential relationships between the sleep indices and neuropsychological measures. Due to evidence supporting an association between CD-4 nadir and risk of developing HAND,27 partial correlations were conducted to determine whether any significant relationships from our initial correlations remained significant after accounting for CD4 Nadir as well as the HIV-MOS total score. Correlations were 2-tailed with an α level of 0.05. T-tests were conducted to explore whether there were sleep differences between the cognitively unimpaired and impaired HIV-seropositive adults. When Levene’s Test for Equality of Variances was violated, t-tests with unequal variances were conducted. Although several analyses were conducted, the analyses did not control for multiple comparisons, because such an approach increases Type II error or decreases the likelihood for observing a significant relationship.28 Given that the relationship between sleep and cognition in HIV is a relatively unexplored area of research, our study’s objective was to take an exploratory approach, identifying potential relationships which future studies can subsequently investigate using alternative, more conservative approaches. Analyses were performed using the Statistical Package for Social Sciences (SPSS) Version 17.28
Results
Clinical Characteristics
Table 3 shows the demographic, health, and neuropsychological test scores of the sample. Two participants were terminated from the study and excluded from analysis due to positive drug toxicology – one at the mid-protocol follow up visit and the other at the final visit. Participants were between the ages of 38 and 63 years (Mean = 49.89; SD = 6.07) and were predominantly Black males, with fifty percent of the participants having less than or equal to a high school education. On aggregate, participants were within normal levels for fatigue, depression, and anxiety. A majority of the participants, however, were considered mildly impaired according to their global score based on the Frascati criteria.27 Given the limited sample size, two groups were created for each measure and included in the analyses [Frascati: impaired (i.e., ANI, MND, and HAD) = 75.0% vs. unimpaired (i.e., normal) = 25.0%].
Table 3.
Demographic, Physical Health, Psychiatric, and Neuropsychological Data (n=32-36)
| % | Range | Mean | SD | |
|---|---|---|---|---|
| Age, years | - | 38-63 | 49.89 | 6.07 |
| Gender, male | 75.0 | - | - | - |
| Race, black | 86.1 | - | - | - |
| Education | ||||
| ≤high school | 50.0 | - | - | - |
| >high school | 50.0 | - | - | - |
| BMI | - | 18-38 | 28.94 | 5.08 |
| Self-reported former IV Drug use history* | 40.0 | - | - | - |
| AHI | 0-78 | 9.09 | 19.61 | |
| Physical health data | ||||
| CD4 Nadir | - | 0-751 | 218.17 | 197.23 |
| CNS CPE | 7.8 | 2.2 | ||
| MOS HIV | - | 8-18 | 14.33 | 3.13 |
| Neuropsychiatric data | ||||
| FSS | - | 9-63 | 33.30 | 13.37 |
| BDI | - | 0-44 | 7.69 | 9.15 |
| STAI-State | - | 19-65 | 36.43 | 9.45 |
| STAI-Trait | - | 20-66 | 35.86 | 11.21 |
| Neuropsychological tests | ||||
| Trail Making Test Part B, sec | - | 27-276 | 98.17 | 61.46 |
| Verbal Fluency, total F,A,S | - | 11-74 | 38.33 | 13.88 |
| Digit Symbol | - | 24-112 | 66.19 | 17.41 |
| WCST | 4-28 | 12.72 | 5.90 | |
| Letter-Number Sequencing | 3-17 | 8.31 | 2.98 | |
| Pegboard-Dominant | 50-171 | 77.14 | 21.82 | |
| Pegboard-Non-dominant | 49-123 | 83.74 | 17.01 | |
| PVT- Lapses | - | 0-52 | 6.06 | 9.81 |
| Frascati | - | - | - | |
| Normal | 25.0 | |||
| ANI | 33.3 | |||
| MND | 33.3 | |||
| HAD | 8.3 | - | - | - |
Notes: AHI = Apnea-hypopnea index; FSS = Fatigue Severity Scale; BDI = Beck Depression Index; STAI-State = State-Trait Anxiety Inventory, subscale for state anxiety; STAI-Trait = State-Trait Anxiety Inventory, subscale for trait anxiety; WCST= Wisconsin Card Sorting Test – Perseverative Errors; PVT = Psychomotor Vigilance Test, 3rd time point; HAD = HIV-associated dementia; MND = mild neurocognitive disorder; ANI = asymptomatic neurocognitive impairment.
