Summary
Agouti-related peptide (AgRP) neurons of the hypothalamus release a fast transmitter (GABA) in addition to neuropeptides (NPY and AgRP). This raises questions as to their respective functions. Acute activation of AgRP neurons robustly promotes food intake, while central injections of AgRP, NPY or GABA agonist results in marked escalation of food consumption with temporal variance. Given the orexigenic capability of all three of these neuroactive substances in conjunction with their coexpression in AgRP neurons, we looked to unravel their relative temporal role in driving food intake. Following acute stimulation of AgRP neurons using DREADD technology, we found that either GABA or NPY is required for rapid stimulation of feeding, and the neuropeptide AgRP, through action on MC4 receptors, is sufficient to induce feeding over a delayed, yet prolonged period. These studies help to elucidate the neurochemical mechanisms of AgRP neurons in controlling temporally distinct phases of eating.
Introduction
As it becomes more and more apparent that many neurons containing fast-acting neurotransmitters co-release slower neuromodulator peptides (van den Pol, 2012), new methodology is necessary to interpret their relative roles and contributions on downstream circuits. Here, we aimed to dissect the functional contributions of the neurotransmitter/neuromodulators (collectively termed “neuromediators”) released by Agouti-related peptide (AgRP) neurons, a small subset of hypothalamic neurons located in the arcuate nucleus (ARC). AgRP neurons release three known neuroactive chemicals, the amino acid transmitter GABA, and the neuropeptides, neuropeptide Y (NPY) and AgRP, from which their name is derived.
Many lines of evidence strongly support a critical role for AgRP neurons in driving food intake, as either optogenetic (Aponte et al., 2011) or pharmacogenetic (Krashes et al., 2011) AgRP neuronal stimulation evokes rapid feeding, and the initial hour of feeding during optogenetic AgRP photoactivation is independent of the melanocortin pathway (Aponte et al., 2011). Conversely, pharmacogenetic inhibition of AgRP neurons blunts food consumption (Krashes et al., 2011) and acute ablation of AgRP neurons in adult animals results in cessation of feeding and ultimately, starvation (Gropp et al., 2005; Luquet et al., 2005) demonstrating their necessity in regulating appetite.
Pharmacological administration of either NPY or AgRP into the hypothalamus induces a robust hyperphagic response in rodents (Clark et al., 1984; Rossi et al., 1998; Semjonous et al., 2009) with distinct temporal dynamics; NPY results in immediate feeding while AgRP increases food intake over a delayed, longer time scale (Semjonous et al., 2009). Moreover, genetic overexpression of the AgRP gene, encoding the neuropeptide, which acts as an antagonist/inverse agonist on downstream melanocortin 4 receptors (MC4R), promotes food intake (Ollmann et al., 1997). Furthermore, GABA receptor agonists administered to the nucleus accumbens shell elicit intense feeding (Stratford and Kelley, 1997). Additionally, GABAergic signaling onto the parabrachial nucleus (PBN), a downstream target of the AgRP circuit, restores appetite following acute AgRP neuron ablation (Wu et al., 2009). Thus, each of these neuromediators has been shown to play a part in enhancing food consumption.
Despite these findings, targeted deletion of Agrp, Npy and/or Slc32a1 (vesicular GABA transporter; VGAT; required for GABA release) all have minimal effects on feeding (Erickson et al., 1996; Qian et al., 2002; Tong et al., 2008). To circumvent these inconsistencies we employed stimulatory DREADD (Designer Receptors Exclusively Activated by Designer Drugs) technology (Alexander et al., 2009; Krashes et al., 2011) to acutely and explicitly activate AgRP neurons, in genetic mouse models that have had release of GABA, NPY or MC4R-signalling disrupted. Using this approach, we are able to test for both necessity and sufficiency of these individual neuromediators in regulating short-term and long-term food intake (Table S1).
