Abstract
The purpose of this study was to investigate the fate of transplanted cells in the central zone of myocardial infarction (MI), and to clarify the relationship between the injection-site impact and the efficacy of cell therapy. MI was created by coronary ligation in female rats. Three weeks later, 3-million labelled male bone marrow mesenchymal stem cells (BMSCs) were directly injected into the border (BZC group) or central zone (CZC group) of MI area. As a control, culture medium was injected into the same sites. Cell survival was evaluated by quantitative real-time polymerase chain reaction, and apoptosis was assayed with TUNEL and caspase-3 staining. Four weeks after transplantation, heart function and cardiac morphometry were evaluated by echocardiography and Masson’s Trichrome staining, respectively. Angiogenesis and myogenesis were detected by immunofluorescence staining. After cell transplantation into the border or central zone, there was no cell migration between the different zones of MI. BMSCs in the CZC group exhibited no difference in apoptotic percentage, in the long-term survival, when compared with those in the BZC group. However, they did effectively promote angiogenesis and cellular myogenic differentiation. Although cell delivery in the central zone of MI had no effect on the recovery of heart function compared with the BZC group, the retained BMSCs could still increase the scar thickness, and subsequently exhibit a trend in the reverse remodelling of ventricular dilation. Hence, we concluded that the central zone of MI should not be ignored during cell-based therapy. Multiple site injection (border+central zone) is strongly recommended during the procedure of cell transplantation.
Keywords: myocardial infarction, mesenchymal stem cells, cell transplantation, cell therapy, ventricular remodelling, apoptosis
Introduction
Cell transplantation has emerged as a promising approach to restoring heart function after MI [1-3]. Intramyocardial injection is considered to be the most effective route for precise cell delivery and therefore the procedure has become an associated with MI patients, during open-chest revascularization surgery.
Various infarct regions have different pathophysiology. The border zone (BZ) of MI area has the same regional myocardial blood flow as that in the uninfarcted area. It would therefore offer more nutrients for cell survival [4]. As a result, in both experimental studies and clinical trials, the BZ of MI has been considered as the optimal site for cell therapy. However, the loss of cardiomyocytes during myocardial ischaemia is primarily located in the central zone (CZ) of the MI area. Hence, CZ is the most demanding area for cell-based regenerative therapy. Owing to the microenvironment of serum deprivation and hypoxia in the scarred CZ, almost all published studies of cell therapy for MI describe cell injection into the BZ. Therefore, until now, the therapeutic effects of BMSCs transplanted in the CZ of MI remain unclear.
This study, to make a thorough inquiry about the therapeutic effect of cell transplantation in the CZ, BMSCs were directly injected into the CZ or BZ of a chronic MI area. We would like to address (1) the cell retention, myogenic differentiation and paracrine function in the CZ of MI, (2) the impact on ventricular remodelling and heart function post-transplantation and (3) the benefits and flaws of BMSCs injection in the CZ of MI.
Methods and materials
Animals
The study was performed in accordance to both the guidelines of the ‘Regulation to the Care and Use of Experimental Animals’ (1996) of the Beijing Council on Animal Care and the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, National Academy Press, Washington, DC, revised 1996). All procedures were approved by the Ethics Committee for Animal Study in Fu Wai hospital. Syngenic Lewis rats were obtained from Vital River Laboratory Animal Inc. (Beijing, China). Among them, 20 male rats weighing 60–80 g served as cell donors and 134 female rats weighing 260–280 g were enrolled in the cell transplantation study
Creation of MI model
Myocardial infraction was induced in female rats by permanent ligation of the left anterior descending artery, as previously described [5]. Three weeks after MI, the surviving rats with left ventricular ejection fraction (LVEF) less than 60% and left ventricular fractional shortening (LVFS) less than 30% were randomly grouped according to the experimental protocol (Fig. 1).
Fig 1.
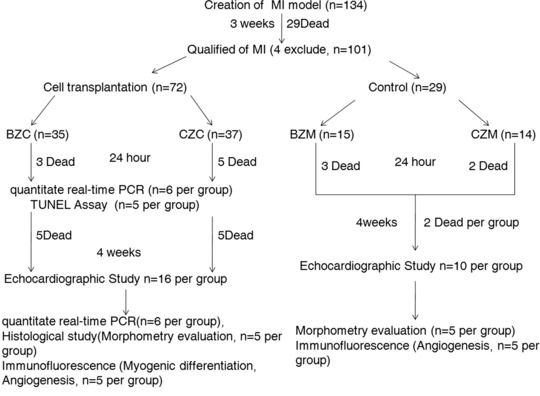
Scheme of experimental protocol.
Cell preparation
BMSCs were isolated from male donor rats and cultured as previously reported [5]. In brief, BMSCs were obtained from the tibias and cultured in DMEM (Grand Island, NY, USA) supplemented with 10% (v/v) foetal bovine serum. The non-adherent cells were removed after 24 hrs and the adherent cells were incubated. The culture medium was replaced with fresh medium every 2 days.
To track the transplanted cells in vivo, BMSCs were labelled with 4′,6-diamidino-2′-phenylin-dole (DAPI; Sigma-Aldrich, St. Louis, MO, USA) before transplantation [6]. Sterile DAPI stock solution was added to the culture medium at a final concentration of 50 μg/ml for 20 min. After labelling, cells were washed six times in PBS to remove excess unbound DAPI.
