Abstract
Electron transfer dissociation (ETD), a technique that provides efficient fragmentation while depositing little energy into vibrational modes, has been widely integrated into proteomics workflows. Current implementations of this technique, as well as other ion–ion reactions like proton transfer, involve sophisticated hardware, lack robustness, and place severe design limitations on the instruments to which they are attached. Described herein is a novel, electrical discharge-based reagent ion source that is located in the first differentially pumped region of the mass spectrometer. The reagent source was found to produce intense reagent ion signals over extended periods of time while having no measurable impact on precursor ion signal. Further, the source is simple to construct and enables implementation of ETD on any instrument without modification to footprint. Finally, in the context of hybrid mass spectrometers, relocation of the reagent ion source to the front of the mass spectrometer enables new approaches to gas phase interrogation of intact proteins.

Since its introduction, electron transfer dissociation (ETD) has become a powerful tool for protein and peptide characterization.1 Joining the tool box of gas phase ion–ion reactions, the extensive fragmentation of peptides induced by ETD is largely complementary to vibrational activation techniques, such as collisionally induced dissociation (CID).2–4 Additionally, workflows involving the interrogation of species with labile post-translational modifications have benefitted from the directed nature of ETD. Despite the unique ability of ETD to access information about labile modifications and provide extensive and complementary fragmentation, the number of publications utilizing ETD has been declining in recent years. We believe that the lack of a robust implementation of ETD has limited the adoption of the technique by more researchers.
The initial implementation of ETD utilized a chemical ionization (CI) source mounted on the rear of a linear ion trap mass spectrometer.5 During an ETD scan event, reagent ions enter the ion trap from the rear of the instrument, while precursor ions are introduced from the atmospheric pressure ionization (API) source. The rear-mounted CI source approach was subsequently extended to the linear trapping quadropole (LTQ) Orbitrap6 series of instruments. Since the Orbitrap mass analyzer is positioned off the axis of the primary ion optical path, a rear-mounted CI source can deliver reagent ions past the Orbitrap and to the linear ion trap. However, when considering other instruments, such as ion cyclotron resonance (ICR) instruments and future instrument layouts, two topological problems arise. First, reagent ions cannot be delivered from the rear of many instruments. Second, the size of a traditional chemical ionization source may prohibit its use on benchtop instruments.
Here, a novel implementation of ion–ion reactions that addresses both of these limitations while also improving robustness is described. By generating reagent ions in the first differentially pumped region of a mass spectrometer and delivering them to the ion–ion reaction region via the same ion path utilized by the API source, the size of the reagent source is drastically reduced and implementation on any instrument that includes an API interface becomes feasible. At the increased pressure in this region (∼1 Torr), the filament that typically generates the electrons used for ionization would burn out rapidly. Replacing the filament with an electrical discharge provides a stable and robust source of electrons and eliminates one of the most prevalent failure modes of traditional CI sources.
Repositioning of the reagent source to the front of the instrument also renders the use of ETD coupled with ion–ion proton transfer (IIPT) for the interrogation of intact proteins feasible. Since these reactions takes place in the ion trap in these instruments, multiple loads of product ions may be accumulated in the C-trap, thereby offsetting the loss in ion current because of the neutralization or transfer of charge by ETD and IIPT, respectively. By reducing the charge state of fragment ions, fragment signals are spread across the mass range. With higher effective peak capacity and reduced resolution requirements, gaining complete sequence information on proteins is drastically simplified by this approach.