Results
DREADD-mediated AgRP stimulation in triple KO mice abrogates feeding response
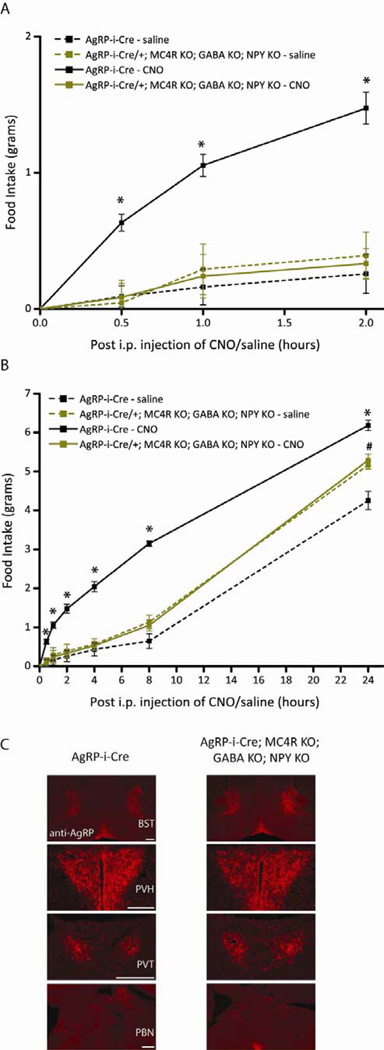
As proof of principle, we targeted a Cre-dependent adeno-associated virus (AAV) expressing the hM3Dq excitatory DREADD (Krashes et al., 2011), specifically to AgRP neurons using either AgRP-ires-Cre control or AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox; Npy−/− triple knock-out (KO) mice lacking GABA release from AgRP neurons as well as ubiquitous deletion of NPY and MC4R, the downstream target of the AgRP neuropeptide (Balthasar et al., 2005; Erickson et al., 1996; Marsh et al., 1999b; Tong et al., 2008). It should be noted that the Mc4r−/− mice are obese due to hyperphagia and reduced energy expenditure (Balthasar et al., 2005), however these studies presented here evaluate feeding behavior after acute AgRP neural activation, making it possible to evaluate the acute role of the AgRP neuropeptide.
Food intake was first measured near the beginning of the light cycle, a time when mice normally refrain from eating and continually monitored throughout the day (food intake was assessed at 0.5-, 1-, 2-, 4-, 8- and 24-hr post-injection). Each trial consisted of saline administration on day 1, CNO administration on day 2 and no injection on day 3, repeated over three trials to demonstrate the robustness/stability of the response (For each series of studies the pooled data from all trials is shown in the main Figure and the data from the first trial is shown in the corresponding Supplemental Figure). Importantly, the results from the pooled trials, in all cases, were similar to the results from the first trial. Even in this calorically replete state, acute activation of AgRP neurons, via clozapine-N-oxide (CNO; 0.3 mg/kg) administration, resulted in voracious feeding in AgRP-ires-Cre mice over the first 2 hr (Figure 1A; Figure S1A) and across a 24 hr window (Figure 1B; Figure S1B) compared to the same mice following a saline injection (Krashes et al., 2011), but failed to evoke food intake in triple KO mice, demonstrating that release of either GABA and/or NPY and/or AgRP action onto MC4Rs is required for increases in food intake following direct AgRP neuronal stimulation (A posthoc Tukey test showed CNO-treated AgRP-ires-Cre mice exhibited significantly elevated levels of food intake compared to saline-treated AgRP-ires-Cre and triple KO and CNO-treated triple KO mice at all time points, P<0.05). Although mice in a Mc4r−/− knockout background consume elevated daily levels of food (Figure 1B), DREADD-mediated AgRP activation in these triple KO mice resulted in comparable food intake observed in the same mice following saline injections.
Figure 1.
Acute pharmaco-genetic activation of AgRP neurons in mice without release of GABA, NPY and AgRP signaling via MC4Rs, collectively, lack DREADD-mediated increases in food intake (Pooled data across multiple trials). All mice in these studies were bilaterally injected with AAV8-DIO-hM3Dq-mCherry in the ARC. (A-B) Activation of AgRP neurons increases food intake in AgRP-ires-Cre mice (black line), but has no effect on food intake in AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox; Npy−/− triple KO mice (light green line). CNO (solid line; 0.3 mg/kg of body weight, i.p.) or saline (dotted line) was injected 3 hr after the start of the “lights on” cycle and food intake was assessed (A) 1-, 2- , (B) 4-, 8- and 24-hr post-injection (PI) over three trials of each treatment. Data shown is from male mice (Error bars indicate mean +/− SEM, n=8 AgRP-ires-Cre mice; n=5 AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox; Npy−/− mice; *P<0.05 AgRP-ires-Cre CNO group versus all other groups; #P<0.05 AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox; Npy−/− groups versus AgRP-ires-Cre saline group). (C) Immunohistochemical analysis of AgRP projections in AgRP-ires-Cre mice (left) and AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox; Npy−/− triple KO mice (right) to (Top-Bottom) the bed nucleus of the stria terminalis (BST), paraventricular hypothalamus (PVH), paraventricular thalamus (PVT) and lateral parabrachial nucleus (PBNl) reveals no gross differences in morphology and/or density (200 μm). See also Figure S1,S4 and Table S1.
