Abstract
The purpose of this report is to summarize the roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma (HCC). Chronic heavy alcohol exposure is a major risk factor for HCC, which is the most frequent type of liver cancer. Alcohol ingestion may initiate and or promote the development of HCC by: 1) acetaldehyde-DNA adduct formation; 2) cytochrome P4502E1-associated reactive oxygen species (ROS) generation, lipid peroxidation, p53 mutation, and conversion of pro-carcinogens to carcinogens; 3) iron accumulation that leads to ROS generation, lipid peroxidation, p53 mutation, and initiation of inflammatory cascade via nuclear factor-KappaB (NF-kB) activation; 4) glutathione depletion leading to oxidative stress; 5) s-adenosylmethionine (SAM) depletion and associated DNA hypomethylation of oncogenes ; 6) retinoic acid depletion and resultant hepatocyte proliferation via up-regulation of activator protein-1 (AP-1); 7) initiating an inflammatory cascade through increased transfer of endotoxin from intestine to liver, Kupffer cell activation via CD14/toll-like receptor-4 (TLR-4), oxidative stress, NF-kB or early growth response-1(Egr-1) activation, and generation of inflammatory cytokines and chemokines; 8) induction of liver fibrosis; and 9) decreasing the number and/or function of Natural Killer cells. Tobacco exposure is also a risk factor for HCC. It may contribute to the initiation and promotion of HCC due the presence of mutagenic and carcinogenic compounds as well as by causing oxidative stress due to generation of ROS and depletion of endogenous antioxidants. Simultaneous exposure to alcohol and tobacco is expected to promote the development of HCC in an additive and/or synergistic manner.
Keywords: hepatocellular carcinoma, alcohol, tobacco, acetaldehyde, CYP2E1, oxidative stress, inflammation, fibrosis
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the most frequent type of liver cancer, accounting for 90% of primary malignant liver tumors in adults (Altekruse et al. 2009; El-Serag and Rudolph 2007). The major risk factors of HCC are viral hepatitis (HCV and HBV), chronic heavy alcohol consumption, tobacco exposure, aflatoxin, and non-alcoholic steatohepatitis. This report will cover the roles of alcohol and tobacco exposure in the development of HCC.
Role of Alcohol in HCC
Chronic alcohol consumption represents a major risk factor for the development of HCC (Hassan et al. 2002; Morgan et al. 2004; Tagger et al. 1999). Of those who drink alcohol heavily for a long time, approximately 10 to 35% develop alcoholic steatohepatitis and about 10 to 20% develop cirrhosis, and of these about 10% develop HCC (Morgan et al. 2004). In an early animal study, mice failed to develop hepatic tumor even after administering 20% alcohol by volume in the water for 15 months (Ketcham et al. 1963). Subsequently in rats, alcohol administered in a liquid diet (Lieber 1994), or through intragastric infusion (Tsukamoto et al. 1995) also failed to induce liver tumors. However, a recent study by Yip-Schneider et al. (2011) has shown, probably for the first time, that chronic ethanol intake, 10% (v/v) for 18 months, can induce hepatic neoplasia in genetically manipulated alcohol-preferring rats. The percent of liver tumor incidence was 0%, 8.3%, and 83.3% at 6, 12, and 18 months respectively. When given free choice between a 10% (v/v) alcohol solution and water, alcohol-preferring rats voluntarily consume 6 to 8 g alcohol/kg BW/d (Chester et al., 2004). This intake of 6 g alcohol/kg BW/d is equivalent to an intake of 1.5 g alcohol/kg BW/d in humans (approximately 8 to 9 standardized alcoholic drinks/70 kg person/d). In comparison, inbred P rats consumed approximately half as much alcohol (3 to 4 g/kg BW/d) as the alcohol-preferring rats, and these rats did not develop neoplastic lesion. From this study, it is clear that both amount of alcohol and duration of alcohol consumption are critical factors for the development of liver tumor. In this study, tumor development in alcohol-preferring rats was associated with mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase (ERK) activation, increased cytochrome P4502E1 (CYP2E1) activity and increased intrahepatic oxidative stress. Alcohol may contribute to the development of HCC through several mechanisms (Brandon-Warner et al. 2012; Cho and Purohit 2006; Mckillop and Schrum 2009; Purohit et al. 2005; Seitz and Stickel 2010) which have been discussed in this report.
Acetaldehyde
In the liver, ethanol is primarily metabolized by alcohol dehydrogenase (ADH) and CYP2E1 to acetaldehyde, which is further metabolized to acetate primarily by mitochondrial low km aldehyde dehydrogenase (ALDH2). Absence of ALDH2 activity in alcohol-exposed Aldh2-null mice has been shown to be associated with higher levels of acetaldehyde in various tissues including liver (Isse et al. 2005). Epidemiological studies have shown that habitual alcohol drinking was likely to lead to an increased risk of HCC when acetaldehyde metabolizing activity (ALDH2) was deficient, suggesting a link between acetaldehyde accumulation and HCC (Munaka et al. 2003; Sakamoto et al. 2006). In addition, genotype ADH1C*1/1 with high capacity to generate acetaldehyde, has been identified as independent risk for the development of alcohol-associated HCC among heavy drinkers (Homann et al. 2006), further supporting a link between acetaldehyde and HCC. Several in vitro and in vivo studies have shown that acetaldehyde is mutagenic and carcinogenic (Seitz and Stickel 2010), but the mechanisms by which acetaldehyde may promote HCC are not clear. Acetaldehyde is known to react with DNA forming acetaldehyde-DNA adducts. Two major DNA adducts identified are N2-ethyl-2-deoxyguanosine (N2Et-dG) and 1, N2-propano-deoxyguanosine (PdG) (Brooks and Theruvathu 2005; Fang and Vaca 1997). DNA adduct formation may be a mechanism by which acetaldehyde could trigger the occurrence of replication errors and/or mutations in oncogenes or tumor suppressor genes. In addition, acetaldehyde can directly inhibit O6-methylguanosyl transferase, an enzyme important for the repair of adducts caused by alkylating agents (Espina et al. 1988). Moreover, acetaldehyde was shown to increase matrix metalloproteinase-9 (MMP-9) gelatinolytic activity and promote cell invasion through the up-regulation of MMP-9 gene transcription in HepG2 cells. The transcription of MMP-9 gene was up-regulated by 10 microM acetaldehyde via inductions of NF-kB and AP-1 activities (Hsiang et al. 2007). Thus, acetaldehyde may increase the prevalence of cell invasion in HCC patients, a metastatic effect of alcohol.