Cell transplantation
Three weeks after MI, the hearts were exposed through the original thoracotomy incision, under anaesthesia. The myocardium extending 0.5–1.0 mm from the infarct scar was considered as the BZ of MI, as previously described [7], and the area covered by pale scar was defined as the CZ of MI (as the schematic diagram depicts in Fig. 2A). After identification of the BZ and CZ, a purse-string suture was placed around the proposed injection site to avoid leakage of cells after injection (Fig. 2A and B). Then 3 χ 106 BMSCs in 60 μl serum-free culture media was intramyocardially injected into the left BZ and right BZ of MI (30 μl in each site), which served as BZC group. Similarly, the same volume of cells was injected into two sites in the CZ of MI, which served as CZC group. The same volume of serum-free culture medium was injected, following the same procedure, in the control groups (BZM or CZM group, respectively). After transplantation, the purse-string suture was tied and left to mark the implantation site. The chest was then closed, and the animals were allowed to recover.
Fig 2.
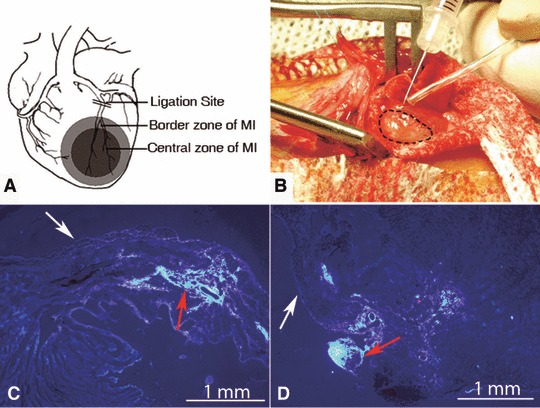
Myocardial infarction area and cell tracking. (A) Left anterior descending artery ligation site and the definition of myocardial infarction area. (B) Illustration of BMSCs transplantation in the recipient heart. The dotted line represent the borderline of the MI. (C, D) 4 weeks after cell transplantation, the engrafted labelled BMSCs (indicated by red arrow) could be easily identified in the injected site of each group. White arrow: the epicardium; MI: myocardial infarction.
Evaluation of left ventricular function
Transthoracic echocardiography was performed at 3 weeks after MI and at 4 weeks after cell transplantation, according to the protocols described previously [8]. Left ventricle end-diastolic and end-systolic dimensions (LVEDD and LVESD, respectively) were measured from the short-axis view of the left ventricle at the mid-papillary level. The LV fractional shortening (LVFS) and LVEF were calculated as follows: LVFS (%) ∇ (LVEDd−LVESd)/LVEDd χ 100 and LVEF (%) ∇ [(LVEDd3−LVESd3)/LVEDd3] χ 100. All measurements were averaged over three consecutive cardiac cycles and were analysed by two independent, experienced investigators who were blinded to the treatment status of the animals.
Heart harvest
At 24 hrs and 4 weeks after transplantation, the rats were deeply anaesthetized and receive a 10% potassium chloride injection to arrest the heart. The hearts were then removed and fixed in 4% paraformaldehyde solution for cardiac morphometry evaluation or cryopreserved in TissueTek® OCT compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) for immunofluorescence study (n ∇ 5 per group). To quantify the number of surviving donor male cells, in the female recipient hearts, and the escaped BMSCs’ distribution in extra-organs, the heart, spleens, lungs, livers and blood were removed and frozen in liquid nitrogen for DNA extraction.
Cardiac morphometry evaluation
The paraformaldehyde fixed hearts were cut into six transverse slices, from apex to base, and paraffin-embedded for histology (n ∇ 5 in each group). Subsequently, partial 5 μm transverse slices from each section were prepared for haematoxylin and eosin staining or Masson’s Trichrome staining. Histological images of the stained sections were photographed with an Olympus DP70 (Olympus Optical Co., Ltd., Tokyo, Japan) and analysed with Image-Pro Plus 5.1 Software (Media Cybernetics, Inc., Bethesda, MD, USA). The following parameters were measured [9]: (1) the thickness of scar and septum (average of five equidistant measurements); (2) areas composed of collagen and non-infarcted muscle, area of LV cavity and area of total LV. To evaluate the degree of LV dilation, the expansion index was calculated as LV cavity area/total LV area χ septum thickness/scar thickness; (3) infarct size was calculated by dividing the sum of the planimetered endocardial and epicardial circumferences of the infarcted area by the sum of the total epicardial and endocardial circumferences of the LV.
DNA preparation and quantization of Y chromosomal DNA by real-time PCR
To quantify the number of surviving donor male cells, in the female recipient hearts (n ∇ 6/group at 1 day and 4 weeks after cell transplantation) and the escaped BMSCs, in the extra-cardiac organs (including lung, liver, blood and spleen), real-time PCR was applied to determine the volume of Y chromosomes, as previously described [10]. At the indicated time points after cell transplantation (1 day and 4 weeks), host female organs were removed under terminal anaesthesia, homogenized and diluted to produce samples for Y chromosome determination. The standard curve was created by mixing 0, 10, 100, 1,000, 10,000 and 100,000 male BMSCs into diluted homogenate of female ventricles before DNA isolation and real-time PCR. The sequences of the PCR primers used for detection of rat male specific sry gene were as follows: (sense 5-CATCGAAGGGTTAAAG TGCCA-3; antisense 5-ATAGTGTGTAGGTT GTTGTCC-3). The real-time PCR conditions were consisted of an initial denaturation step of 10 min. at 95°C, followed by 40 cycles at 95°C, for 15 sec., and at 60°C, for 1 min.