Methods
Reagent Sources
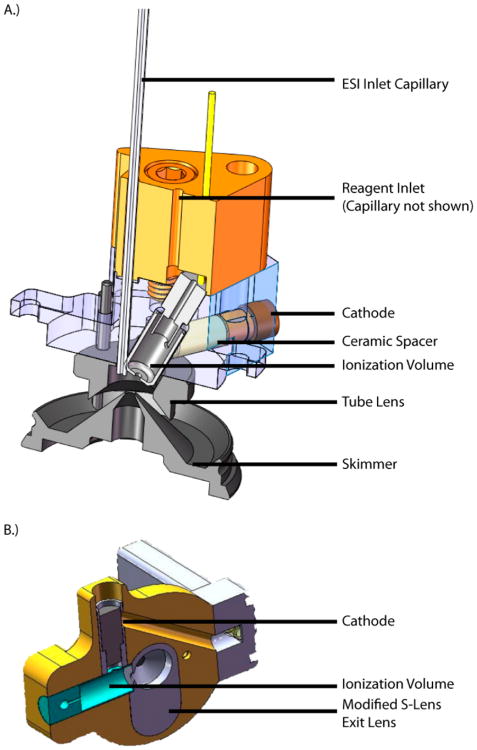
Two reagent sources were designed and constructed to function with both tube lens/skimmer and S-lens inlets. They are presented in Figure 1. In the tube lens/skimmer version, two PEEK pieces house the active components of the glow discharge source. A reagent inlet capillary delivers reagent and carrier gas where shown. A stainless steel ion volume that is 4 mm i.d. defines the ionization region. Press fit against the ion volume is a 4 mm o.d. × 3 mm i.d. ceramic spacer into which fits a stainless steel cathode. A collar on the cathode enforces a spacing of 1.5 mm from the side of the ion volume. A second source with the same internal dimensions was designed to sit directly downstream of a stacked ring ion guide. Here, the S-lens exit lens was increased in thickness to 7 mm to accommodate the ionization source. Thus, the ionization source is integral to the S-lens assembly.
Figure 1.
SolidWorks renderings of the reagent ion sources used with (A) tube lens/skimmer and (B) S-lens atmospheric interfaces.
In both sources, the discharge is powered by a custom current-controlled high voltage supply capable of 1.5 kV and 50 μA (Applied Kilovolt, West Sussex, U.K.). The supply is built to pulse to voltage in less than 5 ms. A 10 MΩ ballast resistor is placed in series between the power supply and the discharge (Vishay, Selb, GMBH). Utilizing several spare digital to analog converters in the instrument, the supply was brought under instrument control, enabling custom scan matrices to be programmed in the proprietary ion trap control language (ITCL) implemented on Thermo mass spectrometers.
Reagent Delivery
The reagent delivery system used for both sources is diagramed in Figure 2. Reagent is delivered to the discharge region through a dedicated reagent inlet capillary. To this capillary is attached a nitrogen gas split flow arrangement. A low flow of nitrogen, controlled by a 10 sccm full range mass flow controller (MKS Instruments, Orland Park, IL), passes through a reagent vial containing the desired reagent (e.g., fluoranthene or azulene). If required for the selected reagent, the vial temperature is regulated by a temperature controller (Omega Engineering, Stamford, CT). A second high flow joins the reagent flow downstream of the reagent vial. This flow is regulated by a 200 sccm full range mass flow controller (MKS Instruments, Orland Park, IL). The combined flow is delivered to the ionization volume.
Figure 2.
Schematic of the reagent inlet system. Two mass flow controllers and a temperature controlled reagent vial deliver a stable stream of vaporized reagent to the discharge ionization source through an independent capillary.