Mice lacking leptin (Lepob/ob) or leptin receptor (Leprdb/db) have severe morphological alterations in their AgRP axonal projections (Bouret et al., 2012). To analyze projection fields of AgRP neurons in both AgRP-ires-Cre and triple KO mice, we performed immunohistochemistry for AgRP protein. Significantly, we detected no gross anatomical differences in either AgRP neural innervation patterns or terminal field projections to different downstream brain regions, such as the bed nucleus of the stria terminalis (BST), paraventricular hypothalamus (PVH), paraventricular thalamus (PVT) and lateral parabrachial nucleus (PBNl) (Figure 1C). Therefore, the absence of increased food intake following CNO-mediated AgRP activation in triple KO mice is not due to obvious axonal projection perturbations.
Activation of AgRP neurons in single KO mice induces feeding
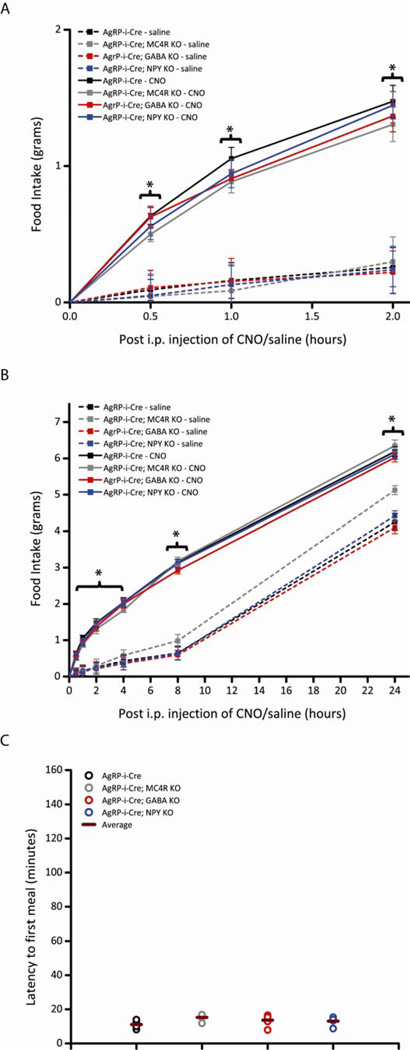
To decipher the relevant neuromediator necessary for the robust increase of food intake following AgRP stimulation, we acutely activated AgRP neurons in mice that either could not release GABA (AgRP-ires-Cre; Vgatflox/flox) or NPY (AgRP-ires-Cre; Npy−/−) or that could not engage MC4Rs (AgRP-ires-Cre; Mc4r−/−). Surprisingly, we found that disruption of each of these individually had no impediment on the CNO-mediated induction of feeding, both over the short-term (Figure 2A; Figure S2A) and long-term (Figure 2B; Figure S2B), as the levels of food intake were comparable in saline versus CNO injected single KO mice (A posthoc Tukey test showed CNO-treated AgRP-ires-Cre and all single KO mice exhibited significantly elevated levels of food intake compared to saline-treated AgRP-ires-Cre and single KO mice at all time points, P<0.05). Importantly, we show acute stimulation of AgRP neurons in Mc4r−/− mice drives feeding beyond the elevated levels normally observed in this strain.
Figure 2.
Acute pharmaco-genetic activation of AgRP neurons in mice without release of GABA, NPY or AgRP signaling via MC4Rs, individually, display intact DREADD-mediated increases in food intake (Pooled data across multiple trials). All mice in these studies were bilaterally injected with AAV8-DIO-hM3Dq-mCherry in the ARC. (A-C) Activation of AgRP neurons increases comparable levels of food intake and latency to first meal in AgRP-ires-Cre mice (black line), AgRP-ires-Cre; Mc4r−/− KO mice (grey line), AgRP-ires-Cre; Vgatflox/flox KO mice (red line) and AgRP-ires-Cre; Npy−/− KO mice (blue line). CNO (solid line; 0.3 mg/kg of body weight, i.p.) or saline (dotted line) was injected 3 hr after the start of the “lights on” cycle and food intake was assessed (A) 1-, 2-, (B) 4-, 8- and 24- hr post-injection (PI) over three trials of each treatment. Data shown is from male mice (Error bars indicate mean +/− SEM, n=8 AgRP-ires-Cre mice; n=5 AgRP-ires-Cre; Mc4r−/− mice; n=7 AgRP-ires-Cre; Vgatflox/flox mice; n=8 AgRP-ires-Cre; Npy−/− mice; *P<0.05 CNO groups versus all saline groups; #P<0.05 AgRP-ires-Cre; Mc4r−/− saline group versus all other saline groups). (C) Latency to first meal following acute pharmaco-genetic activation of AgRP neurons. Each circle represents the average of two trials for each mouse; horizontal bar represents average of all mice. Data shown is from male mice (mean ± SEM, n=7 AgRP-ires-Cre mice; n=5 AgRP-ires-Cre; Mc4r−/− mice; n=5 AgRP-ires-Cre; Vgatflox/flox mice; n=5 AgRP-ires-Cre; Npy−/− KO mice). See also Figure S2 and Table S1.