The retention of transplanted male cells was expressed as [The average of real-time PCR detection of SRY χ The times of dilution DNA] χ [The weight of the initial tissue (mg) χ The weight of tissue extracted for DNA (mg)].
Apoptosis, myogenic differentiation and angiogenesis after cell transplantation
For in situ detection of apoptotic BMSCs, at 1 day and 4 weeks after cell transplantation, we performed the terminal deoxynucleotidyl transferase-mediated dUTP end labelling (TUNEL) assay by using a TdT FragEL-DNA fragmentation detection kit (Roche, Indianapolis, IN, USA) in frozen tissue sections according to the manufacturer’s protocol [11]. The percentage of apoptotic BMSCs was calculated as the number of TUNEL-positive cell number/DAPI-labelled nuclear numbers of BMSCsχ100.
To further evaluate the result of TUNEL assay, primary polyclonal antibodies to activated caspase-3 (Cell Signaling Technology, Beverly, MA, USA; dilution, 1:1600), were used to stain in frozen heart tissue sections obtained at 1 day and 4 weeks after BMSCs transplantation, according to the instructions of the manufacturer (Cell Signaling Technology). Cells were labelled for 2 hrs at room temperature with TRITC-conjugated goat anti-rabbit IgG (Zhongshan Goldenbridge Biotechnology Co. Ltd, Beijing, China). The percentage of apoptotic BMSCs was calculated as the number of caspase-3 positive cell/DAPI-labelled nuclear number of BMSCs χ 100.
To calculate capillary density in the infarct and peri-infarct regions, at 4 week after cell transplantation, the frozen tissue sections were stained with the antibodies against von Willebrand factor (VWF; Abcam, Cambridge, UK), followed by incubation with Fluorescein Isothiocyanate (FITC)-conjugated secondary antibody (Zhongshan Goldenbridge Biotechnology Co. Ltd.). The capillary density was determined as the average number of VWF-positive small vessels, with a diameter less than 100 μm, in a field of a high magnification. Five high-power fields within the scar (containing the two side of BZ and CZ of MI) of each section were randomly selected, and the number of capillaries was averaged and expressed as the number of capillary vessels per high-power field (0.2 mm2).
For detection of myogenic differentiation of BMSCs at 4 weeks after cell transplantation, frozen tissue sections were stained for α-sarcomeric actin (1:100; Abcam) and troponin I (1:150; Santa Cruz Biotech, CA, USA) and then visualized with an FITC or rhodamine 123–conjugated secondary antibody [10].
Statistical analysis
Data are presented as means ± standard deviation. Statistical analysis was performed with the SPSS15.0 software (SPSS Inc., Chicago, IL, USA). Differences among groups were analysed by one-way ANOVA followed by a Bonferroni test. Values of P < 0.05 were considered significant. The mortality, after cell transplantation among different groups, was analysed by chi-square tests.
Results
Surgical mortality
Mortality at the various time points is depicted in Figure 1. All rats that died were excluded from the study. There was no significant difference in mortality among the four groups (P ∇ 0.89).
Evaluation of cardiac function
As shown in Table 1, improved heart function was only observed in the BZC group. LVEF and LVFS in the BZC group were higher than that in the other groups (P < 0.05, respectively), and there was no difference among the CZC, CZM and BZM groups. Although LVEDD and LVESD in the BZC group displayed a tendency toward improved cardiac function as compared with the other three groups, the differences were not statistically significant (P > 0.05).
Table 1.
Evaluation of LV function by echocardiography 4 weeks after BMSCs transplantation
| Cell Transplantation groups | Control groups | |||||||
|---|---|---|---|---|---|---|---|---|
| CZC | BZC | CZM | BZM | |||||
| LVEDd(mm) | 7.62 ± 0.64 | 7.25 ± 0.78 | 7.74 ± 0.69 | 8.11 ± 0.49 | ||||
| LVESd(mm) | 5.86 ± 0.57 | 5.38 ± 0.59 | 5.99 ± 0.67 | 6.49 ± 0.73 | ||||
| LVEF(%) | *51.88 ± 3.88% | 57.48 ± 3.73% | *50.90 ± 3.58% | *48.47 ± 4.65% | ||||
| FS(%) | *21.13 ± 2.19% | 26.42 ± 2.32% | *22.60 ± 1.95% | *22.55 ± 4.35% | ||||
LVEDd: left ventricular end-diastolic diameter; LVESd: left ventricular end-systolic diameter; LVEF: left ventricular ejection fraction; LVFS: left ventricular fractional shortening. *P < 0.05 versus BZC.
Cell survival
DAPI-labelled BMSCs could be easily identified within the damaged myocardium in either the BZC or the CZC groups, after cell injection (Fig. 2). As shown in Figure 3, 24 hrs after cell transplantation, 15.74 ± 4.13% of the initially implanted BMSCs, in the BZC group, were detected. This was significantly higher than that in the CZC group (8.20 ± 2.63%; P < 0.01). Four weeks after cell transplantation, a similar tendency was also found between the BZC and CZC groups (5.57 ± 1.13% versus 1.72 ± 0.41% respectively, P < 0.01).
Fig 3.