Data was obtained during record review at screening. Normative cut-offs: ESS < 10, PSQI <5, ISI <13
Sleep Parameters
The mean PSQI score was suggestive of “poor sleep” based upon the commonly used cutoff score of 5 and mean scores for the ESS and ISI in the abnormal range (Table 4). The mean AHI on the PSG was 9.09, representing a mild level of apnea severity (Table 3). The average PSG sleep latency for the participant sample was relatively low (M = 9.42 minutes) and well within the established normal limits of less than 30 minutes.29 Paired t-tests demonstrated reduced sleep quality during the two-week actigraphic home monitoring as compared to the single-night inpatient PSG monitoring based on several objective markers of overall sleep architecture. The mean actigraphy sleep latency (46.30 ± SE = 5.60) was significantly greater than mean PSG latency (10.02 ± SE = 1.71; t (32) = 6.02, p = 0.000), and mean actigraphy sleep efficiency (66.71± SE = 2.21) (Sleep efficiency = total sleep time/time in bed × 100) was significantly worse than mean PSG sleep efficiency (82.06 ± SE = 1.61; t (32) = -5.73, p = 0.000). In addition, mean PSG sleep duration (406.88 ± 11.58) was significantly longer than mean actigraphic sleep duration (343.08 ± 14.10; t (32) = -3.95, p = 0.000). Mean actigraphy wake after sleep onset time (WASO) minutes (88.87 ± SE = 6.86) trended higher than mean PSG WASO (77.14 ± SE = 7.26), but was not significantly different (t (32) = 1.18, p = 0.245). A subgroup of 13 participants demonstrated a markedly reduced PSG sleep latency (<5 minutes) and a much greater PSG total sleep time (408.39 ± 29.08) compared to their mean actigraphic sleep duration (305.86 ± 24.55) recorded over the two-week outpatient observation. Such a marked reduction in sleep latency and increased total sleep time on the PSG during a stay in the sleep research unit as compared to a home environment is consistent with individuals experiencing chronic partial sleep deprivation due to self-imposed or environmental factors. Our analyses also found that participants with a PSG sleep latency of <5 minutes (11.40 ± 1.63) had significantly greater ESS scores the morning after the PSG compared to the participants with PSG sleep latencies between 5 and 30 minutes (4.18 ± 1.49; t (19) = 3.27, p = 0.004), which supports our chronic partial sleep deprivation hypothesis in the former group.
Table 4.