CYP2E1 induction
Chronic alcohol consumption leads to a several fold induction of CYP2E1, which metabolizes ethanol to acetaldehyde (Lieber 1994; Purohit et al. 2009). CYP2E1-dependent alcohol metabolism leads to increased hepatic oxidative stress due to the generation of ROS including hydroxyethyl radicals (Mckillop and Schrum 2009). The role of alcohol ingestion-associated induction of CYP-2E1 in the generation of ROS and development of alcoholic liver disease (ALD) has been demonstrated in many animal studies (Bradford et al. 2005; Dupont et al. 1998; Gouillon et al. 2000; Morgan et al. 2002). ROS produced by CYP2E1 lead to lipid peroxidation, with the generation of malondialdehyde (MDA) and 4-hydoxynonenal (4-HNE) (Aleynik et al. 1998; Pan and Chung 2002). It has been shown that the concentration of CYP2E1 in the liver is correlated with the generation of hydroxyethyl radicals (HERs) and thus with lipid peroxidation (Dupont et al. 1998). 4-HNE preferentially forms DNA adducts at codon 249 of the human tumor suppressor p53 gene (Hu et al. 2002), supporting the suggestion that this is a targeted mutational site for HCC. In fact, a large fraction of HCC harbors mutation in the gene encoding for the p53, resulting in the loss of p53 function (Tannapfel and Wittekind 2002). The tumor suppressor p53 restrains the expansion of carcinogen-initiated cells by inducing cell cycle arrest and apoptosis. In this regard, Pani et al. (2004) investigated the effect of ethanol on liver injury in p53 deficient mice. Ethanol-induced hepatocyte apoptosis was completely abrogated in the p53 null background, suggesting that the tumor suppressor p53 is necessary for hepatocyte death by ethanol. In addition, marked signs of dysplasia, loss of normal architecture, and malignant transformation were observed in the liver of mutant mice exposed to ethanol, but not in the control-fed littermates nor in ethanol-fed normal mice. These observations suggest that p53-dependent apoptosis restrains the tumorigenic effect of ethanol on liver cells.
Another way by which 4-HNE generated from CYP2E1 induction may promote hepatic tumorogenesis is by inducing the production of etheno DNA adducts which are potentially mutagenic and carcinogenic. Increased levels of etheno-DNA adducts have been detected in the hepatocytes of ALD patients (Frank et al. 2004). In addition, protein-bound 4-HNE and etheno-DNA adducts both strongly correlate with CYP2E1 expression in patients with ALD (Wang et al. 2009). Thus, it is possible that sustained oxidative stress associated with chronic alcohol consumption via induction of CYP2E1 could play an important role in the initiation and promotion of carcinogenesis.
In addition to ROS generation mechanism, CYP2E1 may promote hepatic carcinogenesis via conversion of pro-carcinogens or co-carcinogens such as nitrosamines and azo-compounds, to carcinogens (Guengerich et al. 1994; Lieber 2000; Yang et al. 1990).
Thus, CYP2E1 may promote the development of HCC via generation of ROS, conversion of procarcinogens, and formation of DNA etheno adducts.
Iron accumulation
Chronic alcohol intake is associated with increased accumulation of iron in the hepatocytes and Kupffer cells of the liver (Purohit et al. 2003; Tsukamoto et al. 1999; Valerio et al. 1996). Hepatic iron overload also develops in many individuals who consume alcohol on a chronic basis (Fletcher et al. 1999; Suzuki et al. 2002). Both alcohol and iron are known prooxidants, and oxidative stress is known to play an important role in the development of several diseases including cancer (Klaunig and Kamendulis 2004). The metabolism of alcohol, especially through CYP2E1, can lead to generation of superoxide and hydrogen peroxide. Moreover, hydrogen peroxide can react with ferrous iron (Fe2+) through the Fenton reaction, and generate hydroxyl radicals, which are highly reactive (Petersen 2005). Hydroxyl radicals can react with lipid molecules, initiating chain reactions that lead to lipid peroxidation and generation of products, such as acrolein, crotonaldehyde, MDA, and 4-HNE (Pan and Chung 2002). The 4-HNE is known to cause mutations of p53 gene (a tumor suppressor gene), which may initiate the development of HCC (Hu et al. 2002). Thus, it is possible that sustained combined oxidative stress associated with chronic alcohol consumption and hepatic iron overload could play an important role in the initiation and promotion of carcinogenesis.
Dietary iron exposure has been linked to an increased incidence of HCC in humans (Kew and Asare 2007). In addition, in hereditary hemochromatosis, hepatic iron overload is a major factor in hepatocarcinogenesis (Kowdley 2004). Furthermore, a synergistic effect of alcohol intake and iron accumulation on HCC has been reported in patients with hemochromatosis (Fletcher et al. 2002). The high incidence of human HCC in individuals with the hereditary iron storage disease hemochromatosis supports the suggestion that iron may function as a co-carcinogen in the liver (Deugnier 2003; Huang 2003). An excess of iron accumulated in hepatic macrophage (Kupffer cells) in response to chronic alcohol intake can activate, by means of oxidative stress, nuclear factor-kappa B (NF-kB), which can increase the transcription of the proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha). Thus, in alcoholics, alcohol and iron together may initiate chronic inflammation, which is a known risk factor for liver cancer (Berasain et al. 2009).
Glutathione depletion
It is well established that mitochondria are a leading source of endogenous ROS generation (Kushnarvea et al. 2002; Lenaz 2001; Sato 2007). During the respiration process the majority of oxygen is consumed in the cytochrome-c oxidase complex of the mitochondrial respiratory chain where ROS are not generated. However, within the respiratory chain, the ubiquinone pool of complex III generates superoxide anions as a result of single electron transfer to molecular oxygen (Kushnareva et al. 2002; Lenaz 2001). The superoxide and hydrogen peroxide are precursors of hydroxyl radical formation (Lenaz 2001; Sato 2007). To maintain a balanced mitochondrial redox state, glutathione acts as the endogenous mitochondrial antioxidant by scavenging ROS (Bai and Cederbaum 2001; Garcia-Ruiz and Fernandez-checa 2006; Lash 2006). Glutathione synthesized in the cytoplasm is transported to mitochondria, which does not synthesize its own glutathione. Within the mitochondria, glutathione peroxidase is the only source of hydrogen peroxide metabolism (Bai and Cederbaum 2001; Fernandez-Checa et al. 2002) since mitochondria lacks catalase that breaks down hydrogen peroxide in the cytoplasm. Several studies suggest that ethanol can deplete hepatic glutathione (Bansal et al. 2010; Kumar et al. 2011; Muller et al. 2011). However, in the setting of chronic alcohol consumption, mitochondrial glutathione is depleted (Purohit and Russo 2002a; Tang et al. 2012), at least in part, due to inhibition of glutathione uptake (Fernandez-Checa et al. 1998; Franco et al. 2007; Lash 2006). This can lead to elevated mitochondrial levels of hydrogen peroxide and eventually hydroxyl radicals, which in turn may lead to lipid, protein, and DNA adduct formation, rendering liver vulnerable to carcinogenesis.
S-adenosylmethionine depletion and DNA hypomethylation
DNA methylation is an important determinant in controlling gene expression, whereby hypermethylation has a silencing effect on genes and hypomethylation may lead to increased gene expression. In hepatocarcinogenesis, general hypomethylation may be coupled with areas of regional hypermethylation. Thus, hypermethylation of tumor suppressor genes can result in decreased gene transcription of p53 and HIC-1 (Kanai et al. 1999), and hypomethylation of certain oncogenes such as c-myc and c-N-ras may lead to dedifferentiation and proliferation (Shen et al. 1998; Wainfan et al. 1989; Wainfan and Poirier 1992).