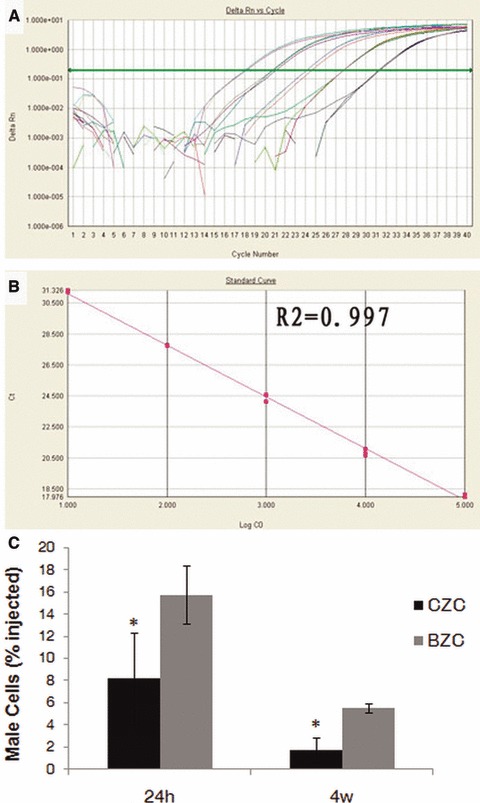
Quantifying transplanted male mesenchymal stem cells (MSCs) in the female recipient hearts by quantitative real-time PCR. (A) Real-time amplification plot showing change in normalized reporter dye fluorescence. (B) Standard curve generated from data in (A) showing relationship between threshold cycle (Ct) and number of male BMSCs. (C) 24 hrs and 4 weeks after cell transplantation, the percentage of surviving cells in BZC group were significantly higher than in CZC group. *P < 0.01 versus BZC.
Cell apoptosis
As shown by TUNEL assay, in the Figure 4, 24 hrs after cell transplantation, the percentage of apoptotic BMSCs was significantly lower in the BZC group than that in the CZC group (28.69% ± 6.59% versus 53.77 ± 8.81%, P < 0.01), and there was no difference at the 4 weeks time point after transplantation (1.71 ± 0.53% versus 2.03 ± 0.89% respectively, P > 0.05). The same results were also obtained with Caspase-3 staining (Fig. 5). The difference in apoptotic percentage between the BZC and CZC group at 24 hrs after cell transplantation was significant (22.80% ± 4.44% versus 44.80% ± 2.39%, P < 0.01). However, there was no difference at 4 weeks after transplantation (1.49% ± 0.07% versus 1.57% ± 0.09%, P > 0.05).
Fig 4.
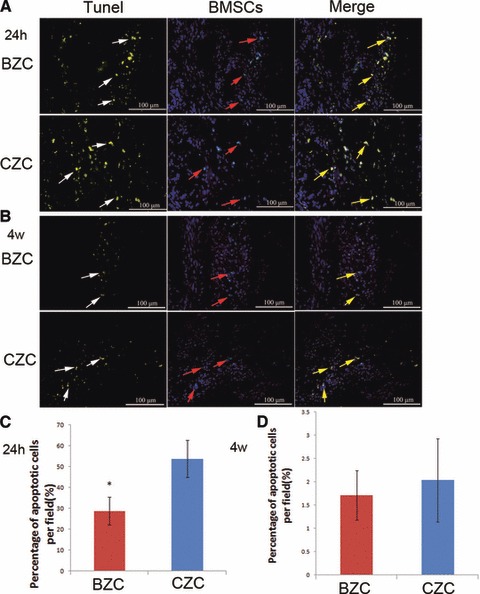
Apoptosis of implanted cells in the infarcted myocardium at 1 day and 4 weeks after cell transplantation by TUNEL assay. (A) 24 hrs after transplantation, the DAPI labelled cells’ nuclei showed blue fluorescence, and the TUNEL-positive nuclei was in Yellow. (B) The 4 weeks after transplantation, the DAPI labelled cells’ nuclei showed blue fluorescence, and the TUNEL-positive nuclei was in Yellow. (C) The BZC group had a significantly lower percentage of TUNEL-positive implanted cell nuclei than the CZC group at 24 hrs after transplantation. *P < 0.01 versus CZC group. (D) There were no difference in percentage of TUNEL-positive implanted cell nuclei between BZC and CZC groups at 4 weeks after transplantation. Red arrow: DAPI-labelled BMSCs; white arrow: TUNEL-positive cells; yellow arrow: DAPI and TUNEL-positive BMSCs; BMSCs: bone marrow mesenchymal stem cells.
Fig 5.