Sleep Questionnaires, Actigraphy, and PSG Data (n=33-36)
| N | Range | Mean | SD | |
|---|---|---|---|---|
|
| ||||
| Sleep Questionnaires: | ||||
| Pittsburgh Sleep Quality Index | 33 | 2-18 | 8.76 | 4.03 |
| Insomnia Severity Index | 35 | 3-26 | 12.57 | 5.57 |
| Epworth Sleepiness Scale | 34 | 0-19 | 9.59 | 4.57 |
|
| ||||
| Actigraphy: | ||||
| Total Sleep Time (TST) | 33 | 105-479 | 343.08 | 80.98 |
| Sleep Latency (SL) | 33 | 14-156 | 46.30 | 32.16 |
| Sleep Efficiency (SE) | 33 | 20-83 | 66.71 | 12.71 |
| Wake After Sleep Onset | 33 | 40-216 | 88.87 | 39.38 |
|
| ||||
| Polysomnography: | ||||
| Total Time in Bed (TIB) | 36 | 417-608 | 493.49 | 41.24 |
| Total Sleep Time | 36 | 238-526 | 406.49 | 63.61 |
| Sleep Latency | 36 | 0-46 | 9.42 | 9.64 |
| Sleep Efficiency | 36 | 55-96 | 82.10 | 8.89 |
| Wake After Sleep Onset | 36 | 17-192 | 77.58 | 40.03 |
| REM Latency (REM L) | 36 | 0-276 | 89.49 | 65.28 |
Notes: Epworth Sleepiness Index evening before PSG; PSG age matched normal values: TST 389-407; SL 8-12; SE 90-92; TIB 422-451; REM L 71-84
Relationship between Sleep and Neuropsychological Performance
As illustrated in Table 5, significant relationships were observed between performance on a number of neuropsychological tests and sleep architectural indices on the PSG. Greater sleep latency and reduced time awake after sleep onset (WASO) on PSG was associated with better performance on Digit Symbol test. Greater PSG sleep efficiency and reduced WASO was associated with faster performance on Trails B. Greater total sleep time and sleep efficiency as well as reduced WASO on PSG were also associated with better performance on Letter-Number Sequence. Lastly, reduced WASO was associated with better performance on the Pegboard dominant hand task. After controlling for the CD4 nadir or MOS-HIV, many of the observed relationships remained significant (Table 5). The sleep questionnaires and actigraphy indices were not significantly associated with any of the neuropsychological measures. However, after adjusting for the CD4 nadir or MOS-HIV, reduced actigraphic sleep latency was associated with worse dominant Pegboard task Sequence performance. Using the Frascati categorization, the only significant difference observed between the unimpaired and impaired groups, across all sleep measures was for the ESS (Unimpaired: 12.50 ± SE = 1.43 vs. Impaired: 8.69 ± SE = 0.86; t (32) = 2.18, p = 0.037), suggesting that the cognitively-unimpaired tended to report more elevated levels of sleepiness than the cognitively-impaired. Based on a large subset of our cohort’s demonstration of sleep patterns suggestive of chronic partial sleep deprivation, we conducted comparative group analyses of cognitive performance based on individuals with markedly short PSG sleep latency (<5 minutes) as compared to individuals with normal sleep latency (5-30 minutes). Participants with reduced sleep latencies demonstrated worse performance on Digits Symbol test (<5 minute: 55.54 ± 3.72 vs. 5-30 minute: 71.18 ± 3.48; t (33) = -2.92, p = 0.006), Pegboard non-dominant (<5 minute: 92.00 seconds ± 4.22 vs. 5-30 minute: 79.64 seconds ± 3.67; t (32) = 2.11, p = 0.043), and Verbal Fluency (<5 minute: 32.69 ± 2.60 vs. 5-30 minute: 41.50 ± 3.30; t (33) = -2.10, p = 0.044; See Figure 1).
Table 5.
Correlation Coefficients for Neuropsychological tests and Sleep Indices (n = 32-36)
| Trail B | Fluency | Digits | WCST | PVT-L | L-N | Peg-D | Peg-ND | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Sleep Questionnaires: | ||||||||
| PSQI | -0.11 | 0.12 | 0.17 | 0.02 | 0.28 | 0.22 | -0.30 | -0.09 |
| ISI | 0.15 | 0.03 | -0.01 | -0.02 | -0.06 | 0.05 | -0.05 | 0.11 |
| ESS | -0.09 | 0.07 | 0.11 | -0.19 | -0.27 | 0.11 | -0.14 | -0.12 |
|
| ||||||||
| Actigraphy: | ||||||||
| Total Sleep Time | -0.21 | -0.05 | 0.16 | -0.21 | 0.11 | 0.07 | 0.04 | -0.22 |
| Sleep Latency | 0.11 | 0.12 | -0.09 | -0.03 | -0.10 | 0.01 | -0.13a,b | 0.10 |
| Sleep Efficiency | -0.28 | -0.09 | 0.30 | -0.22 | -0.04 | 0.15 | -0.06 | -0.10 |
| Wake After Sleep Onset | 0.06 | 0.16 | -0.24 | 0.18 | 0.28 | -0.02 | 0.13 | -0.15 |
|
| ||||||||
| PSG: | ||||||||
| Total Sleep Time | -0.29 | 0.12 | 0.28 | -0.19 | 0.21 | 0.37* | -0.15 | -0.04 |
| Sleep Latency | -0.13 | 0.03 | 0.36*a,b | -0.19 | -0.22 | 0.31 | 0.04 | -0.13 |
| Sleep Efficiency | -0.37*a,b | 0.11 | 0.28 | -0.19 | 0.23 | 0.36*a,b | -0.33 | 0.10 |
| Wake After Sleep Onset | -0.39*a,b | -0.08 | -0.36* | 0.19 | -0.20 | -0.40*a,b | 0.39* | -0.13 |
Notes:
p < .05;
p< .01.