S-adenosylmethionine (SAM) is the principal biologic methyl donor, the precursor for polyamine biosynthesis, and, in liver, a precursor of glutathione (Mato et al. 2002). SAM is synthesized from its precursor methionine through a reaction catalyzed by methionine adenosyltransferase (MAT) (Mato et al. 2002). MATI is primarily expressed in the liver, whereas its isoform MATII is distributed widely in the body. Several lines of evidence suggest a link between SAM deficiency and liver injury, including HCC. In experimental models, SAM deficiency induced by methionine-choline– deficient diet caused DNA hypomethylation and increased DNA strand breaks (DNA instability), and these changes are associated with an increased risk for cancer, (Pogribny et al. 1995). In addition, MAT1A knockout mice, which are deficient in hepatic SAM (76% lower), develop hepatic hyperplasia by 3 months, spontaneous steatohepatitis by 8 months, and HCC by 18 months in most of the knockout mice who were fed a normal diet (Lu et al. 2001; Martinez-Chanter et al. 2002). Furthermore, exogenous SAM administration inhibits the growth of hepatoma cells in culture (Cai et al. 1998) and prevents development of HCC in rats treated with hepatocarcinogen (Pascale et al. 1992; Pascale et al. 1995).
Chronic ethanol has been reported to deplete hepatic SAM levels which are associated with liver cirrhosis or malignant transformation (Lieber et al. 1990; Lu et al. 2000; Purohit and Russo 2002a; Purohit et al. 2005). For example, in rats, ethanol administration to rats for 9 weeks resulted in a relative switch in hepatic MAT expression, decreased SAM levels, hypomethylation of c-myc, increased c-myc expression, and increased DNA strand breaks, supporting the suggestion of an association between reduced SAM levels and malignant degeneration (Lu et al. 2000). In alcohol-induced liver cirrhosis, hepatic MATII activity was decreased which probably resulted in decreased production of SAM (Lieber et al. 1990). These results suggest that ethanol may contribute to the development of HCC via decreasing hepatic SAM levels.
Retinoid metabolism
Vitamin A (retinol) is oxidized by retinol dehydrogenase to retinal, which is further oxidized to retinoic acid by retinal dehydrogenase. In addition, ADH and ALDH participate in the metabolism of retinol to retinal and retinal to retinoic acid, respectively. Retinoic acid is the most physiological active retinoid, and it regulates gene transcription of various regulators of cell proliferation, growth, differentiation, apoptosis, and migration by signaling through its nuclear retinoic acid receptors (RARs and RXRs) (Wang 2005). Depletion of systemic and tissue-specific retinoic acid levels may have important consequences for cell proliferation, differentiation and possibly malignant transformation. Alcoholics have decreased levels of vitamin A (retinol and retinyl ester) in their liver (Leo and Lieber 1982). In addition, ethanol feeding has been shown to decrease hepatic concentrations of retinoic acid in rats (Liu et al. 2001; Wang et al. 1998). Furthermore, ethanol can reduce hepatic levels of retinoic acid by enhancing its catabolism due to induction of CYP2E1 (Chung et al. 2001). CYP2E1 has been shown to play a major role in depleting liver retinoic acid stores by facilitating retinoic acid degradation into polar metabolites (Liu et al. 2001). Treatment with chlormethiazole, a CYP2E1 inhibitor, has been shown to restore hepatic retinoic acid levels decreased by ethanol exposure (Liu et al. 2001), further suggesting a role of CYP2E1 in retinoic acid catabolism.
Alcohol-induced impairment of retinoic acid homeostasis may interfere with its action and signaling. For example, a decrease in retinoic acid levels following chronic ethanol administration in rats was associated with a decrease in MAPK and an increase in levels of phosphorylated c-Jun N-terminal kinase (JNK) (Wang et al. 1998). This was further associated with a functional downregulation of retinoic acid receptors and up to an eightfold higher expression of the AP1 (JUN and FOS) transcriptional complex, resulting in hepatic cell hyperproliferation and a decrease in apoptosis (Wang et al. 1998). Thus, low hepatic retinoic acid levels caused by ethanol administration may lead to increased hepatocyte proliferation via up-regulation of AP-1, with a resultant increased risk for cancer promotion.
Inflammation
Increasing evidence suggests a link between chronic inflammation and cancer development (Coussens and Werb 2002; Karin 2006; Mantovani et al. 2008). This link appears to be strong between chronic hepatitis and development of HCC (Berasain et al. 2009). Although underlying molecular mechanisms by which chronic inflammation triggers the development of HCC are not clear, various molecules such as NF-kB, STAT3, IL-6, IL-1alpha, and EGF receptor ligands have been implicated (Bersain et al. 2009). Several researchers have contributed to the understanding of molecular mechanisms by which chronic alcohol exposure initiates liver inflammation. In animal models, chronic alcohol is known to increased chronic inflammation through enhancing the levels of pro-inflammatory cytokines with disruption of immune surveillance (An et al. 2012; Wang et al. 2012). Chronic ethanol is known to increase intestinal permeability to endotoxin, which plays a central role in initiating and potentiating alcohol-associated liver inflammation (Adachi et al. 1995; Keshavarzian et al. 2001; kirpich et al. 2012; Nanji et al. 1994; Purohit et al. 2008; Wang et al. 2011). By binding to CD14/TLR-4 receptors on Kupffer cells, endotoxin initiates a cascade of events leading to free radical generation, NF-KB activation, and production of inflammatory mediators such as cytokines (e.g., TNF-alpha), chemokines, and adhesion molecules (Purohit and Russo 2002b; Thurman et al. 1998; Uesugi et al. 2001; Wheeler et al. 2001; Yin et al. 2001). An alternate pathway of Kupffer cell activation involves activation of ERK1/2, increased expression of Egr-1, and TNF-alpha production (Thakur et al. 2007). In Kupffer cells, NADPH is a major source of ROS responsible for the activation of NF-kB or Egr-1 transcription factors (Kono et al. 2000; Thakur et al. 2007). Other mechanisms by which alcohol may initiate or promote liver inflammation have been recently reviewed in detail by Gao and Bataller (2011). These include: a) ethanol metabolism-associated generation of reactive oxygen species; b) TLR-4-induced activation of MyD88-independent signaling pathway in Kupffer cells; c) activation of complement C3 and C5 and subsequent activation of Kupffer cells; d) hepatic infiltration of neutrophils; and e) activation of adaptive immunity. Thus, alcohol exposure-induced chronic liver inflammation is likely to contribute to the development of HCC.