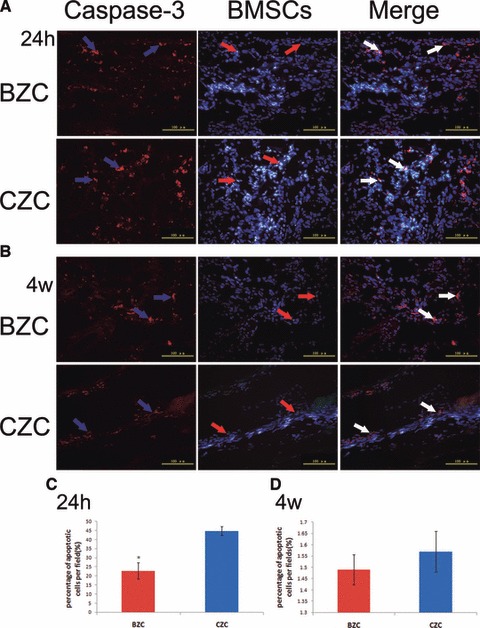
Apoptosis of implanted cells in the infarcted myocardium at 1 day and 4 weeks after cell transplantation by caspase-3 staining. (A) 24 hrs after transplantation, the DAPI-labelled cells’ nuclei showed blue fluorescence, caspase-3 positive staining was in red, and the DAPI+caspase-3 positive staining showed blue fluorescence surrounded by red fluorescence. (B) The 4 weeks after transplantation, the DAPI-labelled cells’ nuclei showed blue fluorescence, caspase-3 positive staining was in red, and the DAPI+caspase-3 positive staining showed blue fluorescence surrounded by red fluorescence. (C) The BZC group had a significantly lower percentage of caspase-3 positive implanted cell nuclei than the CZC group at 24 hrs after transplantation. *P < 0.01 versus CZC group. (D) There were no difference in percentage of caspase-3 positive implanted cell nuclei between BZC and CZC groups at 4 weeks after transplantation. Red arrow: DAPI-labelled BMSCs; blue arrow: caspase-3 positive cells; white arrow: DAPI and caspase-3 positive BMSCs; BMSCs: bone marrow mesenchymal stem cells.
Escaped BMSCs in the extra-cardiac organ
Cell escape often occurs during the early stage of post-transplantation. We quantified the volume of extra-cardiac organ distributed BMSCs at 24 hrs after cell delivery. In the BZC group, the percentage relative to the initial number of injected cells in venous blood, arterial blood, spleen, lung and liver was 2.50 ± 0.42%, 1.54 ± 0.77%, 2.71 ± 1.3%, 2.03 ± 0.52% and 1.50 ± 0.50%, respectively. Simultaneously, in the CZC group, the relative percentage was 2.80 ± 0.47%, 2.06 ± 0.46%, 2.03 ± 1.99%, 1.65 ± 0.75% and 0.93 ± 0.67%. There was no significant difference between the BZC and CZC groups in any of the extracardiac organs (P > 0.05, respectively).
Angiogenesis and myogensis after cell transplantation
As shown in Figure 6, the BZC group had a significant higher capillary density in the BZ of MI (36.06 ± 2.77 vessels/0.2 mm2) than that in the CZC group, BZM or CZM groups (23.75 ± 4.09, 20.88 ± 2.7 and 23 ± 4 vessels/0.2 mm2, respectively) (P < 0.01, respectively). There was no significant difference among the capillary densities of the CZC, BZM and CZM groups. In addition, the implanted cells in the BZ-augmented angiogenesis in the CZ of MI. A higher capillary density, in the CZ (18.63 ± 2.73 vessels/0.2 mm2), was observed in the BZC group than in either the BZM or CZM groups (13.52 ± 3.03, 12.06 ± 2.35 vessels/0.2 mm2, respectively) (P < 0.01, respectively). However, the induced angiogenesis in CZ by implanted cells in the BZC group was still lower than that in the CZC group (18.63 ± 2.73 and 25.53 ± 3.31 vessels/0.2 mm2, respectively, P < 0.01).
Fig 6.
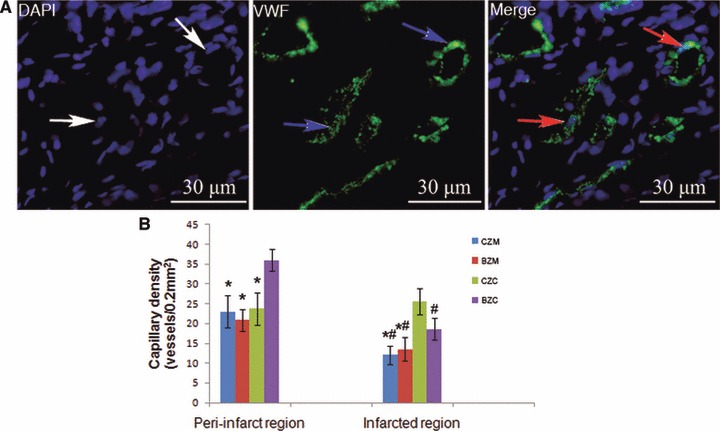
BMSCs transplantation promotes angiogenesis in the infarcted heart and differentiation into vascular endothelium at 4 weeks after transplantation. (A) The green fluorescence represents the VWF factor-positive capillaries (as indicated by the blue arrow) in the BZC group. Some of the transplanted BMSCs were positive for von Willebrand factor and formed vascular structures (as indicated by the red arrow). (B) Cell transplantation groups (BZC and CZC) had a significant higher capillary density than the control groups (CZM and BZM) *P < 0.01 versus BZC group, #P < 0.01 versus CZC group BMSCs: bone marrow mesenchymal stem cells.
Four weeks after transplantation, immunofluorescent analysis demonstrated that a small number of DAPI-labelled BMSCs were positive for α-sarcomeric actin and troponin I, both in the BZC and CZC group (Fig. 7). These results suggested that the engrafted BMSCs could differentiate into cardiomyocyte-like cells. The percentage of positive staining BMSCs in the BZC and CZC groups was 16.60 ± 2.07% and 4.60 ± 1.14% for α-sarcomeric actin, and 12.60 ± 2.17% and 4.20 ± 1.30% for troponin I, respectively. Similarly, the BZC group had dramatically higher percentages of both sarcomeric α-actin and desmin positive staining, implanted cells than that in the CZC group.
Fig 7.