The behavior elicited by AgRP neuronal activation is highly stereotyped with mice engaging in meals, as defined by continuous feeding for >3 min, shortly after CNO injection. These episodes are characterized by mice approaching the food hopper, initiating feeding behavior and continuing to visibly eat for an extended period of time, exceeding 3 minutes (Krashes et al., 2011). We quantified this latency to the first meal following DREADD-mediated AgRP activation by recording how long it took mice to commence in their first meal after a CNO injection. It is important to note that, in the absence of CNO stimulation, mice near the beginning of the light cycle (9 - 11:40 am) fail to engage in >3 min feeding bouts (latency data not shown, but see Figure 2A). Notably, each group of single KO mice began eating shortly post-CNO administration (~15 min, Figure 2C; Figure S2C), comparable to AgRP-ires-Cre mice. Thus, each of these individual neuromediators by themselves is dispensable for the increased food intake after AgRP activation.
GABA and/or NPY are required for rapid onset of food intake while AgRP is sufficient for chronic food intake
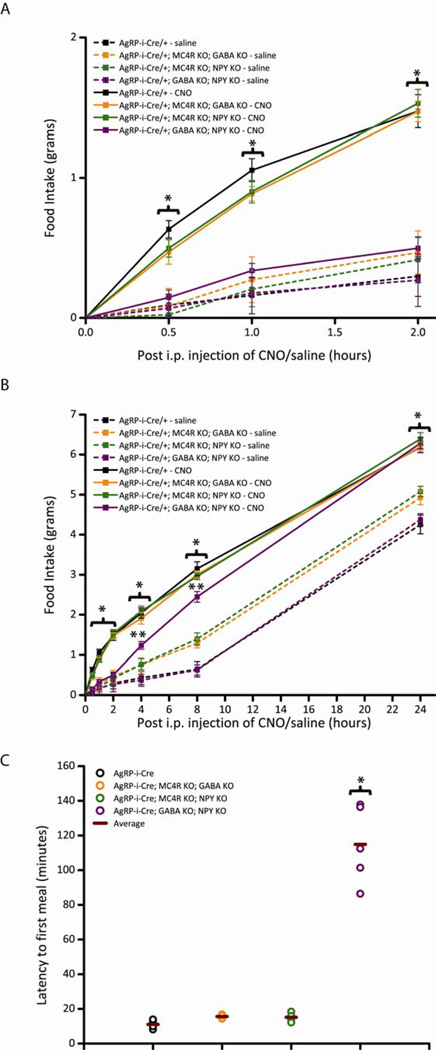
To address both the necessity of transmitter pairs and the sufficiency of each individual neuromediator, we generated each possible double KO combination and assessed the effects on feeding behavior after acute AgRP neuronal activation. Stimulating AgRP neurons in mice with no NPY release and no downstream AgRP signaling via MC4Rs (AgRP-ires-Cre; Mc4r−/−; Npy−/−) or with both disrupted GABA release and downstream AgRP signaling via MC4Rs (AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox) had no consequence on the DREADD-mediated increase in food intake, both over the short-term (Figure 3A; Figure S3A) or long-term (Figure 3B; Figure S3B) (A posthoc Tukey test showed CNO-treated AgRP-ires-Cre and double KO mice exhibited significantly elevated levels of food intake compared to saline-treated AgRP-ires-Cre and double KO mice at all time points, P<0.05). This result demonstrates that AgRP neural release of either NPY or GABA, alone, is sufficient to drive feeding post-AgRP activation, as these mice began eating soon after CNO injection (~ 15 min, Figure 3C; Figure S3C).
Figure 3.