Liver fibrosis/cirrhosis
Chronic heavy alcohol intake is a major cause of liver fibrosis which is characterized by excessive deposition of extracellular matrix (ECM) components, especially collagen, due to increased matrix production and/or decreased matrix degradation. If alcohol consumption is continued, fibrosis may progress to cirrhosis which is a major cause of morbidity and mortality. Alcohol may contribute to liver fibrosis through several mechanisms including, generation of acetaldehyde, increased oxidative stress and associated lipid peroxidation, upregulation of fibrogenic cytokines such as transforming growth factor beta-1(TGF-beta1), induction of hepatocyte apoptotic bodies, and Kupffer cell activation and associated inflammatory cytokine (e.g., (TNF-alpha) production (Purohit and Brenner, 2006; Gao and Bataller, 2011). Chronic alcohol consumption may also promote liver fibrosis by down-regulating the antifibrotic effects of natural killer (NK) cells (Jeong et al., 2008). Liver fibrosis/cirrhosis in turn is strongly associated with HCC, with 90% of HCC cases arising in cirrhotic livers (Seitz and Stickel, 2006), including alcoholic cirrhosis (Fattovich et al., 2004). Potential mechanisms of fibrosis-dependent carcinogenesis include increased integrin signaling by the fibrotic matrix, paracrine signaling between hepatic stellate cells and hepatocytes, increased stromal stiffness, growth factor sequestration by ECM, and reduced tumor surveillance by NK and natural killer T (NKT) cells (Zhang and Friedman, 2012). It is important to note that HCC may develop in the absence of cirrhosis since presence of HCC in non-cirrhotic liver has been reported in human subjects (Okuda et al., 1989; Grando-Lemaire et al., 1999). In addition, recently Yip-Schneider et al. (2011) reported the presence of liver tumors in alcohol-preferring rats in response to chronic alcohol exposure in the absence of cirrhotic changes.
Natural Killer cells
Natural killer (NK) cells, a component of innate immunity, play an important role in the elimination of viral-infected and tumor cells. In the liver these cells have been reported to exhibit antiviral and anti-tumor effects (Gao et al. 2009). Alcohol appears to adversely affect the number and activity of NK cells (Cook et al. 1997; Pan et al. 2006), and thus impairs NK immune surveillance. Ethanol decreased the number and activity of splenic NK cells in a mouse model for binge drinking (Wu et al. 1994). In rats, alcohol administration results in a 10-fold increase in lung metastasis from a cancer that is controlled by NK cells (Ben-Eliyahu et al. 1996). Finally, there is evidence for decreased NK cell number and lytic activity in alcoholics with or without liver disease (Cook et al. 1991; Laso et al. 1997). Taken together these findings suggest that alcohol-induced decreased activity of NK cells may permit the progression of HCC due to their decreased ability to eliminate early tumor cells.
Role of Tobacco Exposure in HCC
Several studies have suggested that tobacco smoking is a significant risk factor for the development of HCC (Hara et al. 2008; Koh et al. 2011; Kuper et al. 2000; Lee et al. 2009; Sakamoto et al. 2008). This connection was further established in a recent study conducted on Singapore Chinese population where a significant dose- and duration-dependent association was observed between tobacco use and HCC risk, even after excluding daily alcohol drinkers from the study (Koh et al. 2011). The liver is a major organ for the metabolism of more than 40 tobacco-related active compounds (Hoffmann et al. 1993), several of which are well-accepted carcinogens such as polycyclic aromatic hydrocarbons, nitrosamines, and aromatic amines (Chen et al. 2002; Staretz et al. 1997). Moreover, several tobacco-related carcinogens, such as N-nitrosodimethylamine and 4-aminobiphenyl, have been directly implicated in the development of liver tumors in animal studies (Dooley et al. 1992; IARC 1987). Cigarette smoke and its main component nicotine have been shown to up regulate CYP2E1 activity in the liver of rodents and human (Joshi and Tyndale 2006; Micu et al. 2003). Since CYP2E1 induction is associated with ROS generation and lipid peroxidation, this may be a mechanism whereby tobacco smoke may contribute to HCC. Long-term exposure of rats to aqueous extract of gutkha (a form of smokeless tobacco) decreased hepatic levels of glutathione and activities of glutathione peroxidase, superoxide dismutase, and catalase, but increased lipid peroxidation in the liver (Avti et al. 2006). Thus, tobacco exposure-associated oxidative stress resulting from the depletion of antioxidants and increased lipid peroxidation may make liver susceptible to tumor development. Taken together, tobacco exposure may contribute to the initiation and promotion of HCC due the presence of mutagenic and carcinogenic compounds as well as by causing oxidative stress due to generation of ROS and depletion of endogenous antioxidants. Tobacco smoking is associated with increased plasma levels of inflammatory cytokines such as TNF-alpha and IL-1beta (Barbieri et al., 2011; Diez Pina et al., 2012) and markers of oxidative stress (Valenca et al., 2008; Barbieri et al., 2011). These mediators can contribute to necro-inflammatory changes in the liver, which in turn may promote the development of HCC.
Summary
Chronic heavy alcohol exposure is a major risk factor for HCC, which is the most frequent type of liver cancer and the fifth most common cancer in the world. In a recent rat study, heavy alcohol exposure for 18 months induced liver tumors in 83.3% of the treated animals, further confirming the tumorogenic effect of alcohol. Alcohol consumption may promote HCC development through various mechanisms including acetaldehyde production, CYP2E1 induction, iron accumulation, glutathione depletion, SAM depletion and associated DNA hypomethylation, dysregulated retinoid metabolism, induction of inflammation, induction of fibrosis and impaired NK cell activity. Acetaldehyde may promote HCC by forming mutagenic/carcinogenic DNA adducts, inhibiting O6-methylguanosyl transferase activity, and up-regulating gene expression of MMP-9 and associated increased cell invasion (metastasis). Alcohol-induced CYP2E1 induction is likely to promote hepatic carcinogenesis through increased ROS generation, lipid peroxidation, especially production of 4HNE which can cause mutation of p53 gene (tumor suppressor) via DNA adduct formation, etheno adduct formation, and conversion of pro-carcinogens to carcinogens. Alcohol-associated hepatic iron accumulation can promote tumor growth by generation of hydroxyl radicals via Fenton reaction, lipid peroxidation, inducing mutation of p53 gene, and initiating inflammatory cascade through activation of NF-kB and subsequent production of inflammatory mediators. Chronic alcohol intake may render liver susceptible to tumor growth by depleting mitochondrial glutathione, an endogenous antioxidant. Chronic ethanol ingestion leads to hepatic SAM depletion which is linked to the development of HCC that is probably caused by hypomethylation of oncogenes (e.g., c-myc) and subsequently their increased expression. Decreased hepatic retinoic acid levels caused by ethanol administration may lead to increased hepatocyte proliferation via up-regulation of AP-1, with a resultant increased risk for cancer promotion. Chronic ethanol exposure may also promote HCC development by initiating an inflammatory cascade through increased transfer of endotoxin from intestine to liver, Kupffer cell activation via CD14/TLR-4 receptors, oxidative stress, NF-kB or Egr-1 activation, and generation of inflammatory cytokines and chemokines. Finally, ethanol may render liver susceptible to tumor development by decreasing the number or function of NK cells.
Tobacco exposure is also a risk factor for HCC. It may contribute to the initiation and promotion of HCC due the presence of mutagenic and carcinogenic compounds as well as by promoting oxidative stress via generation of ROS and depletion of endogenous antioxidants. Simultaneous exposure to alcohol and tobacco is expected to promote the development of HCC in an additive or synergistic manner.