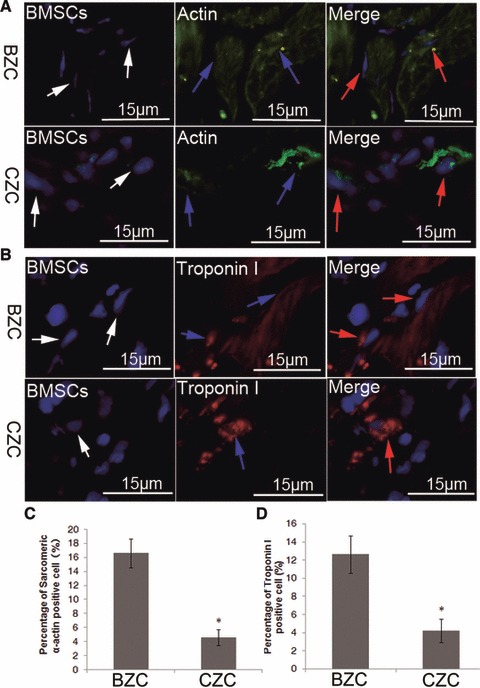
Differentiation of transplanted BMSCs at 4 weeks after cell transplantation, by immunofluorescence staining for α-sarcomeric actin and troponin I, respectively. (A, B) DAPI-labelled BMSCs expressed α-sarcomeric actin and troponin I, (C, D) the transplanted BMSCs in the BZC group had a significantly higher differentiation efficiency than the CZC group, both in the expression of α-sarcomeric actin and troponin I staining. P < 0.01 versus BZC White arrows: DAPI-labelled BMSCs; blue arrow: α-sarcomeric actin or tropnin I positive cells; red arrow: both α-sarcomeric actin and DAPI-positive BMSCs or both tropnin I and DAPI-positive BMSCs; BMSCs: bone marrow mesenchymal stem cells.
Cardiac morphometry evaluation
As shown in Figure 8, the injected BMSCs significantly increased the scar thickness in the BZC and CZC groups (643.30 ± 103.65 μm and 699.70 ± 99.92 μm, respectively) compared with the BZM and CZM groups (473.2 ± 16.72 μm and 473.4 ± 60.73 μm; P < 0.01, respectively). The scar thickness in the CZC group was significantly thicker than that in the BZC group (P < 0.01). The Hochman–Choo expansion index in the BZC (0.89 ± 0.12) group was significantly reduced compared with that in the CZC, BZM and CZM groups (1.18 ± 0.13, 1.44 ± 0.09, 1.31 ± 0.14, respectively) (P < 0.01, respectively), and there was no difference among these three groups (P > 0.05). In addition, the BZC group had a significantly reduced scar size (34.28 ± 1.96%) from that in the CZC, BZM and CZM groups (43.53 ± 3.37%, P < 0.05; 46.55 ± 5.36%, P < 0.01; 47.20 ± 4.86%, P < 0.01; respectively).
Fig 8.
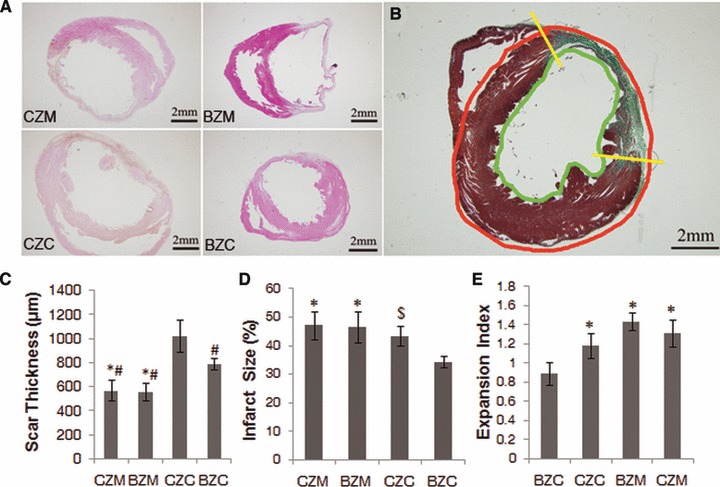
Morphological measurements at 4 weeks after transplantation. (A) Cell transplantation reversed post-MI remodelling. (B) The mean infarct size per section was calculated after determining the inner (green line) and outer (red line) LV infarct length. Yellow bars divide infarcted from non-infarcted myocardium. (C) The cell transplantation group (BZC and CZC groups) had a significantly increased scar thickness compared with that of the control groups (BZM and CZM groups). (D) BZC had a significant reduced infarct size compared with CZC, BZM and CZM. (E) The expansion index was also reduced in the BZC group, but there were no difference among the groups of CZC, BZM and CZM. $P < 0.01 versus BZC, #P < 0.01 versus CZC, *P < 0.01 versus BZC; $P < 0.05 versus BZC.
Discussion
BMSCs are a popular cell source for cell-based regenerative therapy. Safety and efficacy studies with BMSC transplantation are already being done world-wide [1], because they are easily obtained and expanded under in vitro culture conditions, while maintaining their multi-lineage differentiation potential. Therefore, in this study we applied the BMSCs as the cell resource for myocardial regeneration. The phenotype and differentiation potential of BMSCs in this study has been verified in our recent published research [5, 12, 13]. Although BMSCs transplantation has been considered as a new therapeutic strategy for cardiovascular disease, some potential hurdles must be considered to improve the outcome of cell therapy. In the end, there is the possibility that cell injection sites might dramatically influence the therapeutic efficacy of cell transplantation for MI.