Acute pharmaco-genetic activation of AgRP neurons in mice without release of GABA and NPY, collectively, display delayed DREADD-mediated increases in food intake (Pooled data across multiple trials). All mice in these studies were bilaterally injected with AAV8-DIO-hM3Dq-mCherry in the ARC. (A-C) Activation of AgRP neurons increases comparable levels of food intake (A,B) and latency to first meal (C) in AgRP-ires-Cre mice (black line), AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox double KO mice (orange line) and AgRP-ires-Cre; Mc4r−/−; Npy−/− double KO mice (green line). In contrast, activation of AgRP neurons in AgRP-ires-Cre; Vgatflox/flox; Npy−/− double KO mice (purple line) resulted in highly attenuated short-term feeding (A), increased latency to first meal (C) and late-onset hyperphagia (B). CNO (solid line; 0.3 mg/kg of body weight, i.p.) or saline (dotted line) was injected 3 hr after the start of the “lights on” cycle and food intake was assessed (A) 1-, 2-, (B) 4-, 8- and 24-hr post-injection (PI) over three trials of each treatment. Data shown is from male mice (Error bars indicate mean +/− SEM, n=8 AgRP-ires-Cre mice; n=5 AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox mice; n=5 AgRP-ires-Cre; Mc4r−/−; Npy−/− mice; n=8 AgRP-ires-Cre; Vgatflox/flox; Npy−/− mice; *P<0.05 AgRP-ires-Cre, AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox and AgRP-ires-Cre; Mc4r−/−; Npy−/− CNO groups versus AgRP-ires-Cre; Vgatflox/flox; Npy−/− CNO and all saline groups; #P<0.05 AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox and AgRP-ires-Cre; Mc4r−/−; Npy−/− saline groups versus all other saline groups; &P<0.05 AgRP-ires-Cre; Vgatflox/flox; Npy−/− CNO group versus all saline groups). (C) Latency to first meal following acute pharmaco-genetic activation of AgRP neurons. Each circle represents the average of two trials for each mouse; horizontal bar represents average of all mice. Data shown is from male mice (mean ± SEM, n=7 AgRP-ires-Cre mice; n=5 AgRP-ires-Cre; Mc4r−/−; Vgatflox/flox mice; n=5 AgRP-ires-Cre; Mc4r−/−; Npy−/− mice; n=5 AgRP-ires-Cre; Vgatflox/flox; Npy−/− mice; *P<0.05 AgRP-ires-Cre; Vgatflox/flox; Npy−/− group versus all other groups). See also Figure S3 and Table S1.
In stark contrast, we found that DREADD-mediated AgRP activation in mice with perturbed GABA and NPY release (AgRP-ires-Cre; Vgatflox/flox; Npy−/−) abrogated short-term feeding (Figure 3A; Figure S3A). (A posthoc Tukey test showed CNO-treated AgRP-ires-Cre mice exhibited significantly elevated levels of food intake compared to saline-treated AgRP-ires-Cre and AgRP-ires-Cre; Vgatflox/flox; Npy−/− mice at all time points, P<0.05 and CNO-treated AgRP-ires-Cre; Vgatflox/flox; Npy−/− mice at time points 0.5, 1, 4 and 8, P<0.05). These mice initiated food intake ~2 hr post-CNO injection (Figure 3C; Figure S3C). This suggests that either GABA or NPY is required for the acute feeding phase and the neuropeptide AgRP is insufficient to mediate this early response. Remarkably however, these double KO mice exhibited late on-set hyperphagia over 24 hr, ultimately reaching similar quantitative levels of food intake as all the other mice tested (Figure 3B; Figure S3B). Importantly, although actions of AgRP neuropeptide independent of the MC4R have been described (Fu and van den Pol, 2008; Marsh et al., 1999a)), we show here that the rise in food consumption subsequent of acute AgRP activation is dependent on AgRP peptide signaling through MC4Rs because it is absent in the triple KO mice (Figure 1).