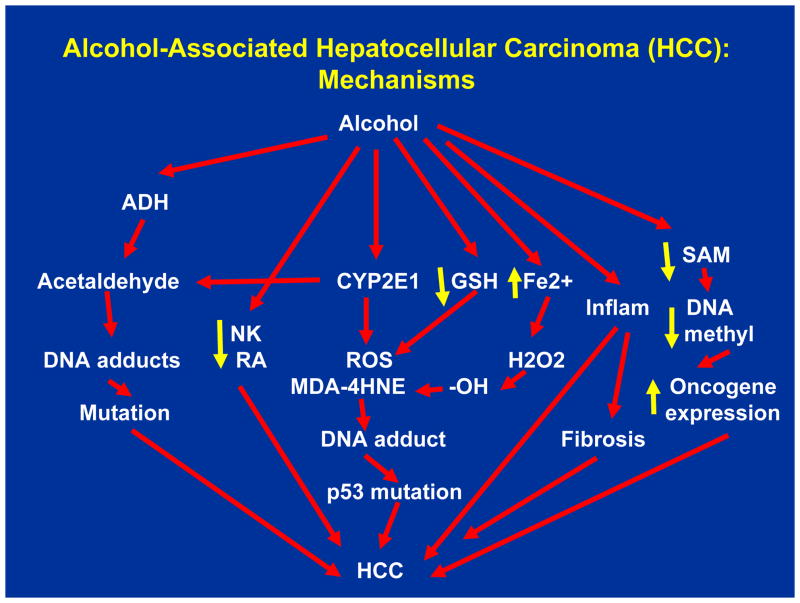
Figure.
Alcohol-Associated Hepatocellular Carcinoma (HCC): Mechanisms Acetaldehyde generated from the metabolism of alcohol, catalyzed by alcohol dehydrogenase (ADH) and cytochrome P4502E1 (CYP2E1), can form mutagenic/carcinogenic DNA adducts that may promote HCC. Alcohol may promote HCC by depleting hepatic Natural Killer cells (NK) and retinoic acid (RA). Alcohol-induced CYP2E1 generates reactive oxygen species (ROS), which can react with lipid molecules to form malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE). 4-HNE can react with DNA forming DNA adducts, which may result in p53 mutation and subsequent promotion of HCC. Alcohol may promote HCC by depleting antioxidant glutathione (GSH). Alcohol-associated accumulation of iron in the liver can generate hydroxyl (-OH) radicals by reacting with H2O2. –OH radicals can react with lipids and form MDA and 4-HNE, which can promote HCC via formation of DNA adducts and inducing p53 mutation. Alcohol-associated liver inflammation has potential to promote HCC, directly as well as indirectly via promoting liver fibrosis. Finally, alcohol-induced hepatic s-adenosylmethionine (SAM) depletion can result in DNA hypomethylation, increased oncogene expression, and promotion of HCC.
Footnotes
Conflict of interest statement: The authors declare that there are no conflicts of interest to report.
References
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–24. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:192–6. [PubMed] [Google Scholar]
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An L, Wang X, Cederbaum AI. Cytokines in alcoholic liver disease. Arch Toxicol. 2012 Feb 25; doi: 10.1007/s00204-012-0814-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Avti PK, Kumar S, Pathak CM, Vaiphei K, Khanduja KL. Smokeless tobacco impairs the antioxidant defense in liver, lung, and kidney of rats. Toxicol Sci. 2006;89:547–53. doi: 10.1093/toxsci/kfj041. [DOI] [PubMed] [Google Scholar]
- Bai J, Cederbaum AI. Mitochondrial catalase and oxidative injury. Biol Signals Recept. 2001;10:189–99. doi: 10.1159/000046887. [DOI] [PubMed] [Google Scholar]
- Bansal S, Liu CP, Sepuri NB, Anandatheerthavarada HK, Selvaraj V, Hoek J, Milne GL, Guengerich FP, Avadhani NG. Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augments alcohol-mediated oxidative stress. J Biol Chem. 2010;285:24609–19. doi: 10.1074/jbc.M110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri SS, Zacchi E, Amadio P, Gianellini S, Mussoni L, Weksler BB, Tremoli E. Cytokines present in smokers’ serum interact with smoke components to enhance endothelial dysfunction. Cardiovasc Res. 2011;90:475–83. doi: 10.1093/cvr/cvr032. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG, Yirmiya R, Taylor AN. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med. 1996;2:457–60. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–21. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, Bleye L, Krausz KW, Gonzalez FJ, Koop DR, Rusyn I. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336–44. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- Brandon-Warner E, Walling TL, Schrum LW, McKillop IH. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol Clin Exp Res. 2012;36:641–53. doi: 10.1111/j.1530-0277.2011.01660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–93. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cai J, Mao Z, Hwang JJ, Lu SC. Differential expression of methionine adenosyltransferase genes influences the rate of growth of human hepatocellular carcinoma cells. Cancer Res. 1998;58:1444–50. [PubMed] [Google Scholar]
- Chen SY, Wang LY, Lunn RM, Tsai WY, Lee PH, Lee CS, Ahsan H, Zhang YJ, Chen CJ, Santella RM. Polycyclic aromatic hydrocarbon-DNA adducts in liver tissues of hepatocellular carcinoma patients and controls. Int J Cancer. 2002;99:14–21. doi: 10.1002/ijc.10291. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Zweifel M, Froehlich JC. Effects of stress on alcohol consumption in rats selectively bred for high or low alcohol drinking. Alcohol Clin Exp Res. 2004;28:385–93. doi: 10.1097/01.alc.0000117830.54371.7a. [DOI] [PubMed] [Google Scholar]
- Cho CH, Purohit V. Alcohol, Tobacco, and Cancer. New York: Karger; 2006. [Google Scholar]
- Chung J, Liu C, Smith DE, Seitz HK, Russell RM, Wang XD. Restoration of retinoic acid concentration suppresses ethanol-enhanced c-Jun expression and hepatocyte proliferation in rat liver. Carcinogenesis. 2001;22:1213–9. doi: 10.1093/carcin/22.8.1213. [DOI] [PubMed] [Google Scholar]
- Cook RT, Garvey MJ, Booth BM, Goeken JA, Stewart B, Noel M. Activated CD-8 cells and HLA DR expression in alcoholics without overt liver disease. J Clin Immunol. 1991;11:246–53. doi: 10.1007/BF00918182. [DOI] [PubMed] [Google Scholar]
- Cook RT, Li F, Vandersteen D, Ballas ZK, Cook BL, LaBrecque DR. Ethanol and natural killer cells. I. Activity and immunophenotype in alcoholic humans. Alcohol Clin Exp Res. 1997;21:974–80. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deugnier Y. Iron and liver cancer. Alcohol. 2003;30:145–50. doi: 10.1016/s0741-8329(03)00129-0. [DOI] [PubMed] [Google Scholar]
- Díez Piña JM, Fernández Aceñero MJ, Llorente Alonso MJ, Díaz Lobato S, Mayoralas Alises S, Pérez Rodríguez E, Alvaro Álvarez D, Flórez Horcajada A, Pérez Rojo R. Tumor necrosis factor as an early marker of inflammation in healthy smokers. Med Clin (Barc) 2012;139:47–53. doi: 10.1016/j.medcli.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Dooley KL, Von Tungeln LS, Bucci T, Fu PP, Kadlubar FF. Comparative carcinogenicity of 4-aminobiphenyl and the food pyrolysates, Glu-P-1, IQ, PhIP, and MeIQx in the neonatal B6C3F1 male mouse. Cancer Lett. 1992;62:205–9. doi: 10.1016/0304-3835(92)90097-f. [DOI] [PubMed] [Google Scholar]
- Dupont I, Lucas D, Clot P, Ménez C, Albano E. Cytochrome P4502E1 inducibility and hydroxyethyl radical formation among alcoholics. J Hepatol. 1998;28:564–71. doi: 10.1016/s0168-8278(98)80279-1. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Espina N, Lima V, Lieber CS, Garro AJ. In vitro and in vivo inhibitory effect of ethanol and acetaldehyde on O6-methylguanine transferase. Carcinogenesis. 1988;9:761–6. doi: 10.1093/carcin/9.5.761. [DOI] [PubMed] [Google Scholar]
- Fang JL, Vaca CE. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis. 1997;18:627–32. doi: 10.1093/carcin/18.4.627. [DOI] [PubMed] [Google Scholar]