Fluency = Verbal Fluency, Total F,A,S; Digits = Digit Symbol55; WCST = Wisconsin Card Sort Test59; PVT-MRT = Psychomotor Vigilance Test (3rd time point), mean reaction time; PVT-L = Psychomotor Vigilance Test (3rd time point), lapses; L-N = Letter-Number; Peg-D = Pegboard Dominant; Peg-ND = Pegboard Non-Dominant61; PSG = Polysomnography; PSQI = Pittsburgh Sleep Quality Index; ISI=Insomnia Severity Index; ESS = Epworth Sleepiness Index, evening before PSG;
Remains significant after controlling for CD4 Nadir;
Remains significant after controlling for CD4 Nadir and MOS-HIV (MOS Total).
Figure 1.
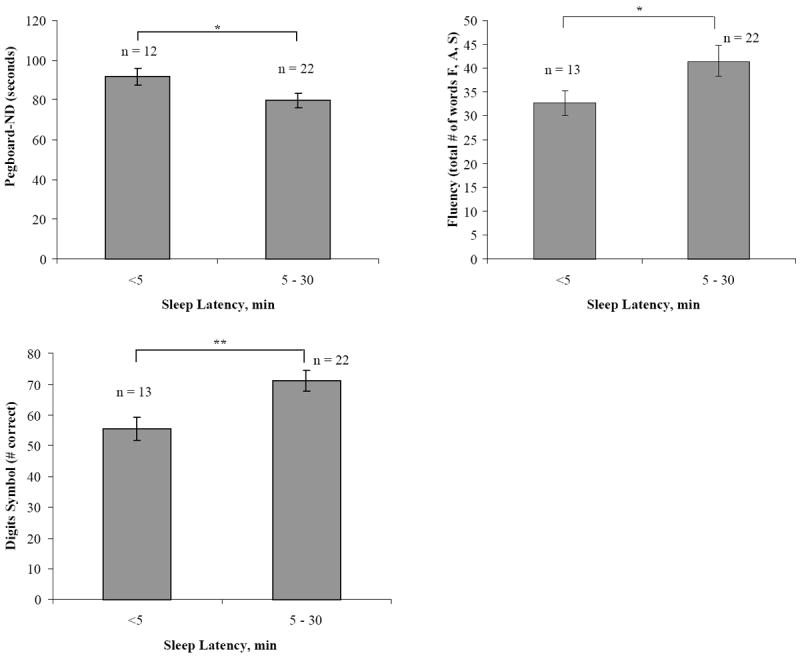
Significant Sleep Latency Group Differences in Cognitive Performance
Discussion
Despite the widespread use of cART, cognitive impairment continues to be a prevalent concern in both symptomatic and asymptomatic individuals with chronic HIV infection.1 The relationship between sleep and cognitive performance assessed by comprehensive neuropsychological and sleep function evaluation in an HIV cohort has not been previously reported. Our results suggest that both subjective and objective indices of sleep continuity and quality may have a significant relationship with cognitive performance in HIV+ individuals.