To date, there is no information to indicate the fate of implanted cells in the CZ of MI or their therapeutic efficacy on the heart function recovery after MI. As showed in Figure 8, the thickness of the CZ of MI was less than 1 mm, and therefore was very easy to penetrate the scar into the ventricular cavity during cell injection. A failed injection would cause bleeding from the injection site, and could also cause the cells to be washed from the left side of the heart cavity and pumped into the systemic circulation, which may cause safety issues [14, 15]. However, in this study, during the procedure of cell delivery, we did not observe any bleeding in the CZC group. Moreover, in the early stage of post-transplantation, there was no difference in the extracardiac cell redistribution. Hence, we concluded that it was feasible and safe to deliver the cells into the CZ of MI.
The major findings of this study were that (1) BMSCs implanted in the CZ of MI effectively promote angiogenesis and cellular myogenic differentiation also occurred; (2) Cell apoptosis in the CZ arrived the peak at the early stage after cell injection but it was then equal to the cells in BZ in the long run; (3) although cell delivery in the CZ was invalid on the heart function improvement, the retained BMSCs could increase the scar thickness, and subsequently exhibited a trend in the reverse remodelling of ventricular dilation.
It is well-demonstrated that poor cell survival limits the efficacy of cell transplantation. It has been reported that intromyocardially injected cells decreased rapidly from 34–80% at 0 hr to 0.3–3.5% at 6 weeks in rat MI model. This implies that ischaemic microenvironment of the infracted myocardium could not be conducive of BMSCs survival [14]. In a common sense, the adverse factors of the deprivation of oxygen and nutrient supply, the mechanical tension from myocardial motion, and various cytotoxic factors in the CZ of MI, can result in large-scale cell apoptosis and death [16, 17]. Therefore, the CZ of MI has been considered as the restricted area for cell transplantation. In this study, although the amount of traceable cells were dramatically reduced after cell injection in the CZC group, 8.20 ± 2.63% of the initial cells could be detected at 1 day after transplantation. Moreover, there was no difference in apoptotic percentage in the long-term survival of transplanted BMSCs between the BZC and CZC groups. Neoangiogenesis plays an important role in the inhibition of cell apoptotic death. Before cell transplantation, the blood supply in the BZ is five times more than that of the CZ of MI [4]. As a result, at 24 hrs after transplantation, the apoptotic percentage of BMSCs in BZC group was about 50% of that in CZC group. However, with the development of neovacularization due to BMSCs injection, capillary density in the CZ of CZC group dramatically increased and supplied more nutrients and growth factors. With the improved blood supply in CZ of CZC group, the apoptotic percentage of BMSCs at 4 weeks decreased to about 1/26 of that at 24 hrs after transplantation. For the BZC group, the viable BMSCs of BZC group was two times more than that of CZC group at 4 weeks after transplantation. The increment of blood supply in BZ was still not adequate to support a large number of viable cells. Therefore, the apoptotic percentage of BZC group was eventually similar to that in CZC group, only decreasing to 1/16 at 24 hrs after transplantation.
Previous studies have confirmed that failure of collagen network formation inside necrotic tissue is the main histological factor of aneurysm formation [18]. The preservation of connective tissue components of myocardium would decrease the incidence of ventricular aneurysms. Dixon et al. found that matrix metalloproteinase (MMP) and tissue inhibitor of matrix metalloproteinase (TIMP) levels, critical determinants of collagen turnover, were influenced in a BMSCs’ concentration dependent manner [19]. In our study, BMSCs were directly injected into the CZ of MI, so the influence of BMSCs on collagen formation in the CZ seems stronger than that in BZC group. The expression of MMP–TIMP axis would be significantly altered with the retained injected cells, and thereby the myocardial collagen in CZ was presumed to have increased to thicken the scar. In a clinical setting, the thicker scar in CZ would avoid the formation of ventricular aneurysms and contribute to heart function recovery.
Cell proliferation and differentiation of transplanted cells usually occurs weeks after transplantation. Local cardiac environment plays a determinant role in the myogenic differentiation of BMSCs [20]. As shown in the Figure 8, there were a large number of viable myocytes existing in the BZ, and with the help of abundant blood supply and viable cardiomyocytes contact, about 10% of the total traceable cells could differentiate into the cardiac phenotype, in BZC group. Although, the CZ of chronic MI was surrounded by green stained collagen, we demonstrated that a number of viable myocytes still existed and dispersed in the CZ of MI in CZC group. Currently, it is generally accepted that paracrine secretion might be the primary contributor to restore damaged myocardium after cell transplantation [21-23]. Because telocytes seem to ‘nurse’ cardiac progenoitor cells, working in tandem with them, and playing a role in paracrine secrection [24-26], we infer that telocytes might act together with BMSCs and prompt regeneration of myocardium. As far as we know, multiple bioactive molecules (including vascular endothelial growth factor, VEGF) secreted by the implanted cells or telocytes [27] have the potential to induce neoangiogenesis and regenerate the damaged tissues. Without a doubt, after cell injection, oxygen and nutrient supply are improved, which contributes to the long-term survival of cardiomyocytes in the CZ of MI.
There are some limitations in this study. We applied a rat chronic MI model to mimic the open-chest direct cell injection. The infarction area in the current model usually accounted for 30% of the whole left ventricle free wall. In a clinical setting, besides cell-based therapy, ventricular reconstruction procedure would be necessary. Secondly, we did not add a group that received the cell injection in both BZ and CZ of MI. Owing to the small heart size, the combined injection could bias the results.