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35–50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Fernández-Checa JC, Colell A, García-Ruiz C. S-Adenosyl-L-methionine and mitochondrial reduced glutathione depletion in alcoholic liver disease. Alcohol. 2002;27:179–83. doi: 10.1016/s0741-8329(02)00229-x. [DOI] [PubMed] [Google Scholar]
- Fernández-Checa JC, Kaplowitz N, García-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- Fletcher LM, Dixon JL, Purdie DM, Powell LW, Crawford DH. Excess alcohol greatly increases the prevalence of cirrhosis in hereditary hemochromatosis. Gastroenterology. 2002;122:281–9. doi: 10.1053/gast.2002.30992. [DOI] [PubMed] [Google Scholar]
- Fletcher LM, Halliday JW, Powell LW. Interrelationships of alcohol and iron in liver disease with particular reference to the iron-binding proteins, ferritin and transferrin. J Gastroenterol Hepatol. 1999;14:202–14. doi: 10.1046/j.1440-1746.1999.01836.x. [DOI] [PubMed] [Google Scholar]
- Franco R, Panayiotidis MI, Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem. 2007;282:30452–65. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A, Seitz HK, Bartsch H, Frank N, Nair J. Immunohistochemical detection of 1, N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepato-carcinogenesis. Carcinogenesis. 2004;25:1027–31. doi: 10.1093/carcin/bgh089. [DOI] [PubMed] [Google Scholar]
- Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–28. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21 (Suppl 3):S3–6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–8. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- Grando-Lemaire V, Guettier C, Chevret S, Beaugrand M, Trinchet JC. Hepatocellular carcinoma without cirrhosis in the West: epidemiological factors and histopathology of the non-tumorous liver. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:508–13. doi: 10.1016/s0168-8278(99)80044-0. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Shimada T, Yun CH, Yamazaki H, Raney KD, Thier R, Coles B, Harris TM. Interactions of ingested food, beverage, and tobacco components involving human cytochrome P4501A2, 2A6, 2E1, and 3A4 enzymes. Environ Health Perspect. 1994;102 (Suppl 9):49–53. doi: 10.1289/ehp.94102s949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Tanaka K, Sakamoto T, Higaki Y, Mizuta T, Eguchi Y, Yasutake T, Ozaki I, Yamamoto K, Onohara S, Kawazoe S, Shigematsu H, Koizumi S. Case-control study on cigarette smoking and the risk of hepatocellular carcinoma among Japanese. Cancer Sci. 2008;99:93–7. doi: 10.1111/j.1349-7006.2007.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–13. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Rivenson A, Chung FL, Wynder EL. Potential inhibitors of tobacco carcinogenesis. Ann N Y Acad Sci. 1993;686:140–60. doi: 10.1111/j.1749-6632.1993.tb39169.x. [DOI] [PubMed] [Google Scholar]
- Homann N, Stickel F, König IR, Jacobs A, Junghanns K, Benesova M, Schuppan D, Himsel S, Zuber-Jerger I, Hellerbrand C, Ludwig D, Caselmann WH, Seitz HK. Alcohol dehydrogenase 1C*1 allele is a genetic marker for alcohol-associated cancer in heavy drinkers. Int J Cancer. 2006;118:1998–2002. doi: 10.1002/ijc.21583. [DOI] [PubMed] [Google Scholar]
- Hsiang CY, Wu SL, Chen JC, Lo HY, Li CC, Chiang SY, Wu HC, Ho TY. Acetaldehyde induces matrix metalloproteinase-9 gene expression via nuclear factor-kappaB and activator protein 1 signaling pathways in human hepatocellular carcinoma cells: Association with the invasive potential. Toxicol Lett. 2007;171:78–86. doi: 10.1016/j.toxlet.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–9. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res. 2003;533:153–71. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
- IARC Working Group. Overall evaluations of carcinogenicity: an updating of the IARC monographs volumes 1 to 42. IARC Monogr Eval Carcinogen Risks Humans. 1987;S7:1–440. [PubMed] [Google Scholar]
- Isse T, Matsuno K, Oyama T, Kitagawa K, Kawamoto T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity show high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol Clin Exp Res. 2005;29:1959–64. doi: 10.1097/01.alc.0000187161.07820.21. [DOI] [PubMed] [Google Scholar]
- Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–58. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi M, Tyndale RF. Regional and cellular distribution of CYP2E1 in monkey brain and its induction by chronic nicotine. Neuropharmacology. 2006;50:568–75. doi: 10.1016/j.neuropharm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hui AM, Sun L, Ushijima S, Sakamoto M, Tsuda H, Hirohashi S. DNA hypermethylation at the D17S5 locus and reduced HIC-1 mRNA expression are associated with hepatocarcinogenesis. Hepatology. 1999;29:703–9. doi: 10.1002/hep.510290338. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–8. [PubMed] [Google Scholar]
- Ketcham AS, Wexler H, Mantel N. Effects of alcohol in mouse neoplasia. Cancer Res. 1963;23:667–70. [PubMed] [Google Scholar]
- Kew MC, Asare GA. Dietary iron overload in the African and hepatocellular carcinoma. Liver Int. 2007;27:735–41. doi: 10.1111/j.1478-3231.2007.01515.x. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;36:835–46. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Koh WP, Robien K, Wang R, Govindarajan S, Yuan JM, Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. Br J Cancer. 2011;105:1430–5. doi: 10.1038/bjc.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, Gäbele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowdley KV. Iron, hemochromatosis, and hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):S79–86. doi: 10.1016/j.gastro.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Kumar KJ, Chu FH, Hsieh HW, Liao JW, Li WH, Lin JC, Shaw JF, Wang SY. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol. 2011;136:168–77. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Kuper H, Tzonou A, Kaklamani E, Hsieh CC, Lagiou P, Adami HO, Trichopoulos D, Stuver SO. Tobacco smoking, alcohol consumption and their interaction in the causation of hepatocellular carcinoma. Int J Cancer. 2000;85:498–502. [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–53. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash LH. Mitochondrial glutathione transport: physiological, pathological and toxicological implications. Chem Biol Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laso FJ, Madruga JI, Girón JA, López A, Ciudad J, San Miguel JF, Alvarez-Mon M, Orfao A. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25:1096–100. doi: 10.1002/hep.510250508. [DOI] [PubMed] [Google Scholar]
- Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38:1497–511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–64. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med. 1982;307:597–601. doi: 10.1056/NEJM198209023071006. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology. 1994;106:1085–105. doi: 10.1016/0016-5085(94)90772-2. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Alcohol: its metabolism and interaction with nutrients. Annu Rev Nutr. 2000;20:395–430. doi: 10.1146/annurev.nutr.20.1.395. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Casini A, DeCarli LM, Kim CI, Lowe N, Sasaki R, Leo MA. S-adenosyl-L-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology. 1990;11:165–72. doi: 10.1002/hep.1840110203. [DOI] [PubMed] [Google Scholar]
- Liu C, Russell RM, Seitz HK, Wang XD. Ethanol enhances retinoic acid metabolism into polar metabolites in rat liver via induction of cytochrome P4502E1. Gastroenterology. 2001;120:179–89. doi: 10.1053/gast.2001.20877. [DOI] [PubMed] [Google Scholar]
- Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci U S A. 2001;98:5560–5. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Huang ZZ, Yang H, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G178–85. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Martínez-Chantar ML, Corrales FJ, Martínez-Cruz LA, García-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–4. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- Mato JM, Corrales FJ, Lu SC, Avila MA. S-Adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29:222–32. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- Micu AL, Miksys S, Sellers EM, Koop DR, Tyndale RF. Rat hepatic CYP2E1 is induced by very low nicotine doses: an investigation of induction, time course, dose response, and mechanism. J Pharmacol Exp Ther. 200;306:941–7. doi: 10.1124/jpet.103.052183. [DOI] [PubMed] [Google Scholar]
- Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–34. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Müller LG, Pase CS, Reckziegel P, Barcelos RC, Boufleur N, Prado AC, Fett R, Block JM, Pavanato MA, Bauermann LF, da Rocha JB, Burger ME. Hepatoprotective effects of pecan nut shells on ethanol-induced liver damage. Exp Toxicol Pathol. 2011 Sep 14; doi: 10.1016/j.etp.2011.08.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Munaka M, Kohshi K, Kawamoto T, Takasawa S, Nagata N, Itoh H, Oda S, Katoh T. Genetic polymorphisms of tobacco- and alcohol-related metabolizing enzymes and the risk of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2003;129:355–60. doi: 10.1007/s00432-003-0439-5. [DOI] [PubMed] [Google Scholar]
- Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver disease. Proc Soc Exp Biol Med. 1994;205:243–7. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- Okuda K, Nakashima T, Kojiro M, Kondo Y, Wada K. Hepatocellular carcinoma without cirrhosis in Japanese patients. Gastroenterology. 1989;97:140–6. doi: 10.1016/0016-5085(89)91427-3. [DOI] [PubMed] [Google Scholar]
- Pan J, Chung FL. Formation of cyclic deoxyguanosine adducts from omega-3 and omega-6 polyunsaturated fatty acids under oxidative conditions. Chem Res Toxicol. 2002;15:367–72. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]
- Pan HN, Sun R, Jaruga B, Hong F, Kim WH, Gao B. Chronic ethanol consumption inhibits hepatic natural killer cell activity and accelerates murine cytomegalovirus-induced hepatitis. Alcohol Clin Exp Res. 2006;30:1615–23. doi: 10.1111/j.1530-0277.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- Pani G, Fusco S, Colavitti R, Borrello S, Maggiano N, Cravero AA, Farré SM, Galeotti T, Koch OR. Abrogation of hepatocyte apoptosis and early appearance of liver dysplasia in ethanol-fed p53-deficient mice. Biochem Biophys Res Commun. 2004;325:97–100. doi: 10.1016/j.bbrc.2004.09.213. [DOI] [PubMed] [Google Scholar]
- Pascale RM, Marras V, Simile MM, Daino L, Pinna G, Bennati S, Carta M, Seddaiu MA, Massarelli G, Feo F. Chemoprevention of rat liver carcinogenesis by S-adenosyl-L-methionine: a long-term study. Cancer Res. 1992;52:4979–86. [PubMed] [Google Scholar]
- Pascale RM, Simile MM, De Miglio MR, Nufris A, Daino L, Seddaiu MA, Rao PM, Rajalakshmi S, Sarma DS, Feo F. Chemoprevention by S-adenosyl-L-methionine of rat liver carcinogenesis initiated by 1,2-dimethylhydrazine and promoted by orotic acid. Carcinogenesis. 1995;16:427–30. doi: 10.1093/carcin/16.2.427. [DOI] [PubMed] [Google Scholar]
- Petersen DR. Alcohol, iron-associated oxidative stress, and cancer. Alcohol. 2005;35:243–9. doi: 10.1016/j.alcohol.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Pogribny IP, Basnakian AG, Miller BJ, Lopatina NG, Poirier LA, James SJ. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res. 1995;55:1894–901. [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42:349–61. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology. 2006;43:872–8. doi: 10.1002/hep.21107. [DOI] [PubMed] [Google Scholar]
- Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Exp Clin Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Khalsa J, Serrano J. Mechanisms of alcohol-associated cancers: introduction and summary of the symposium. Alcohol. 2005;35:155–60. doi: 10.1016/j.alcohol.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Purohit V, Russo D. Role of S-adenosyl-L-methionine in the treatment of alcoholic liver disease: introduction and summary of the symposium. Alcohol. 2002a;27:151–4. doi: 10.1016/s0741-8329(02)00232-x. [DOI] [PubMed] [Google Scholar]
- Purohit V, Russo D. Cellular and molecular mechanisms of alcoholic hepatitis: introduction and summary of the symposium. Alcohol. 2002b;27:3–6. doi: 10.1016/s0741-8329(02)00211-2. [DOI] [PubMed] [Google Scholar]