Furthermore, many participants demonstrated patterns strongly suggestive of chronic partial sleep deprivation, which may be the result of behaviorally-induced insufficient sleep syndrome (BISS).30 According to the ICSD-2 classification, the key diagnostic features of this syndrome refer to an individual who reports customary sleep times (at home during their “normal routine”) measured by history, sleep diary, or actigraphy which is significantly shorter than the sleep time recorded when placed in a non-customary setting (e.g., PSG in sleep research facility). Due to this self-imposed chronic partial sleep debt, when the individual is provided an ad libitum sleep opportunity in a research facility (despite the unfamiliar environment and cumbersome recording techniques), he or she will often show “supra” efficient sleep architectural patterns, including very high sleep efficiency, markedly short sleep onset latency, and minimal sleep fragmentation on PSG. Similar to the patterns characterized by those with BISS, many of the individuals in our cohort demonstrated markedly reduced sleep latencies of <10 minutes with sleep efficiencies often greater than 90% on their PSGs. In turn, many of these same participants demonstrated significantly shorter sleep durations on their two-week home monitoring compared to their PSG and much shorter durations than the recommended 7.5-8.5 hours needed for most adults to function optimally. Study participants demonstrating patterns suggestive of chronic partial sleep deprivation reported more complaints of daytime sleepiness and performed significantly poorer on several measures of cognitive function compared to participants without these suggestive patterns.
Interestingly, self-reported daytime sleepiness, based on the ESS, was significantly higher in the cognitively-unimpaired group (using Frascati criteria) than in the impaired group. The ESS inquires about the likelihood of “dozing” in specific scenarios, such as while in a movie theatre or meeting or while driving. Since most of our cohort was not actively employed and did not own an automobile, these scenarios may not have occurred customarily enough for our cohort to accurately respond. Based on our findings, future studies evaluating the relationship between sleep and cognition amongst HIV+ individuals should consider the participant’s historical sleep behaviors, attitudes, daily habits, and relevant sociodemographic factors (i.e., employment and residential status, common means of transportation).
Some studies have suggested that demographics, such as female gender, age, low BMI, and history of intravenous drug use, may increase the risk for future cognitive impairment in seropositive individuals. Overall health status may also be a potential issue, with two large longitudinal cohort studies (CHARTER and MACS) reporting increased risk for HAND development in aviremic subjects who suffered with “incidental” comorbidities (examples include low reading level, school problems, brain trauma, history of cerebrovascular events, epilepsy, CNS opportunistic diseases, major depression, psychotic disorder, and substance abuse) compared to aviremic participants without these comorbidities.1, 3
Obtaining ideal sleep quality and quantity is another critical component for both optimal cognitive performance and immune function.31 HIV enters the brain early after infection, and disturbances in sleep patterns during the asymptomatic stage of HIV infection were recognized and reported very early in the HIV/AIDS epidemic.32 The release of cytokines including MCP-1 (CCL2), TNF-alpha, IL-1beta, IL-6, IFN-gamma, and IL-15 in the brain is thought to lead to neuronal injury and dysfunction33 and has recently been correlated with the development of HAND.34,35 In addition, many of these same cytokines such as TNF-alpha, IL-1 and IFN-alpha can modulate sleep wake patterns and can be associated with significant sleep architectural changes.36-38 Studies are warranted to look at the effect of sleep loss on CNS immune activity, especially since there is evidence to suggest that sleep disruption can increase blood-brain barrier permeability for pro-inflammatory substances.1, 39,40 Thus, identification of the sleep-wake patterns and disturbances specific to HIV+ individuals may prove fertile in furthering our understanding of HAND manifestation and suggest future preventative and therapeutic avenues.