Instruments
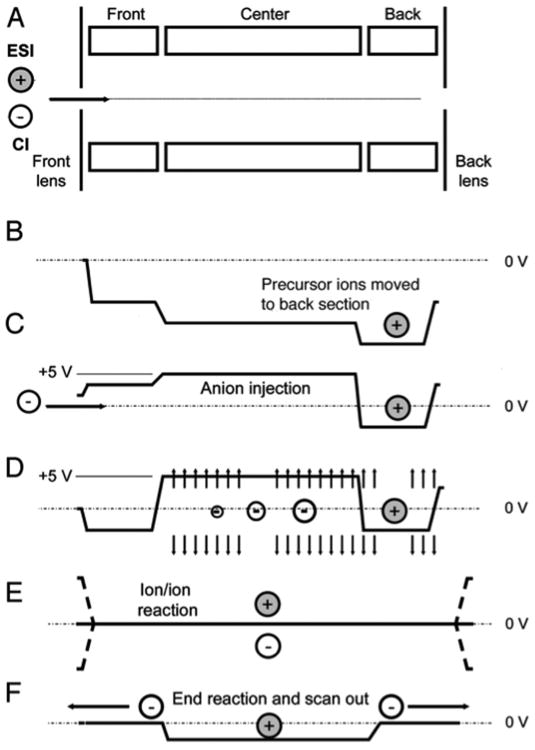
A standalone LTQ XL, an LTQ Velos, an LTQ Orbitrap XL, an LTQ FT-ICR Ultra (ThermoFisher Scientific, Germany) and an LTQ Orbitrap Velos Pro were fitted with reagent inlet systems, ion sources and power supplies as described previously. The LTQ Orbitrap Velos Pro and LTQ Velos utilized an S-lens source (panel B, Figure 1) and all other instruments utilized the tube lens/skimmer source (panel A, Figure 1). The instrument control software was modified to enable the ion–ion reactions as depicted in Figure 3. The electrical discharge was switched on only during reagent injection.
Figure 3.
Voltage changes occurring on a linear ion trap during an ion–ion reaction when reagent ions enter from the front of the ion trap. (A) A 3-section linear ion trap. (B–D) The voltages used to sequester ions and prevent mixing prior to charge sign independent trapping (E) and scan out (F).
Reagents
Azulene and fluoranthene were purchased from Sigma-Aldrich (St. Louis, MO) at 99% purity. SF6 was purchased form GTS-Welco (Allentown, PA). Ubiquitin from bovine red blood cells was purchased from Sigma-Aldrich (St. Louis, MO) at >90% purity. Ubiquitin was resuspended in 40% ACN with 0.1% AcOH for direct infusion experiments.
Stability Evaluation
The LTQ Velos, equipped with an S-lens source, was operated continuously in a mode that scans the reagent signal (in this case, fluoranthene) for 200 days. After 200 days, intensity data for the reagent signal was collected every 30 min for the next 186 days, while the source operated continuously, yielding a total study time of ∼13 months.
ETD and IIPT
Ubiquitin was directly infused at a concentration of 2.5 pmol/μL. SF6 at 10 ppm in N2 was introduced to the makeup nitrogen line depicted in Figure 2 through a 25 μm × 30 cm fused silica restrictor (2 psig applied to restrictor inlet). The +13 charge state was waveform isolated and subjected to reactions with fluoranthene radical anions for 10 ms and SF6&minus ions for 20 ms. The instrument programming was modified to enable the products from 10 consecutive reactions to be transferred and stored in the C-trap prior to detection in the Orbitrap mass analyzer. Reagent and precursor target values were 2 × 105. The resolution was set at 60 000 at m/z 400.
Data Analysis
Data was analyzed using a research version of ProSight PC capable of simultaneously searching for b, y, c and z ions. Further, this version has been modified to search for “off by n” errors that are caused by hydrogen radical transfers between fragments following electron transfer or caused by improper isotopic distribution matching by either THRASH or Xtract.
Results
Several researchers have investigated implementations of reagent ion sources that produce reagent ions at the front end of a mass spectrometer. Because of the elevated pressure compared to a traditional CI source, filament-based sources were immediately abandoned in favor of other approaches. These implementations relied on atmospheric pressure chemical ionization (APCI) sources or negative ESI followed by an in situ decarboxylation reaction to generate ETD reagent ions.7,8 Both of these approaches lacked the ability to generate the most efficient ETD reagent species. Because the chemistry governing the in situ generation of a reagent ion from a decarboxylation reaction is limiting, a discharge-based approach was selected for these investigations.
In our initial attempts at performing ETD with an APCI source, two major limitations were noted. First, the voltage required to initiate an APCI discharge was relatively high (several kilovolts), making the discharge difficult to pulse on and off. The additional time required to pulse the discharge made any approach with an atmospheric discharge difficult to implement. Further, the structure of a corona discharge (used for ionization in APCI sources) led to the formation of a putative stable species via the proposed reaction depicted in Figure 4 as the major product observed in the mass spectrometer when using fluoranthene as the reagent (confirmed by accurate mass detection in an Orbitrap mass analyzer). Other reagents, such as azulene, undergo similar reactions.