Compromising GABA and NPY release from AgRP neurons delays physiological food intake
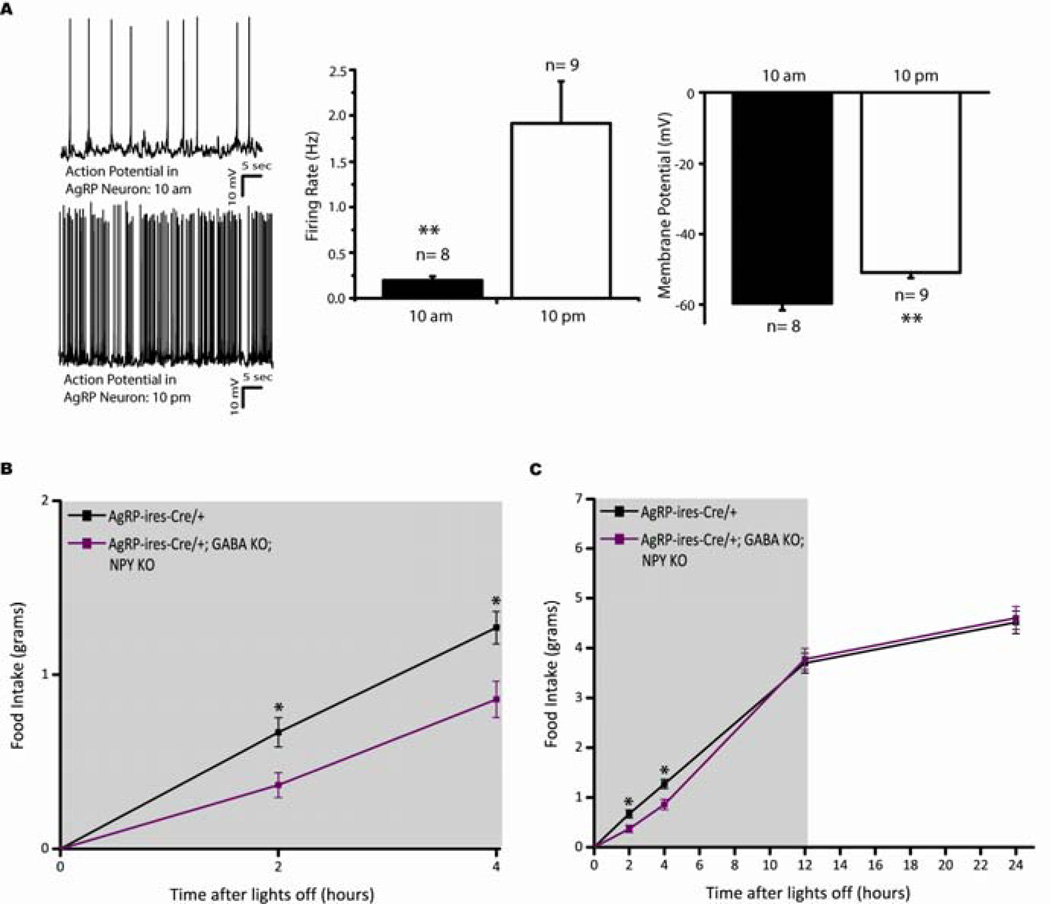
The above studies employed DREADD technology to artificially activate AgRP neurons, but to investigate this biphasic feeding due to differential AgRP neuromediator release in a physiological state, we assessed dark cycle food intake, the active feeding period in rodents, in both AgRP-ires-Cre and AgRP-ires-Cre; Vgatflox/flox; Npy−/− double KO mice. Importantly, using Npy-hrGFP mice to visualize AgRP neurons (Liu et al., 2012), we demonstrate enhanced AgRP neuron activity during the dark cycle compared to basal levels near the beginning of the light cycle (Figure 4A). Membrane potential and firing rate of AgRP neurons are significantly elevated during the dark cycle (calorically deficient state) compared to the onset of the light cycle (calorically replete state) (Figure 4A), consistent with a recent finding that AgRP neurons receive approximately double the frequency of spontaneous excitatory post-synaptic currents at the beginning of the dark period compared to the fed state (Yang et al., 2011).
Figure 4.
Mice with compromised release of both GABA and NPY from AgRP neurons display a delayed physiological increase in dark cycle food intake. (A) Light cycle (10 am; n=8) versus dark cycle (10 pm; n=9) electrophysiological properties of AgRP neurons (Error bars indicate mean + SEM; *P<0.05). Top, representative trace of AgRP neuron in the light cycle. Bottom, representative trace of AgRP neuron in the dark cycle. Right, quantitative analyses of firing rate and membrane potential of AgRP neurons in the light cycle (black bar) versus dark cycle (white bar). (B-C) Dark cycle food intake measurements assessed at (B) 2-, 4-, (C) 12-and 24-hr after lights are shut off. Grey background indicates dark cycle period. Data shown is from male mice (Error bars indicate mean +/− SEM, n=9 AgRP-ires-Cre mice; n=9 AgRP-ires-Cre; Vgatflox/flox; Npy−/− mice; *P<0.05).
In vivo feeding measurements revealed that the double KO mice exhibited decreased food consumption during the first 2- and 4-hr window of the dark cycle, compared to littermate controls (Figure 4B) (A posthoc Tukey test showed AgRP-ires-Cre exhibited significantly elevated levels of food intake compared to double KO mice at 2 and 4 hr, P<0.05). Despite this early temporal reduction in food intake in the double KO mice, both groups ate comparable amounts of food over the entire 12-hr dark cycle and 24 hr (Figure 4C). Assuming hunger signaling flows through AgRP neural output, and given their increased activity during the dark cycle (Figure 4A) these results would suggest that the AgRP neuropeptide is sufficient to drive comparable levels of feeding over a delayed, prolonged temporal scale in a physiological condition.
Discussion
Given the discrepancies between pharmacological and genetic deletion experiments, we took an alternative approach to assign prandial function to the distinct neuromediators released by AgRP neurons. By employing DREADD technology to rapidly and remotely stimulate AgRP neural activity with genetic mouse lines harboring transgenes to perturb GABA-, NPY- and/or AgRP-signaling, our findings suggest the following conclusions. First, the complete absence of CNO-stimulated feeding in triple KO mice indicates that at least one of these neuroactive chemicals is necessary to drive AgRP-mediated feeding behavior. Secondly, it demonstrates that there are no other functionally relevant mediators released by AgRP neurons that promote food intake in our assay.