While this study yielded several important findings, its ability to detect subtle cognitive differences based on a four-tiered Frascati classification may have been limited by its relatively small sample size. Conducting this type of study utilizing multi-method sleep assessments and observations across several time points requires an inordinate amount of financial and personnel resources. Even though our study sample of predominantly Black males may place limits on generalizability, CDC statistics continue to show that Black males are disproportionately affected by HIV as compared to other demographic groups.41 Significantly lower mean sleep durations have also been reported for black men compared to other demographic groups in general population studies,41 and similar sleep behavioral patterns suggestive of self-imposed chronic partial sleep deprivation were also revealed in our cohort. Providing counseling on the importance of adequate sleep opportunity may prove to be an effective health care management strategy. Although HIV+ individuals undergoing treatment with Efavirenz were excluded in this study to minimize confounding medication-related effects on sleep 42,43, evidence regarding the impact of Efavirenz on sleep is mixed with some reports actually demonstrating no significant difference in sleep disturbance among individuals on Efavirenz compared to other cART regimens 4,44. Moreover, even among the studies reporting an association between sleep disruption and Efavirenz, the findings ranged from significant changes in sleep architecture in some studies while others may have only found significant differences in reports of “unusual dream”45,46. With the wide prevalence of Efavirenz use in modern cART, future studies may consider including those undergoing treatment with Efavirenz in order provide further insight and generalizability. Thus, we anticipate that the findings revealed in this study will serve as additional support for conducting future and larger-scale studies to evaluate the relationship between sleep and cognition across multiple demographic groups living with HIV and undergoing varying cART regimens.
Even in the context of optimally-controlled viral replication (low or undetectable viral loads), patients may still continue to experience mild or asymptomatic forms of neurocognitive dysfunction at a surprisingly high rate.47 The stringent inclusion criteria and intricate protocol employed in this study certainly biased towards the inclusion of subjects who were more stable cognitively. However, individuals demonstrating even milder forms of impairment such as ANI are at higher risk for eventual progression to more severe forms of HAND.3 Moreover, deficits sufficient to impact activities of daily living that are essential for disease management, including medication adherence, have been shown in seropositive individuals, especially those under the age of 50, regardless of their level of cognitive impairment.48 Thus, identifying confounding factors, such as sleep disturbances that might influence the manifestation of neurocognitive impairment, is critical in the post-cART era.
Acknowledgments
FUNDING SOURCES: This publication was made possible by Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. This study was also supported by award 5P30MH075673-S02 from the National Institute of Mental Health (NIMH) (PI JCM), a Developmental Grant from JHU NIMH Center for Novel Therapeutics of HIV-associated Cognitive Disorders (to author CG, PI JCM), and a Developmental Grant from JHU Center for Mind-Body Research (CMBR) (PI Jennifer Haythornthwaite PI to author CG). The recruitment of participants was assisted by an existing cohort, funded by NIMH, the Central Nervous System HIV Antiretroviral Therapy Effects Research (CHARTER). The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 RR 025005. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We acknowledge Dr. Adam Spira for his invaluable contributions to this project.
References
- 1.McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- 2.Wright EJ, Nunn M, Joseph J, Robertson K, Lal L, Brew BJ. NeuroAIDS in the asia pacific region. J Neurovirol. 2008;14(6):465–473. doi: 10.1080/13550280802235932. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid S, Dwyer J. Insomnia in HIV infection: A systematic review of prevalence, correlates, and management. Psychosom Med. 2005;67(2):260–269. doi: 10.1097/01.psy.0000151771.46127.df. [DOI] [PubMed] [Google Scholar]