Figure 4.
Proposed mechanism for the formation of a stable anionic species that is observed experimentally.
These limitations led to the pursuit of an electrical discharge that operated at a lower voltage, enabling rapid switching, and that had a structure that could be exploited to prevent the formation of these nitrogen adducts. There are three major discharge regimes: Townsend, glow, and arc discharge. All of these discharges rely on a large electric field causing a gas to become electrically conductive. However, there are major differences in the structures and characteristics of these discharges. For our purposes, a glow discharge was selected.
The point at which the voltage applied to an electrode system induces a self-sustaining glow discharge is called the sparking potential (Vs in Figure 5). Freidrich Paschen derived an expression relating the properties of the gas, electrodes, and dimensions of the discharge apparatus to this potential:9
Figure 5.
Paschen's curve for air on a nickel cathode. The minimum voltage required to light a discharge with proper spacing of 6 mm at 1 Torr is slightly over 200 V.
where Vs is the sparking potential in volts, p is pressure in mmHg, and d is discharge distance in mm. A and B are constants related to the gas occupying the discharge region and have units of (mm-mmHg)−1 and V/mm-mmHg, respectively. Finally, γ is a constant indicating the likelihood of an incoming cation to release an electron from the cathode and is dimensionless. The plot in Figure 5 corresponds to air on a nickel cathode. Values of A and B are 14.6 mmHg−1 and 36.5 V/mm-mmHg. γ is 0.036 for air on a nickel cathode.
The expression indicates that there is a minimum in the sparking potential at a particular value of the product of the pressure and the distance between electrodes (pd) (Figure 5). Note that at 1 Torr, a distance of ∼6 mm between electrodes results in a sparking potential of just over 200 V. It is this realization that led to the repositioning of the reagent source to the first differentially pumped region of the mass spectrometer, since this region is maintained between 1 and 3 Torr. At such reduced pressure, discharge voltages as low as 400 V (applied through a ballast resistor) were sufficient to light the discharge.
In practice, the discharge turns on and stabilizes within 5 ms and may be extinguished in less than 1 ms. These rapid switching times can be absorbed by other events in a scan, resulting in no additional scan time. Further, a pulsed arrangement prevents the discharge source from impacting ions being delivered to the mass analyzer via the API source.
Once the discharge had been moved to the first differentially pumped region, the major product observed when using fluoranthene was still the nitrogen adduct. Electrical discharges in nitrogen produce atomic nitrogen via the dissociative recombination of an electron with N2+.10 Therefore, to prevent the formation of the nitrogen adduct, newly formed radical anions had to be segregated from the region of high atomic nitrogen concentration. To do this, the dimensions of the discharge source were tailored to take advantage of the structure of glow discharges.
The structure of a glow discharge is relatively complex.9 Nearest the cathode is a region of high electric field and negative space charge known as the Aston dark space. Here, there is a large concentration of electrons but they lack the energy sufficient to ionize the surrounding gas and thus generate no light. Following this region is the cathode glow. Cations, generated within the discharge, recombine after collision with the cathode and then relax to the ground state, emitting light. It is also in this region that the majority of atomic nitrogen is created. Next is the cathode dark space, a region of high electric field and positive space charge. In this region, cations are accelerated toward the cathode and electrons are accelerated away from it. After being accelerated through this region, some electrons have sufficient energy to ionize surrounding gas molecules, resulting in the next region, the negative glow. This is the most intensely lit region of the discharge due to the amount of ionization and excitation occurring here. Because of the ionizing collisions occurring in the negative glow, there is a large concentration of thermal electrons within and just past the negative glow. It is this region that is best utilized for ionizing reagent molecules. By placing an orifice between the cathode glow and the negative glow, atomic nitrogen may be separated from newly ionized reagent, preventing the formation of nitrogen adducts.