- Purohit V, Russo D, Salin M. Role of iron in alcoholic liver disease: introduction and summary of the symposium. Alcohol. 2003;30:93–7. doi: 10.1016/s0741-8329(03)00132-0. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Hara M, Higaki Y, Ichiba M, Horita M, Mizuta T, Eguchi Y, Yasutake T, Ozaki I, Yamamoto K, Onohara S, Kawazoe S, Shigematsu H, Koizumi S, Tanaka K. Influence of alcohol consumption and gene polymorphisms of ADH2 and ALDH2 on hepatocellular carcinoma in a Japanese population. Int J Cancer. 2006;118:1501–7. doi: 10.1002/ijc.21505. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Higaki Y, Hara M, Ichiba M, Horita M, Mizuta T, Eguchi Y, Yasutake T, Ozaki I, Yamamoto K, Onohara S, Kawazoe S, Shigematsu H, Koizumi S, Tanaka K. Interaction between interleukin-1beta-31T/C gene polymorphism and drinking and smoking habits on the risk of hepatocellular carcinoma among Japanese. Cancer Lett. 2008;271:98–104. doi: 10.1016/j.canlet.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Sato N. Central role of mitochondria in metabolic regulation of liver pathophysiology. J Gastroenterol Hepatol. 2007;22 (Suppl 1):S1–6. doi: 10.1111/j.1440-1746.2007.04963.x. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349–60. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: role of genetics in ethanol metabolism. Genes Nutr. 2010;5:121–8. doi: 10.1007/s12263-009-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Fang J, Qiu D, Zhang T, Yang J, Chen S, Xiao S. Correlation between DNA methylation and pathological changes in human hepatocellular carcinoma. Hepatogastroenterology. 1998;45:1753–9. [PubMed] [Google Scholar]
- Staretz ME, Murphy SE, Patten CJ, Nunes MG, Koehl W, Amin S, Koenig LA, Guengerich FP, Hecht SS. Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N′-nitrosonornicotine in human hepatic microsomes. Drug Metab Dispos. 1997;25:154–62. [PubMed] [Google Scholar]
- Suzuki Y, Saito H, Suzuki M, Hosoki Y, Sakurai S, Fujimoto Y, Kohgo Y. Up-regulation of transferrin receptor expression in hepatocytes by habitual alcohol drinking is implicated in hepatic iron overload in alcoholic liver disease. Alcohol Clin Exp Res. 2002;26(8 Suppl):26S–31S. doi: 10.1097/01.ALC.0000026830.27338.23. [DOI] [PubMed] [Google Scholar]
- Tagger A, Donato F, Ribero ML, Chiesa R, Portera G, Gelatti U, Albertini A, Fasola M, Boffetta P, Nardi G. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer. 1999;81:695–9. doi: 10.1002/(sici)1097-0215(19990531)81:5<695::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Tang Y, Gao C, Xing M, Li Y, Zhu L, Wang D, Yang X, Liu L, Yao P. Quercetin prevents ethanol-induced dyslipidemia and mitochondrial oxidative damage. Food Chem Toxicol. 2012;50:1194–200. doi: 10.1016/j.fct.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Tannapfel A, Wittekind C. Genes involved in hepatocellular carcinoma: deregulation in cell cycling and apoptosis. Virchows Arch. 2002;440:345–52. doi: 10.1007/s00428-002-0617-x. [DOI] [PubMed] [Google Scholar]
- Thakur V, McMullen MR, Pritchard MT, Nagy LE. Regulation of macrophage activation in alcoholic liver disease. J Gastroenterol Hepatol. 2007;22 (Suppl 1):S53–6. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Arteel GE, Yin M, Connor HD, Wall C, Raleigh JA, Frankenberg MV, Adachi Y, Forman DT, Brenner D, Kadiiska M, Mason RP. The role of gut-derived bacterial toxins and free radicals in alcohol-induced liver injury. J Gastroenterol Hepatol. 1998;13 (Suppl):S39–50. doi: 10.1111/jgh.1998.13.s1.39. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Horne W, Kamimura S, Niemelä O, Parkkila S, Ylä-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–30. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Lin M, Ohata M, Giulivi C, French SW, Brittenham G. Iron primes hepatic macrophages for NF-kappaB activation in alcoholic liver injury. Am J Physiol. 1999;277(6 Pt 1):G1240–50. doi: 10.1152/ajpgi.1999.277.6.G1240. [DOI] [PubMed] [Google Scholar]
- Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–8. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- Valenca SS, Silva Bezerra F, Lopes AA, Romana-Souza B, Marinho Cavalcante MC, Lima AB, Gonçalves Koatz VL, Porto LC. Oxidative stress in mouse plasma and lungs induced by cigarette smoke and lipopolysaccharide. Environ Res. 2008;108:199–204. doi: 10.1016/j.envres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Valerio LG, Jr, Parks T, Petersen DR. Alcohol mediates increases in hepatic and serum nonheme iron stores in a rat model for alcohol-induced liver injury. Alcohol Clin Exp Res. 1996;20:1352–61. doi: 10.1111/j.1530-0277.1996.tb01134.x. [DOI] [PubMed] [Google Scholar]
- Wainfan E, Dizik M, Stender M, Christman JK. Rapid appearance of hypomethylated DNA in livers of rats fed cancer-promoting, methyl-deficient diets. Cancer Res. 1989;49:4094–7. [PubMed] [Google Scholar]
- Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res. 1992;52(7 Suppl):2071s–2077s. [PubMed] [Google Scholar]
- Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in Alcoholic Liver Disease. Annu Rev Nutr. 2012;32:343–68. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD. Alcohol, vitamin A, and cancer. Alcohol. 2005;35:251–8. doi: 10.1016/j.alcohol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Wang XD, Liu C, Chung J, Stickel F, Seitz HK, Russell RM. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-Jun and c-Fos) expression in rat liver. Hepatology. 1998;28:744–50. doi: 10.1002/hep.510280321. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kirpich I, Liu Y, Ma Z, Barve S, McClain CJ, Feng W. Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am J Pathol. 2011;179:2866–75. doi: 10.1016/j.ajpath.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Millonig G, Nair J, Patsenker E, Stickel F, Mueller S, Bartsch H, Seitz HK. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology. 2009;50:453–61. doi: 10.1002/hep.22978. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gäbele E, Rusyn I, Yamashina S, Froh M, Adachi Y, Iimuro Y, Bradford BU, Smutney OM, Connor HD, Mason RP, Goyert SM, Peters JM, Gonzalez FJ, Samulski RJ, Thurman RG. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–9. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Wolcott RM, Pruett SB. Ethanol decreases the number and activity of splenic natural killer cells in a mouse model for binge drinking. J Pharmacol Exp Ther. 1994;271:722–9. [PubMed] [Google Scholar]
- Yang CS, Yoo JS, Ishizaki H, Hong JY. Cytochrome P450IIE1: roles in nitrosamine metabolism and mechanisms of regulation. Drug Metab Rev. 1990;22:147–59. doi: 10.3109/03602539009041082. [DOI] [PubMed] [Google Scholar]
- Yin M, Gäbele E, Wheeler MD, Connor H, Bradford BU, Dikalova A, Rusyn I, Mason R, Thurman RG. Alcohol-induced free radicals in mice: direct toxicants or signaling molecules? Hepatology. 2001;34:935–42. doi: 10.1053/jhep.2001.28888. [DOI] [PubMed] [Google Scholar]
- Yip-Schneider MT, Doyle CJ, McKillop IH, Wentz SC, Brandon-Warner E, Matos JM, Sandrasegaran K, Saxena R, Hennig ME, Wu H, Waters JA, Klein PJ, Froehlich JC, Schmidt CM. Alcohol induces liver neoplasia in a novel alcohol-preferring rat model. Alcohol Clin Exp Res. 2011;35:2216–25. doi: 10.1111/j.1530-0277.2011.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–75. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]