- 5.Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the united states. Sleep Breath. 2010;14(1):63–70. doi: 10.1007/s11325-009-0281-3. [DOI] [PubMed] [Google Scholar]
- 6.Norman SE, Chediak AD, Freeman C, et al. Sleep disturbances in men with asymptomatic human immunodeficiency (HIV) infection. Sleep. 1992;15(2):150–155. doi: 10.1093/sleep/15.2.150. [DOI] [PubMed] [Google Scholar]
- 7.Norman SE, Chediak AD, Kiel M, Cohn MA. Sleep disturbances in HIV-infected homosexual men. AIDS. 1990;4(8):775–781. doi: 10.1097/00002030-199008000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Cruess DG, Antoni MH, Gonzalez J, et al. Sleep disturbance mediates the association between psychological distress and immune status among HIV-positive men and women on combination antiretroviral therapy. J Psychosom Res. 2003;54(3):185–189. doi: 10.1016/s0022-3999(02)00501-9. [DOI] [PubMed] [Google Scholar]
- 9.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 10.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5(6):463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 11.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25(1):117–129. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 12.Killgore WD, Balkin TJ, Wesensten NJ. Impaired decision making following 49 h of sleep deprivation. J Sleep Res. 2006;15(1):7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 13.Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15(3):261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 14.Harrison Y, Jones K, Waterhouse J. The influence of time awake and circadian rhythm upon performance on a frontal lobe task. Neuropsychologia. 2007;45(8):1966–1972. doi: 10.1016/j.neuropsychologia.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Bolla KI, Lesage SR, Gamaldo CE, et al. Polysomnogram changes in marijuana users who report sleep disturbances during prior abstinence. Sleep Med. 2010;11(9):882–889. doi: 10.1016/j.sleep.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27(3):423–433. [PubMed] [Google Scholar]
- 17.Moyle G, Fletcher C, Brown H, Mandalia S, Gazzard B. Changes in sleep quality and brain wave patterns following initiation of an efavirenz-containing triple antiretroviral regimen. HIV Med. 2006;7(4):243–247. doi: 10.1111/j.1468-1293.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 18.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/S1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 19.Johns MW. A new method for measuring daytime sleepiness: The epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 20.Gamaldo CE, Spira AP, Hock RS, et al. Sleep, function and HIV: A multi-method assessment. AIDS Behav. 2013 doi: 10.1007/s10461-012-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30(5):603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 22.Marder K, Albert SM, McDermott MP, et al. Inter-rater reliability of a clinical staging of HIV-associated cognitive impairment. Neurology. 2003;60(9):1467–1473. doi: 10.1212/01.wnl.0000064172.46685.82. [DOI] [PubMed] [Google Scholar]
- 23.Concha M, Selnes OA, McArthur JC, et al. Normative data for a brief neuropsychologic test battery in a cohort of injecting drug users. Int J Addict. 1995;30(7):823–841. doi: 10.3109/10826089509067009. [DOI] [PubMed] [Google Scholar]
- 24.Selnes OA, Jacobson L, Machado AM, et al. Normative data for a brief neuropsychological screening battery. multicenter AIDS cohort study. Percept Mot Skills. 1991;73(2):539–550. doi: 10.2466/pms.1991.73.2.539. [DOI] [PubMed] [Google Scholar]
- 25.Heaton R, Miler SW, Taylor MJ, Grant I. A Professional Manual of the Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, Florida: Psychological Assessement Resources, Inc; 2004. [Google Scholar]
- 26.Stewart AL, Ware JE. Measuring Function and Well-being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1993. [Google Scholar]
- 27.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 29.Hohagen F, Rink K, Kappler C, et al. Prevalence and treatment of insomnia in general practice. A longitudinal study. Eur Arch Psychiatry Clin Neurosci. 1993;242(6):329–336. doi: 10.1007/BF02190245. [DOI] [PubMed] [Google Scholar]
- 30.The International Classification of Sleep Disorder: Diagnostic and Coding Manual. Vol. 2. Westchester, IL: 2005. [Google Scholar]