The orifice serves a second function. As the current through a glow discharge is increased, the current density at the cathode remains constant. Therefore, the area of the cathode covered by the plasma must increase. If the current is further increased after the plasma is covering the entire cathode surface, then the current density must rise. The increase in current density comes with a requisite increase in applied potential. This regime is known as the abnormal glow and is commonly used in sputter sources for various manufacturing processes. An orifice that is smaller than the surface area of the cathode forces the plasma to occupy a smaller area. In effect, it forces early onset of the abnormal discharge regime. The energetic collisions of cations with the cathode under these conditions prevent accumulation of material on the electrode surface, ensuring a stable and long-lasting source of reagent ions (the electrode is essentially self-cleaning).
The most effective reagents were used to assess the characteristics of the new reagent source. It was found to be very bright, producing reagent signals that facilitate <1 ms reagent injection times to a target value of 2e5 charges, which corresponds to a saturated condition. Further, the spectra in Figure 6 are displayed with no isolation to demonstrate the purity of the reagent generated. While the focus of these studies was ETD, the source is equally capable of generating reagents for both IIPT and negative ETD (nETD).
Figure 6.
Reagent ion spectra of both azulene (left) and fluoranthene (right) and a plot of reagent stability using fluoranthene.
The stability of the reagent source was also examined for a period of over 1 year (Figure 6). During this time, the reagent source produced an intense and stable reagent signal. Note that the overall intensity is reduced when compared to the reagent spectra. Because of the high ion flux generated by the source, the gain of the detectors was intentionally lowered to prevent detector aging on the time scale of the experiment, resulting in this apparently lower signal.
To accurately calculate injection times, automatic gain control (AGC) was implemented for the reagent. However, to eliminate scan time overhead, AGC was only performed once every 30 min, placing high demands on the stability of the reagent signal. Using a makeup flow arrangement accomplishes two goals. First, the low flow through the vial allows the reagent to establish equilibrium between the gas and liquid/solid phases, regardless of the amount of reagent present in the vial. Without this equilibrium, the reagent signal will decay slowly over time as reagent is consumed because the rate of evaporation/sublimation is surface area dependent. The split flow also drastically reduces the amount of reagent that is consumed. One hundred milligrams of reagent can provide continuous operation for >6 months currently, although modifications to this delivery system should enable the use of even less reagent.
By moving the reagent source to the front of an Orbitrap LTQ Velos Pro mass spectrometer, new experimental modes become viable. Proton transfer reactions have been utilized previously to control the charge state of proteins and their fragment ions following electrospray ionization. However, reducing the charge states of fragment ions also results in a concomitant reduction in sensitivity. The advantage, of course, is that fragment ions are concentrated in fewer charge states and spread across the mass spectral space, thereby reducing complexity and increasing effective peak capacity. The instrument configuration described above enables the ions resulting from multiple rounds of ETD followed by proton transfer to be accumulated in the C-trap prior to detection in the Orbitrap mass analyzer. In this way, the loss of charge via the ETD and IIPT reactions is compensated by multiple injections.
In this mode, the selected ions can be those generated across the available mass range (200 – 4000 amu) or those from a small segment within this range. In either case, the observed enhancement in signal-to-noise (S/N) increases linearly with each C-trap fill. In contrast, S/N enhancement observed by transient or spectral averaging increases linearly with the square root of the number of scans recorded. In short, multiple C-trap fills enabled by front-end ETD provides dramatic signal enhancement for spectra recorded on a chromatographic time scale.