Third, the absence of any impairment in single KO mice indicates that there is redundancy in the effects of these three mediators. Whether this redundancy is due to similar roles played by one or both of the remaining mediators, or is due to compensation caused by chronic genetic KO of a given mediator, is not resolved by our study. Interestingly, compensation has been described in AgRP neurons as adaptive mechanisms were observed after both cell-specific ablation (Gropp et al., 2005; Luquet et al., 2005) in neonatal animals or slow progressive loss of AgRP neurons (Xu et al., 2005). Also, it was recently suggested, based upon optogenetic assessment of connectivity, that Npy−/− mice have strengthening of their AgRP neuron → PVH neuron GABAergic transmission (Atasoy et al., 2012). Such compensation could explain how GABA substitutes for absence of NPY in rapid stimulation of feeding. On the other hand, it is presently unknown how NPY substitutes for the absence of GABAergic transmission.
Fourth, the near complete absence of feeding during the first 2 hr of stimulation of double KO mice lacking NPY and GABA signaling indicates a) that AgRP by itself is unable to activate feeding quickly, but is able to do so chronically, consistent with the delayed kinetics of MC4R-signalling following proopiomelanocortin (POMC) neural manipulations (Aponte et al., 2011; Atasoy et al., 2012; Zhan et al., 2013) and b) that either GABA or NPY, both of which are inhibitory transmitters, are required for rapid feeding, and are able to compensate for each other in these constitutive genetic deletion backgrounds. This ability of GABA and NPY to compensate for each other with regard to rapid feeding is surprising given that one is a synaptic fasting-acting neurotransmitter while the other is a neuropeptide. Interestingly, acute brain slice electrophysiology has demonstrated the fast temporal dynamics of NPY application onto downstream POMC neurons (Cowley et al., 2001). Additionally, it was recently shown that NPY puncta in the axons of hypothalamic neurons colocalized with the sites of GABA transmitter release (Ramamoorthy et al., 2011), implying that NPY may be released synaptically, as suggested by immunohistochemistry (Broberger et al., 1998) and in vivo manipulations (Atasoy et al., 2012). In agreement with this observation, electron microscopy verified co-expression of NPY and GABA in axon terminals and revealed that these boutons formed synapses onto POMC perikarya (Cowley et al., 2001).
To assess the physiological function of these neuroactive substances, we monitored food intake during the dark cycle, a time when mice normally consume most of their food. Of note, the impaired feeding response in AgRP-ires-Cre; Vgatflox/flox; Npy−/− double KO mice over the first few hours of dark cycle feeding, when the basal firing rate of AgRP neurons is high, suggest that their role in rapidly activating feeding is physiologically relevant – not just restricted to pharmaco-genetic activation of AgRP neurons. Furthermore, although double KO mice show postponed feeding behavior during the first 2- and 4-hr of the dark cycle, food intake levels were comparable to control mice by the end of the entire 12-hr dark period, suggesting either the neuropeptide AgRP is sufficient to evoke chronic feeding or induction of food intake can occur via AgRP neural-independent circuit mechanisms.
Finally, the absence of any stimulation of feeding in triple KO mice combined with the late onset, but robust stimulation of feeding in GABA/NPY double KO mice (which retain MC4R signaling) strongly suggests that the hyperphagic response caused by released AgRP is due to inhibition of MC4Rs and not MC3Rs or other non-melanocortin receptor-mediated effects. This delayed, yet dynamic role for AgRP in promoting chronic food intake in comparison with the rapid effects of NPY align with the temporal differences of drug-elicited feeding episodes observed following pharmacological injection studies (Semjonous et al., 2009).
Experimental Procedures
Animals
All animal care and experimental procedures were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. AgRP-ires-Cre (Tong et al., 2008), Vgatflox/flox (Tong et al., 2008), Npy−/− (Erickson et al., 1996) and Mc4r−/− (Balthasar et al., 2005) mice were previously described. Knock out mice were all bred to the homozygous state for experiments.
Stereotaxic injections
Mice were injected as previously described (Krashes et al., 2011). Briefly, 200 nL bilateral injections of AAV8-DIO-hM3dq-mCherry (titer 1.2 × 1012 genomes copies per mL) were made in the ARC. All injected mice were positive for bilateral hits (Figure S4).
Food intake studies
Food intake studies on chow were performed as previously described (Krashes et al., 2011). For light cycle DREADD-mediated AgRP activation studies, 10- to 12- wk male mice were injected with either saline or CNO (0.3 mg/kg; i.p.) at 9 am, and food intake was monitored at 0.5-, 1-, 2-, 4-, 8- and 24-hr post-injection. Studies were run as within-subject design with each trial consisting of saline administration on day 1, CNO administration on day 2 and no injection on day 3, repeated over three trials. An averaged consumption value across trials for each animal for each time point was obtained by averaging the 3 values for that time point from each of the three trials. This averaged value was then used, in conjunction with similarly obtained values from the other animals within the same treatment/genotype group, to create a mean +/− SEM, and statistically analyzed. For latency studies, a lab timer (Control Company) was used to record the time it took mice to engage in a continuous 3 min meal immediately following CNO injection. For dark cycle physiological studies, food intake was monitored at 2-, 4-, 12- and 24-hr after lights were turned off (6 pm) in 10- to 12- wk male mice.