Taken together, our data demonstrated that cell delivery in the CZ of chronic MI effectively increased scar thickness, promoted angiogenesis and promoted cellular myogenic differentiation. Therefore, the CZ of MI should not be ignored during cell-based therapy. Multiple site injections (BZ+CZ) are strongly recommended during the procedures of cell transplantation.
Acknowledgments
This study was supported by the National Scientific Foundation of China (81070099), Fok Ying Tung Education Foundation (121041), National Scientific Foundation of Zhejiang province (Y2110641) and Natural Science Foundation of Wenzhou, Zhejiang, China (No. h20090019). The authors also thank Mr. Jacob Bauer from Vanderbilt University, Nashville, Tennessee, for his excellent language editing.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Nesselmann C, Ma N, Bieback K, et al. Mesenchymal stem cells and cardiac repair. J Cell Mol Med. 2008;12:1795–810. doi: 10.1111/j.1582-4934.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Oorschot AA, Smits AM, Pardali E, et al. Low oxygen tension positively influences cardiomyocyte progenitor cell function. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01270.x. ; doi: 10.1111/j.1582-4934.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen Z, Zheng S, Zhou C, et al. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. J Cell Mol Med. 2011;15:1032–43. doi: 10.1111/j.1582-4934.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson BM, Gorman JH, Moainie SL, et al. Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1160–7; discussion 8–71. doi: 10.1016/s0735-1097(02)02121-6. [DOI] [PubMed] [Google Scholar]
- 5.Hou M, Yang KM, Zhang H, et al. Transplantation of mesenchymal stem cells from human bone marrow improves damaged heart function in rats. Int J Cardiol. 2007;115:220–8. doi: 10.1016/j.ijcard.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Su W, Zhang H, Jia Z, et al. Cartilage-derived stromal cells: is it a novel cell resource for cell therapy to regenerate infarcted myocardium? Stem Cells. 2006;24:349–56. doi: 10.1634/stemcells.2005-0168. [DOI] [PubMed] [Google Scholar]
- 7.Palojoki E, Saraste A, Eriksson A, et al. Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2001;280:H2726–31. doi: 10.1152/ajpheart.2001.280.6.H2726. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, Zhu SJ, Wang W, et al. Transplantation of microencapsulated genetically modified xenogeneic cells augments angiogenesis and improves heart function. Gene Ther. 2008;15:40–8. doi: 10.1038/sj.gt.3303049. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Zhu SJ, Wang D, et al. Intramyocardial injection of tannic acid attenuates postinfarction remodeling: a novel approach to stabilize the breaking extracellular matrix. J Thorac Cardiovasc Surg. 2009;137:216–22, 22e1–2. doi: 10.1016/j.jtcvs.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Hou JF, Shen Y, et al. Low level laser irradiation precondition to create friendly milieu of infarcted myocardium and enhance early survival of transplanted bone marrow cells. J Cell Mol Med. 2010;14:1975–87. doi: 10.1111/j.1582-4934.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Hou J, Shi L, et al. Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells Dev. 2009;18:947–54. doi: 10.1089/scd.2008.0352. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Jiang Q, Zhang H, et al. Intravenous administration of bone marrow mesenchymal stromal cells is safe for the lung in a chronic myocardial infarction model. Regen Med. 2011;6:179–90. doi: 10.2217/rme.10.104. [DOI] [PubMed] [Google Scholar]
- 13.Xin Y, Wang YM, Zhang H, et al. Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: implications for tissue engineering heart valve construction. Artif Organs. 2010;34:215–22. doi: 10.1111/j.1525-1594.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Chen H, Wang W, et al. Cell survival and redistribution after transplantation into damaged myocardium. J Cell Mol Med. 2010;14:1078–82. doi: 10.1111/j.1582-4934.2010.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Jin P, Wang L, et al. Impact of escaped bone marrow mesenchymal stromal cells on extracardiac organs after intramyocardial implantation in a rat myocardial infarction model. Cell Transplant. 2010;19:1599–607. doi: 10.3727/096368910X513982. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Methot D, Poppa V, et al. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 17.Muller-Ehmsen J, Whittaker P, Kloner RA, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–16. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 18.Castagnino HE, Toranzos FA, Milei J, et al. Preservation of the myocardial collagen framework by human growth hormone in experimental infarctions and reduction in the incidence of ventricular aneurysms. Int J Cardiol. 1992;35:101–14. doi: 10.1016/0167-5273(92)90061-7. [DOI] [PubMed] [Google Scholar]
- 19.Dixon JA, Gorman RC, Stroud RE, et al. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120:S220–9. doi: 10.1161/CIRCULATIONAHA.108.842302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Yu X, Lin Q, et al. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42:295–303. doi: 10.1016/j.yjmcc.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 22.Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–36. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 23.Timmers L, Lim SK, Hoefer IE, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011;6:206–14. doi: 10.1016/j.scr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popescu LM. Telocytes—a novel type of interstitial cells. In: Braisant OHWH, Kang I, Allegaert K, et al., editors. Recent researches in modern medicine – HISTEM’11. Cambridge. WSEAS Press 2011 424–32.
- 26.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popescu LM. Telocytes and stem cells: a tandem in cardiac regenerative medicine. Curr Med Chem. 2011;18:94. [Google Scholar]