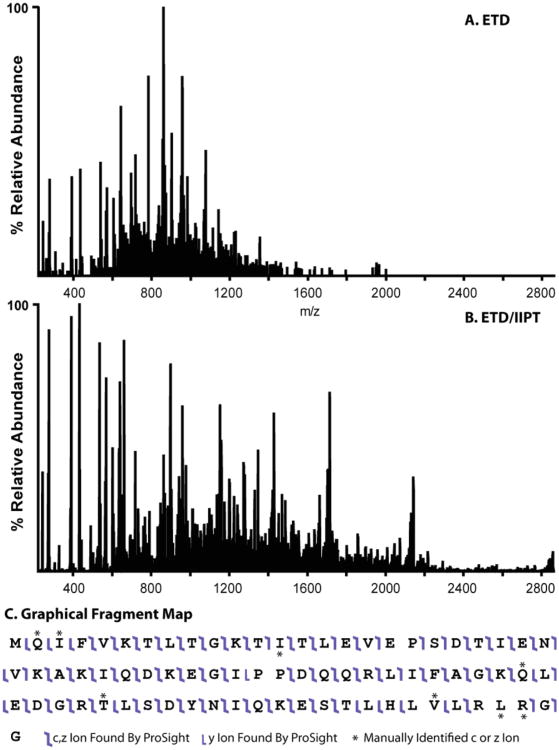
Figure 7 demonstrates the power of such a technique. Panel A is a single scan spectrum resulting from ETD alone. While this spectrum provides nearly full sequence coverage, the density of fragment ions complicates spectral interpretation. Panel B is the ETD spectrum of ubiquitin followed by IIPT. The spectrum is the average of 14 scans of 10 fills to the C-trap prior to detection. The spectrum demonstrates nearly 100% sequence coverage with fragment ion charge states ranging from +1 to +6. Because of charge reduction via IIPT, the sequence ions are spread over a greater m/z range. All of this dramatically eases spectral interpretation.
Figure 7.
Front-end ETD spectrum of ubiquitin (A) compared with front-end ETD/IIPT of ubiquitin (B). Sequence coverage for the ETD/IIPT spectrum is presented in panel C.
Conclusion
A reagent ion source with many favorable characteristics was developed and evaluated. The electrical discharge-based ion source produced stable and intense radical anionic reagents ions for ETD, radical cationic reagents for nETD and anionic species for IIPT over extended periods of time. Further, the simple construction of the source lowers materials costs and enables implementation on nearly any instrument without alteration to the instrument's footprint. The improved robustness and reduced cost of this reagent source should encourage stronger adoption of electron-based fragmentation techniques. Further, novel approaches that utilize sequential ion–ion reactions will become an important technique for intact protein characterization.
Acknowledgments
The authors thank Neil L. Kelleher and Ryan T. Fellers for granting access to the developmental search tools used to analyze the ubiquitin spectrum. We also thank our colleagues at Thermo Fisher Scientific for financial support of and intellectual contribution to this work. We also appreciate the financial support of NIH Grants GM037537 and NIH AI033993.
Footnotes
Notes: The authors declare no competing financial interest
References
- 1.Udeshi ND, Compton PD, Shabanowitz J, Hunt DF, Rose KL. Nat Protoc. 2008;3:1709–1717. doi: 10.1038/nprot.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alley WR, Jr, Mechref Y, Novotny MV. Rapid Commun Mass Spectrom. 2009;23:161–170. doi: 10.1002/rcm.3850. [DOI] [PubMed] [Google Scholar]
- 3.Han H, Xia Y, Yang M, McLuckey SA. Anal Chem. 2008;80:3492–3497. doi: 10.1021/ac7022734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan JM, Pitteri SJ, Chrisman PA, McLuckey SA. J Proteome Res. 2005;4:628–632. doi: 10.1021/pr049770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc Natl Acad Sci U S A. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAlister GC, Phanstiel D, Good DM, Berggren WT, Coon J. J Anal Chem. 2007;79:3525–3534. doi: 10.1021/ac070020k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang TY, Emory JF, O'Hair RA, McLuckey SA. Anal Chem. 2006;78:7387–7391. doi: 10.1021/ac061409v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DK, Jr, McAlister GC, Good DM, Coon JJ, Muddiman DC. Anal Chem. 2007;79:7916–7919. doi: 10.1021/ac071444h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobine JD. Gaseous Conductors, Theory and Engineering Applications. 1st. McGraw-Hill Book Company; New York; 1941. [Google Scholar]
- 10.Guberman SL. Presented at the Workshop on Planetary Atmospheres; Nov 6–7, 2007. [Google Scholar]