Immunohistochemistry
This procedure was performed as previously described (Bouret et al., 2012). Rabbit anti-AgRP (1:2000; Phoenix Pharmaceuticals) primary antibody and Alexa Fluor 594 donkey anti-mouse Ig (H+L) (Invitrogen; 1:200) secondary antibody were used to analyze AgRP projections. Fluorescent images were captured and processed under identical parameters with an Olympus VS120 Slide Scanner microscope.
Electrophysiology
The protocols of slice preparation and whole cell recording are previously described (Krashes et al., 2011; Liu et al., 2012). 5- to 7-wk Npy-hrGFP mice were used to visualize AgRP neurons.
Statistics
Statistical analyses were performed using KaleidaGraph (Synergy Software). DREADD-mediated feeding studies were run as within-subject design and a final consumption value for each animal obtained from an average of 3 trials. Data was analyzed using a two-way repeated-measures ANOVA, interaction of 'genotype' and 'treatment'. A P value of < 0.05 (*,# and $) was considered significant in these studies. Error bars indicate mean +/− SEM We analyzed all the data with a repeated measures ANOVA with 2 between subject factors (genotype, treatment) and one repeated factor (timepoint). We found main effects of genotype (F5,82=6.929, P<0.05), treatment (F1,86=403.216, P<0.05) and timepoint (F5,82=2907.265, P<0.05) and a three-way interaction between these factors (F35,347=3.169, P<0.05).
For dark cycle physiological feeding studies, 2 genotypes were assessed for food intake across 4 timepoints. Data was analyzed with a repeated measures ANOVA with 1 between subject factor (genotype), and one repeated measure (timepoint). We found a main effect of timepoint (F4,30=171.813, P<0.05), no significant effect of genotype (F1,33=1.210, P=0.28) and a significant interaction between genotype and timepoint (F4,30=11.866, P<0.05).
Supplementary Material
Research Highlights.
GABA, NPY and AgRP via MC4Rs, are required for DREADD stimulation-mediated feeding
Loss of each individual mediator does not impair feeding, suggesting redundancy
GABA or NPY is required for rapid stimulation of feeding
AgRP via MC4R signaling is sufficient for the delayed chronic feeding response
Acknowledgments
This research was funded by the following NIH grants to BBL: R01 DK096010, R01 DK089044, R01 DK071051, R01 DK075632, R37 DK053477 BNORC Transgenic Core P30 DK046200 and BADERC Transgenic Core P30 DK0547521; to MJK: F32 DK089710. We thank members of Lowell lab for helpful discussions. We thank Daniel Cusher and Yanfang Li for genotyping the various mouse lines, Jenna Carroll and Yikun Guo for perfusions and tissue removal, and Bryan L. Roth and Sarah C. Rogan for generating the AAV-FLEX-hM3Dq-mCherry plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Bates SH, Chen S, Myers MG, Jr, Simerly RB. Distinct roles for specific leptin receptor signals in the development of hypothalamic feeding circuits. J Neurosci. 2012;32:1244–1252. doi: 10.1523/JNEUROSCI.2277-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nature neuroscience. 2005;8:1289–1291. doi: 10.1038/nn1548. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Hollopeter G, Huszar D, Laufer R, Yagaloff KA, Fisher SL, Burn P, Palmiter RD. Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nature genetics. 1999a;21:119–122. doi: 10.1038/5070. [DOI] [PubMed] [Google Scholar]
- Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Effects of neuropeptide Y deficiency on hypothalamic agouti-related protein expression and responsiveness to melanocortin analogues. Brain research. 1999b;848:66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Molecular and cellular biology. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy P, Wang Q, Whim MD. Cell type-dependent trafficking of neuropeptide Y-containing dense core granules in CNS neurons. J Neurosci. 2011;31:14783–14788. doi: 10.1523/JNEUROSCI.2933-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Semjonous NM, Smith KL, Parkinson JR, Gunner DJ, Liu YL, Murphy KG, Ghatei MA, Bloom SR, Small CJ. Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance. International journal of obesity. 2009;33:775–785. doi: 10.1038/ijo.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Morton GJ, Ogimoto K, Stanhope K, Graham J, Baskin DG, Havel P, Schwartz MW, Barsh GS. Effects of hypothalamic neurodegeneration on energy balance. PLoS biology. 2005;3:e415. doi: 10.1371/journal.pbio.0030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.