- 31.Pearson VE, Allen RP, Dean T, Gamaldo CE, Lesage SR, Earley CJ. Cognitive deficits associated with restless legs syndrome (RLS) Sleep Med. 2006;7(1):25–30. doi: 10.1016/j.sleep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Darko DF, Miller JC, Gallen C, et al. Sleep electroencephalogram delta-frequency amplitude, night plasma levels of tumor necrosis factor alpha, and human immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1995;92(26):12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010;37(3):542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63(11):2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 35.Ragin AB, Wu Y, Ochs R, et al. Biomarkers of neurological status in HIV infection: A 3-year study. Proteomics Clin Appl. 2010;4(3):295–303. doi: 10.1002/prca.200900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clinton JM, Davis CJ, Zielinski MR, Jewett KA, Krueger JM. Biochemical regulation of sleep and sleep biomarkers. J Clin Sleep Med. 2011;7(5 Suppl):S38–42. doi: 10.5664/JCSM.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158(9):4454–4464. [PubMed] [Google Scholar]
- 38.Mayhan WG. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002;927(2):144–152. doi: 10.1016/s0006-8993(01)03348-0. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Lv S, Zhou Y, Fu J, Li C, Liu P. Tumor necrosis factor-alpha affects blood-brain barrier permeability in acetaminophen-induced acute liver failure. Eur J Gastroenterol Hepatol. 2011;23(7):552–558. doi: 10.1097/MEG.0b013e3283470212. [DOI] [PubMed] [Google Scholar]
- 40.Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont) 2009;6(11):18–22. [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention (CDC) HIV prevalence estimates--united states, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–1076. [PubMed] [Google Scholar]
- 42.Jena A, Sachdeva RK, Sharma A, Wanchu A. Adverse drug reactions to nonnucleoside reverse transcriptase inhibitor-based antiretroviral regimen: A 24-week prospective study. J Int Assoc Physicians AIDS Care (Chic) 2009;8:318–22. doi: 10.1177/1545109709343967. [DOI] [PubMed] [Google Scholar]
- 43.Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav. 2011;15:1803–18. doi: 10.1007/s10461-011-9939-5. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen A, Calmy A, Delhumeau C, Mercier IK, Cavassini M, Fayet-Mello A, et al. A randomized crossover study to compare efavirenz and etravirine treatment. AIDS. 2011;25:57–63. doi: 10.1097/QAD.0b013e32833f9f63. [DOI] [PubMed] [Google Scholar]
- 45.Rihs TA, Begley K, Smith DE, Sarangapany J, Callaghan A, Kelly M, et al. Efavirenz and chronic neuropsychiatric symptoms: A cross-sectional case control study. HIV Med. 2006;7:544–8. doi: 10.1111/j.1468-1293.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 46.Clifford DB, Evans S, Yang Y, Acosta EP, Ribaudo H, Gulick RM, et al. Long-term impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals (ACTG 5097s) HIV Clin Trials. 2009;10:343–55. doi: 10.1310/hct1006-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 48.Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, Lam M, et al. Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry. 2009;17:281–90. doi: 10.1097/JGP.0b013e31819431bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spielberger CD, Gorsuch RL, Lushene RE. Manual for State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 50.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46(10):1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 51.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 52.Grossman HA, Sullivan PS, Wu AW. Quality of life and HIV: Current assessment tools and future directions for clinical practice. AIDS Read. 2003;13(12):583–90. 595–7. [PubMed] [Google Scholar]
- 53.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 54.Wechsler D. Wechsler Adult Intelligence Scale - Revised Manual. New York: The Psycholgical Corporation; 1981. [Google Scholar]
- 55.Reitan RM, Wolfson D. The Halstead - Reitan Neuropsychological Test Battery. Tucson, Arizona: Neuropsychology Press; 1985. [Google Scholar]
- 56.Brandt J, Benedict R. HVLT-R. Lutz, Florida: Psychological Assessment Resources, Inc; 2001. [Google Scholar]
- 57.Benedict R. Brief Visuospatial Memory Test-Revised. Jannali NSW: Psychological Assessments Australia; 2006. [Google Scholar]
- 58.Heaton RK PAR staff. WCST-64:CV2. Research Edition Lutz, Florida: Psychological Assessments Resources, Inc; 2000. [Google Scholar]
- 59.Benton AL, Hamsher KdeS. Multilingual Aphasia Examination. Iowa City, IA: University of Iowa; 1976. [Google Scholar]
- 60.Grooved Pegboard Test. Lafayette, IN: Lafayette Instruments; 1989. [Google Scholar]
- 61.Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]


